Analytical Method Development of Benzisothiazolinone, a Biocide, Using LC–MS/MS and a Pharmacokinetic Application in Rat Biological Matrices
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Conditions and MS Detections
2.2. Method Validation
2.2.1. Specificity
2.2.2. Linearity and Sensitivity
2.2.3. Precision and Accuracy
2.2.4. Dilution Integrity
2.2.5. Extraction Recovery and Matrix Effects
2.2.6. Stability
2.3. Application of the Developed Method for BIT Pharmacokinetic Studies in Rats
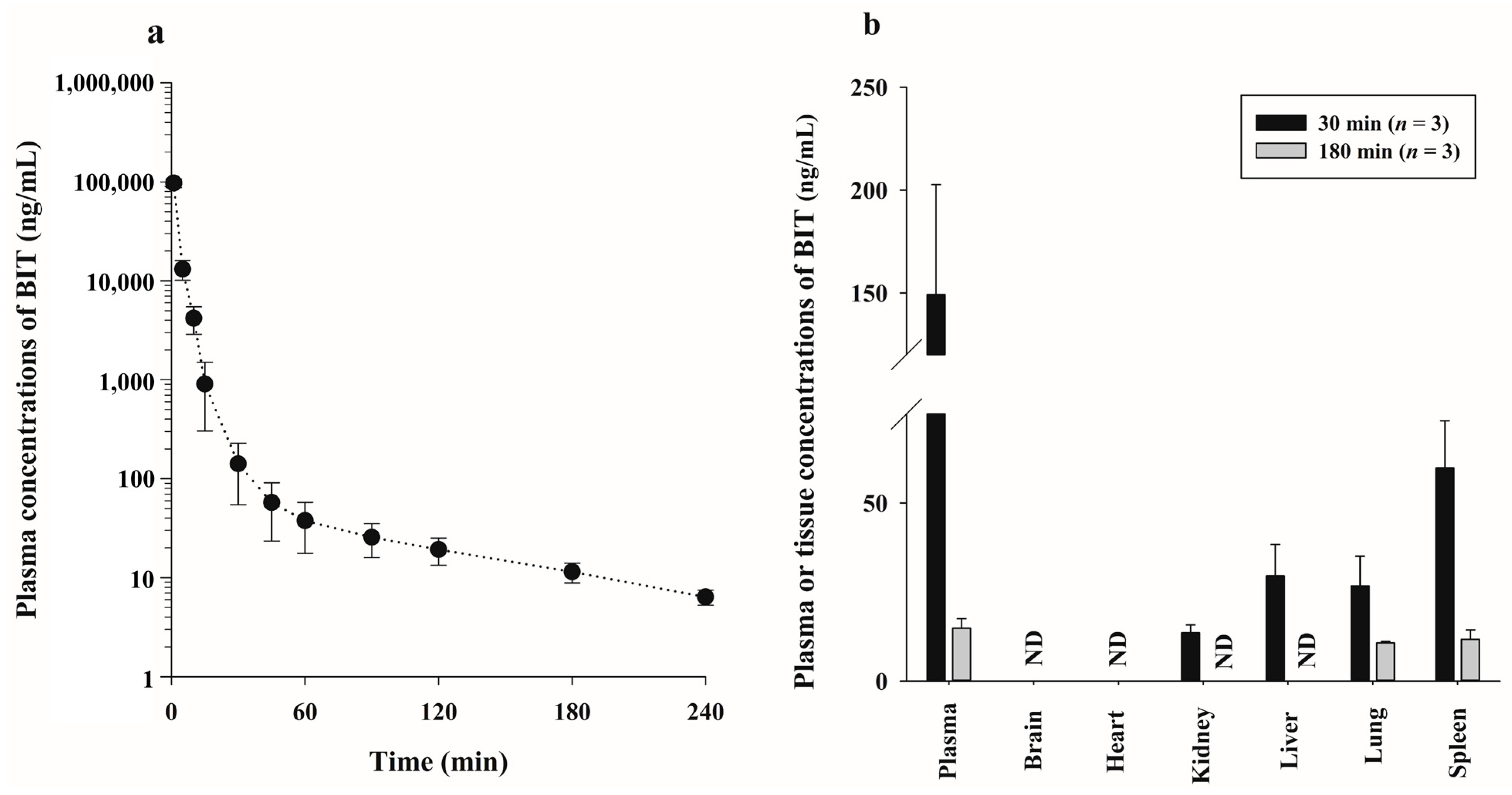
2.3.1. Intravenous Injection of BIT in Rats
2.3.2. Tissue Distribution after Intravenous Injection of BIT
2.3.3. Dermal Application of BIT on Rats
3. Materials and Methods
3.1. Materials and Reagents
3.2. Animals
3.3. LC–MS/MS Conditions
3.4. Stock Solutions, Calibration Standards, and Quality Controls
3.5. Sample Preparation
3.6. Method Validation
3.6.1. Specificity
3.6.2. Linearity and Sensitivity
3.6.3. Precision and Accuracy
3.6.4. Dilution Integrity
3.6.5. Extraction Recovery and Matrix Effects
3.6.6. BIT Stability
3.7. Application in BIT Pharmacokinetic Studies in Rats
3.7.1. Intravenous Injection of BIT in Rats
3.7.2. Tissue Distribution after Intravenous Injection of BIT in Rats
3.7.3. Dermal Application
3.8. Pharmacokinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Thomsen, A.V.; Schwensen, J.F.; Bossi, R.; Banerjee, P.; Gimenez-Arnau, E.; Lepoittevin, J.P.; Liden, C.; Uter, W.; White, I.R.; Johansen, J.D. Isothiazolinones are still widely used in paints purchased in five European countries: A follow-up study. Contact Dermat. 2018, 78, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalto-Korte, K.; Ackermann, L.; Henriks-Eckerman, M.L.; Valimaa, J.; Reinikka-Railo, H.; Leppanen, E.; Jolanki, R. 1,2-benzisothiazolin-3-one in disposable polyvinyl chloride gloves for medical use. Contact Dermat. 2007, 57, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.M.; Nelson, M.L.; Unice, K.M.; Keenan, J.J.; Paustenbach, D.J. Estimation of the safe use concentrations of the preservative 1,2-benzisothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem. Toxicol. 2013, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Schwensen, J.F.; Lundov, M.D.; Bossi, R.; Banerjee, P.; Gimenez-Arnau, E.; Lepoittevin, J.P.; Liden, C.; Uter, W.; Yazar, K.; White, I.R.; et al. Methylisothiazolinone and benzisothiazolinone are widely used in paint: A multicentre study of paints from five European countries. Contact Dermat. 2015, 72, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Schneider, K.; Gartiser, S.; Heger, W.; Mangelsdorf, I. Consumer exposure to biocides--identification of relevant sources and evaluation of possible health effects. Environ. Health 2010, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwensen, J.F.; Menne, T.; Andersen, K.E.; Sommerlund, M.; Johansen, J.D. Occupations at risk of developing contact allergy to isothiazolinones in Danish contact dermatitis patients: Results from a Danish multicentre study (2009–2012). Contact Dermat. 2014, 71, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Meysman, T.; Goossens, A. Occupational allergic contact dermatitis caused by benzisothiazolinone in printing ink and soap. Contact Dermat. 2017, 76, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-T.; Lee, M.; Seo, G.-B.; Kim, H.-M.; Shim, I.; Lee, D.-H.; Kim, T.; Seo, J.K.; Kim, P.; Choi, K. Cytotoxic Effects of Air Freshener Biocides in Lung Epithelial Cells. Nat. Prod. Commun. 2013, 8, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.; Choi, Y.S.; Na, H.G.; Bae, C.H.; Song, S.-Y.; Kim, H.G.; Kim, Y.-D. Benzisothiazolinone upregulates the MUC5AC expression via ERK1/2, p38, and NF-κB pathways in airway epithelial cells. Toxicol. Res. 2019, 8, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hidalgo, E.; Schneider, D.; von Goetz, N.; Delmaar, C.; Siegrist, M.; Hungerbühler, K. Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Environ. Int. 2018, 118, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, Y.J.; Kim, K.-B. Analytical method development of methylisothiazolinone, a preservative, in rat plasma using LC-MS/MS. J. Chromatogr. B 2018, 1100-1101, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Alsohaimi, I.H.; Khan, M.R.; Azam, M. Simultaneous Determination of Isothiazolinones and Parabens in Cosmetic Products Using Solid-Phase Extraction and Ultra-High Performance Liquid Chromatography/Diode Array Detector. Pharmaceuticals 2020, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hidalgo, E.; Sottas, V.; von Goetz, N.; Hauri, U.; Bogdal, C.; Hungerbuhler, K. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermat. 2017, 76, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.-B.; Wang, T.-J.; Song, H.; Li, B. Analysis of isothiazolinone biocides in paper for food packaging by ultra-high-performance liquid chromatography–tandem mass spectrometry. Food Addit. Contam. Part A 2010, 27, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Marrero-Aleman, G.; Borrego, L.; Antuna, A.G.; Macias Montes, A.; Perez Luzardo, O. Isothiazolinones in cleaning products: Analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and market. Contact Dermat. 2020, 82, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Rafoth, A.; Gabriel, S.; Sacher, F.; Brauch, H.-J. Analysis of isothiazolinones in environmental waters by gas chromatography–mass spectrometry. J. Chromatogr. A 2007, 1164, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Nicol, E.; Bouchonnet, S. Photodegradation of benzisothiazolinone: Identification and biological activity of degradation products. Chemosphere 2020, 240, 124862. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Bioanalytical Method Validation. Available online: https://www.fda.gov/media/70858/download (accessed on 11 January 2023).
- ICH. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 11 January 2023).
- Bae, S.K.; Yang, K.H.; Aryal, D.K.; Kim, Y.G.; Lee, M.G. Pharmacokinetics of Amitriptyline and One of Its Metabolites, Nortriptyline, in Rats: Little Contribution of Considerable Hepatic First-Pass Effect to Low Bioavailability of Amitriptyline Due to Great Intestinal First-Pass Effect. J. Pharm. Sci. 2009, 98, 1587–1601. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.G.; Kim, M.G.; Shin, B.S.; Kim, K.B.; Lee, J.B.; Paik, S.H.; Yoo, S.D. Simultaneous determination of phenoxyethanol and its major metabolite, phenoxyacetic acid, in rat biological matrices by LC-MS/MS with polarity switching: Application to ADME studies. Talanta 2015, 144, 29–38. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guideline for the Testing of Chemicals. Available online: https://www.oecd-ilibrary.org/docserver/9789264071063-en.pdf?expires=1623402038&id=id&accname=guest&checksum=14408C560774C43FCC42A61F30845B21 (accessed on 11 January 2023).




| Matrices | Spiked Concentration (ng/mL) | Intra-Day (n = 6) | Inter-Day (n = 5) | Extraction Recovery Mean ± SD (%) | Matrix Effects Mean ± SD (%) | ||
|---|---|---|---|---|---|---|---|
| Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | Accuracy (%) | ||||
| Plasma | 2 | 11.3 | 100 | 8.66 | 104 | ||
| 4 | 4.31 | 99.4 | 4.33 | 101 | 90.8 ± 7.26 | 108 ± 3.58 | |
| 400 | 3.34 | 109 | 8.18 | 102 | 91.6 ± 6.00 | 107 ± 6.54 | |
| 1600 | 5.37 | 108 | 6.52 | 103 | 93.5 ± 6.25 | 102 ± 3.60 | |
| Urine | 2 | 4.92 | 99.1 | 9.59 | 101 | ||
| 4 | 5.51 | 101 | 8.36 | 100 | 92.7 ± 2.72 | 100 ± 6.30 | |
| 400 | 3.96 | 95.6 | 4.95 | 103 | 91.3 ± 1.67 | 97.8 ± 3.48 | |
| 1600 | 4.39 | 108 | 3.82 | 109 | 90.4 ± 2.25 | 101 ± 4.66 | |
| IS | 10 | 99.1 ± 4.54 | 102 ± 3.50 | ||||
| Matrices | Spiked Concentration (ng/mL) | Intra-Day (n = 6) | Inter-Day (n = 5) | Extraction Recovery Mean ± SD (%) | Matrix Effects Mean ± SD (%) | ||
|---|---|---|---|---|---|---|---|
| Precision (RSD, %) | Accuracy
(%) | Precision (RSD, %) | Accuracy (%) | ||||
| Brain | 10 | 12.8 | 96.4 | 12.9 | 96.3 | ||
| 30 | 5.65 | 99.8 | 11.4 | 100 | 86.6 ± 3.32 | 110 ± 3.58 | |
| 150 | 3.31 | 102 | 8.23 | 103 | 85.6 ± 4.43 | 103 ± 2.34 | |
| 800 | 6.08 | 105 | 5.38 | 107 | 90.3 ± 3.49 | 103 ± 8.44 | |
| Liver | 10 | 7.25 | 100 | 10.2 | 101 | ||
| 30 | 5.40 | 95.7 | 4.92 | 106 | 85.8 ± 6.50 | 109 ± 3.08 | |
| 150 | 4.23 | 94.9 | 9.29 | 99.3 | 85.9 ± 5.65 | 105 ± 4.45 | |
| 800 | 5.26 | 101 | 8.34 | 103 | 86.5 ± 5.38 | 110 ± 3.66 | |
| Lung | 10 | 8.64 | 99.4 | 7.12 | 107 | ||
| 30 | 3.72 | 102 | 9.65 | 104 | 87.8 ± 3.38 | 110 ± 5.61 | |
| 150 | 3.62 | 91.8 | 5.63 | 100 | 89.2 ± 3.02 | 105 ± 4.91 | |
| 800 | 7.24 | 97.7 | 4.92 | 106 | 84.4 ± 5.09 | 107 ± 6.33 | |
| Kidney | 10 | 10.2 | 98.7 | 8.16 | 106 | ||
| 30 | 4.59 | 101 | 10.2 | 100 | 83.0 ± 4.32 | 103 ± 3.14 | |
| 150 | 5.15 | 98.8 | 6.45 | 106 | 86.2 ± 5.84 | 105 ± 5.81 | |
| 800 | 4.53 | 90.3 | 4.42 | 100 | 84.7 ± 3.31 | 107 ± 3.66 | |
| Heart | 10 | 14.4 | 99.4 | 8.09 | 103 | ||
| 30 | 4.25 | 94.5 | 5.45 | 101 | 87.4 ± 4.14 | 103 ± 3.56 | |
| 150 | 5.40 | 94.2 | 6.60 | 96.2 | 84.5 ± 3.91 | 98.5 ± 8.34 | |
| 800 | 4.83 | 109 | 10.3 | 98.2 | 84.9 ± 5.35 | 104 ± 7.80 | |
| Spleen | 10 | 13.7 | 98.0 | 12.3 | 96.5 | ||
| 30 | 8.96 | 103 | 5.08 | 95.5 | 86.7 ± 6.70 | 104 ± 5.49 | |
| 150 | 4.51 | 92.4 | 5.47 | 102 | 89.0 ± 3.83 | 102 ± 7.54 | |
| 800 | 5.96 | 105 | 10.4 | 99.4 | 93.3 ± 3.76 | 105 ± 5.08 | |
| Storage Conditions | Concentration (ng/mL) | Precision (RSD, %) | Accuracy (%) | |
|---|---|---|---|---|
| Spiked | Measured (Mean ± SD) | |||
| Plasma | ||||
| Bench-top stability | 4 | 4.04 ± 0.230 | 5.76 | 101 |
| (6 h at room temperature) | 400 | 376 ± 22.8 | 5.70 | 93.9 |
| 1600 | 1490 ± 74.1 | 4.63 | 92.8 | |
| Long-term stability | 4 | 4.31 ± 0.182 | 4.55 | 108 |
| (2 months at −80 °C) | 400 | 448 ± 15.6 | 3.89 | 112 |
| 1600 | 1720 ± 118 | 7.36 | 107 | |
| Freeze–thaw stability | 4 | 3.77 ± 0.371 | 9.28 | 94.1 |
| (three freeze–thaw cycles) | 400 | 373 ± 21.7 | 5.41 | 93.2 |
| 1600 | 1540 ± 58.8 | 3.68 | 95.9 | |
| Post-preparative/autosampler stability | 4 | 3.99 ± 0.526 | 13.2 | 99.8 |
| (24 h at 4 °C) | 400 | 381 ± 24.2 | 6.05 | 95.3 |
| 1600 | 1560 ± 98.0 | 6.13 | 97.6 | |
| Urine | ||||
| Bench-top stability | 4 | 3.84 ± 0.288 | 7.52 | 95.9 |
| (at room temperature for 6 h) | 400 | 390 ± 19.0 | 4.87 | 97.5 |
| 1600 | 1770 ± 23.4 | 1.32 | 111 | |
| Long-term stability | 4 | 3.87 ± 0.264 | 6.83 | 96.8 |
| (at −80 °C for 2 months) | 400 | 412 ± 16.9 | 4.11 | 103 |
| 1600 | 1720 ± 139 | 8.10 | 108 | |
| Freeze–thaw stability | 4 | 4.04 ± 0.183 | 4.52 | 101 |
| (three freeze–thaw cycles) | 400 | 385 ± 25.9 | 6.74 | 96.2 |
| 1600 | 1740 ± 46.6 | 2.68 | 108 | |
| Post-preparative/autosampler stability | 4 | 3.84 ± 0.204 | 5.31 | 96.1 |
| (at 4 °C for 24 h) | 400 | 373 ± 21.1 | 5.65 | 93.2 |
| 1600 | 1750 ± 44.7 | 2.56 | 109 | |
| Parameters (Units) | 10 mg/kg (n = 6) |
|---|---|
| AUCt (μg·min/mL) 1 | 359 ± 49.8 |
| AUCinf (μg·min/mL) 2 | 360 ± 49.9 |
| t1/2 (min) 3 | 79.2 ± 10.4 |
| CL (mL/min/kg) 4 | 28.3 ± 3.79 |
| MRT (min) 5 | 4.60 ± 0.621 |
| Vdss (mL/kg) 6 | 129 ± 19.0 |
| Ae0–24 h (% dose) 7 | 0.0506 ± 0.0136 |
| GI24 h (% dose) 8 | 0.0324 ± 0.00639 |
| Parameters (Units) | Dermal Application Amount |
|---|---|
| 10 mg/Rat (n = 8) | |
| Dermal absorbed amount for 4 h (mg) | 0.720 ± 0.122 |
| AUCt (μg·min/mL) 1 | 8.58 ± 0.905 |
| AUCinf (μg·min/mL) 2 | 10.6 ± 2.39 |
| t1/2 (min) 3 | 101 ± 15.7 |
| Cmax (ng/mL) 4 | 41.1 ± 7.87 |
| Tmax (min) 5 | 245 (240–255) |
| F (%) 6 | 11.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.J.; Huang, Z.; Lee, C.B.; Chae, S.U.; Bae, S.H.; Bae, S.K. Analytical Method Development of Benzisothiazolinone, a Biocide, Using LC–MS/MS and a Pharmacokinetic Application in Rat Biological Matrices. Molecules 2023, 28, 845. https://doi.org/10.3390/molecules28020845
Jo SJ, Huang Z, Lee CB, Chae SU, Bae SH, Bae SK. Analytical Method Development of Benzisothiazolinone, a Biocide, Using LC–MS/MS and a Pharmacokinetic Application in Rat Biological Matrices. Molecules. 2023; 28(2):845. https://doi.org/10.3390/molecules28020845
Chicago/Turabian StyleJo, Seong Jun, Zhouchi Huang, Chae Bin Lee, Soon Uk Chae, Soo Hyeon Bae, and Soo Kyung Bae. 2023. "Analytical Method Development of Benzisothiazolinone, a Biocide, Using LC–MS/MS and a Pharmacokinetic Application in Rat Biological Matrices" Molecules 28, no. 2: 845. https://doi.org/10.3390/molecules28020845
APA StyleJo, S. J., Huang, Z., Lee, C. B., Chae, S. U., Bae, S. H., & Bae, S. K. (2023). Analytical Method Development of Benzisothiazolinone, a Biocide, Using LC–MS/MS and a Pharmacokinetic Application in Rat Biological Matrices. Molecules, 28(2), 845. https://doi.org/10.3390/molecules28020845







