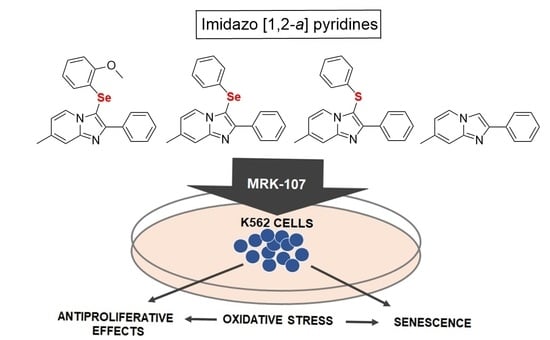
Selenylated Imidazo[1,2-a]pyridine Induces Cell Senescence and Oxidative Stress in Chronic Myeloid Leukemia Cells
Abstract
1. Introduction
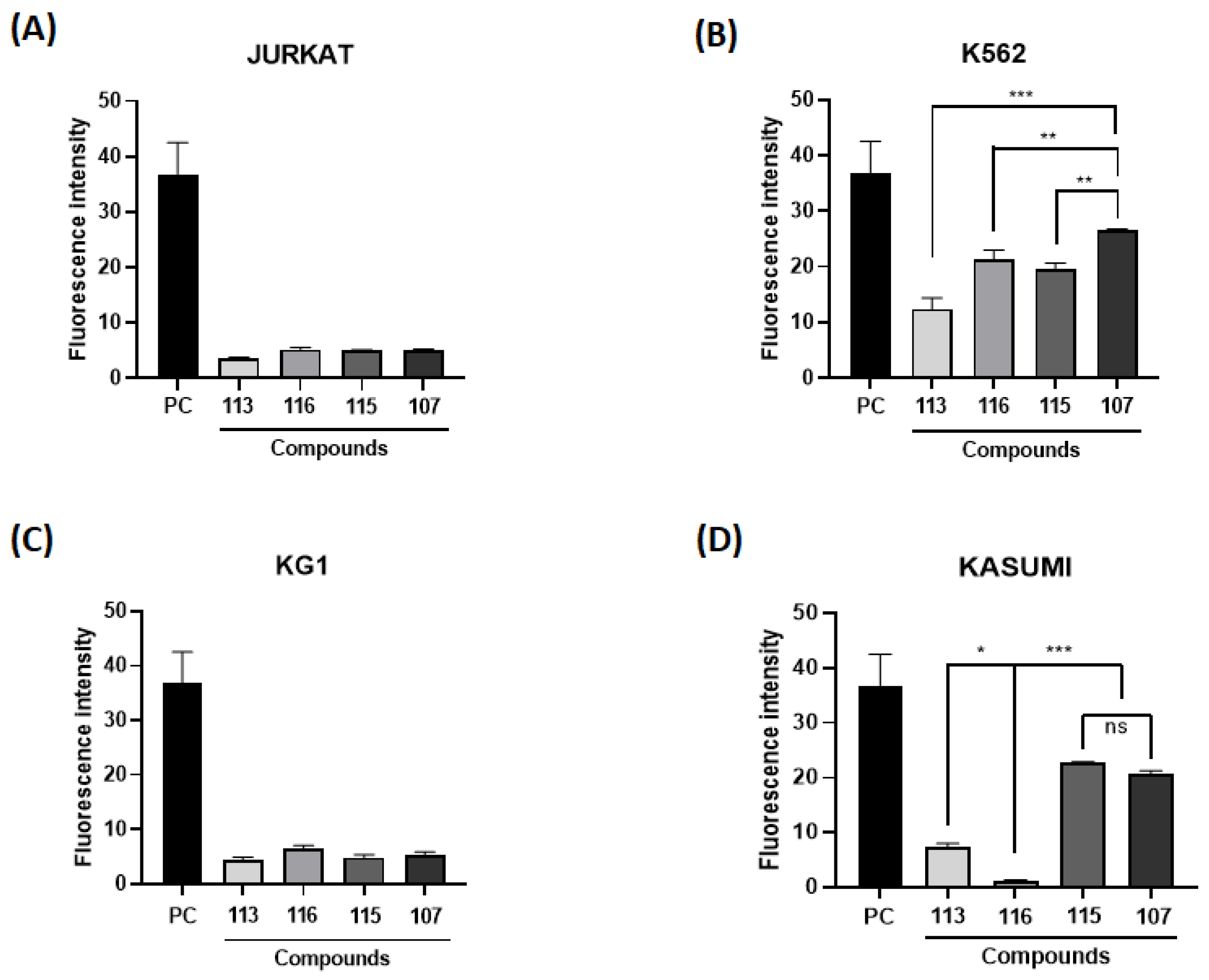
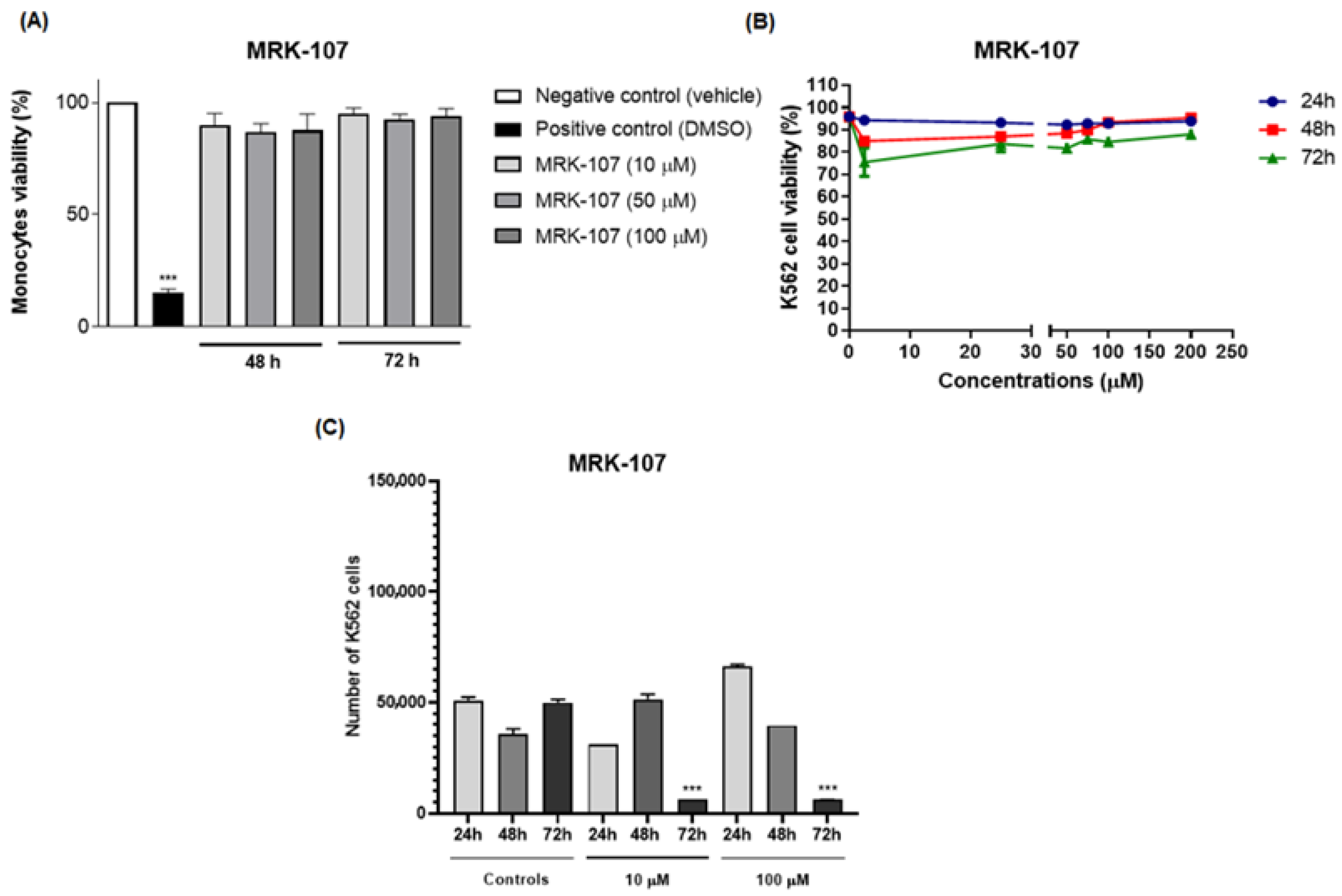
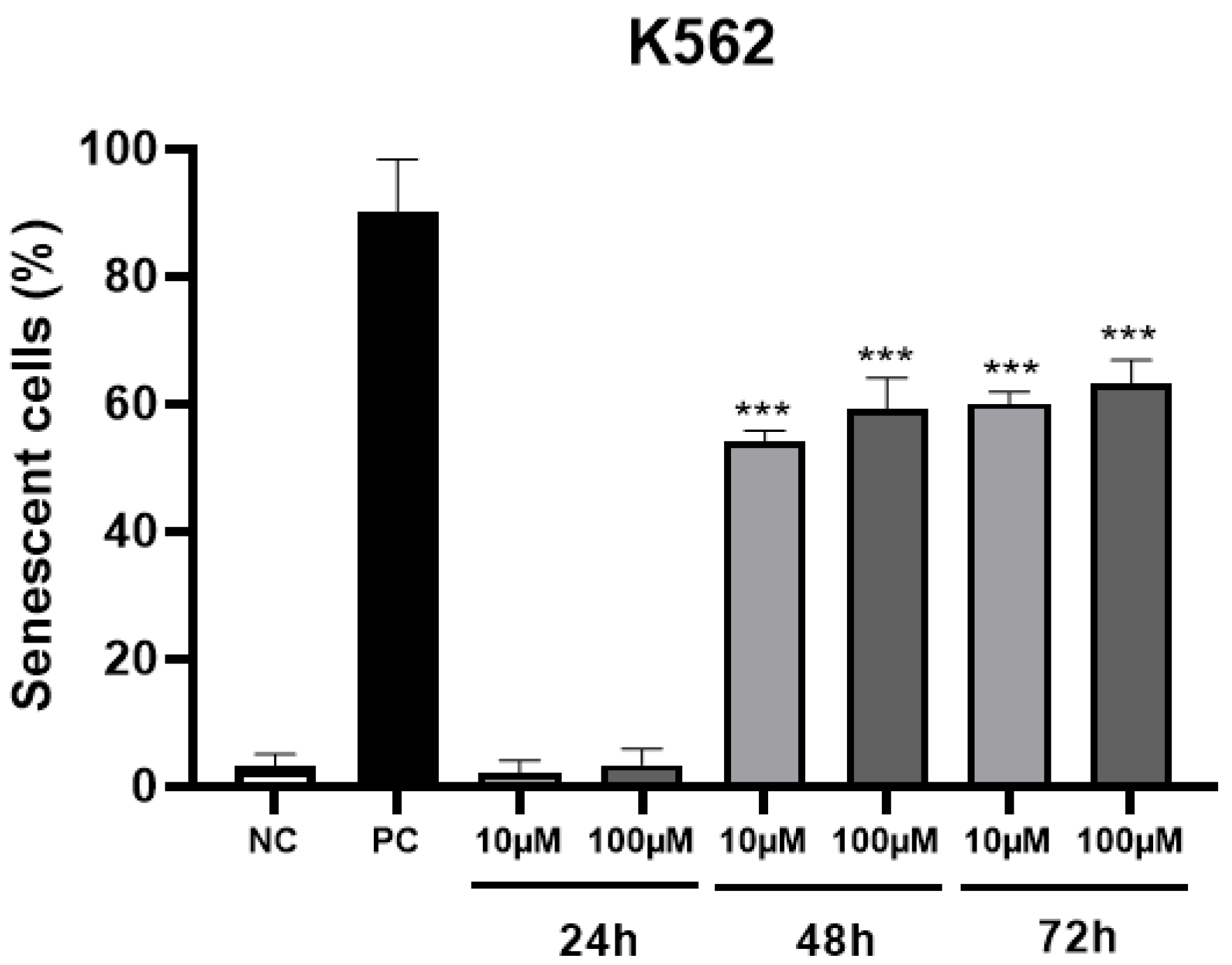
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Imidazo[1,2-a]pyridines and Chalcogenated Derivatives
4.2. Oral Bioavailability and Toxicity of Compounds: In Silico Analysis
4.3. Cell Culture
4.4. Redox Effect Screening: Measurement of Intracellular ROS
4.5. Cytotoxicity Assay
4.6. Cell Death Assay
4.7. Cell Proliferation Assay
4.8. Senescence Assay
4.9. Oxidative Stress Markers
4.9.1. Lipid Peroxidation Assessment
4.9.2. Reduced Glutathione Assay (Non-Protein Thiols)
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juliusson, G.; Hough, R. Leukemia. Prog. Tumor Res. 2016, 43, 87–100. [Google Scholar] [PubMed]
- Whiteley, A.E.; Price, T.T.; Cantelli, G.; Sipkins, D.A. Leukaemia: A model metastatic disease. Nat. Rev. Cancer 2021, 21, 461–475. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017. 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf (accessed on 16 November 2022).
- Cortes, J.; Silver, R.; Kantarjian, H. Chronic myeloid leucemia, In Abeloff’s Clinical Oncology, 4th ed.; Abeloff, M., Armitage, J., Niederhuber, J., Kastan, M., McKenna, G., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 2279–2293. [Google Scholar]
- Kantarjian, H.; O’Brien, S. The chronic leukemias: Chronic myelogenous leucemia. In Cecil Medicine, 23rd ed.; Goldman, L., Ausiello, D., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 1397–1408. [Google Scholar]
- Huang, X.; Cortes, J.; Kantarjian, H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 2012, 118, 3123–3127. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.J.; Jung, K.H.; Son, M.K.; Yan, H.H.; Hong, S.; Hong, S.-S. A novel imidazopyridine PI3K inhibitor with anticancer activity in non-small cell lung cancer cells. Oncol. Rep. 2013, 30, 863–869. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Z.; Zhang, Y.; Shan, X.; Jiang, L.; Zhao, Y.; Liang, G. Synthesis and anti-in flammatory evaluation of novel benzimidazole and imidazopyridine derivatives. ACS Med. Chem. Lett. 2013, 4, 69–74. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, K.H. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2011, 5, 299–304. [Google Scholar] [CrossRef]
- Zou, B.; Nagle, A.; Chatterjee, A.K.; Leong, S.Y.; Tan, L.J.; Sim, W.L.S.; Mishra, P.; Guntapalli, P.; Tully, D.C.; Lakshminarayana, S.B.; et al. Lead Optimization of Imidazopyrazines: A New Class of Antimalarial with Activity on Plasmodium Liver Stages. ACS Med. Chem. Lett. 2014, 5, 947–950. [Google Scholar] [CrossRef]
- Yu, Y.N.; Han, Y.; Zhang, F.; Gao, Z.; Zhu, T.; Dong, S.; Ma, M. Design, synthesis, and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3K/mTOR dual inhibitors. J. Med. Chem. 2020, 63, 3028–3046. [Google Scholar] [CrossRef]
- Frizon, T.E.A.; Cararo, J.H.; Saba, S.; Dal-Pont, G.C.; Michels, M.; Braga, H.; Pimentel, T.; Dal-Pizzol, F.; Valvassori, S.S.; Rafique, J. Synthesis of Novel Selenocyanates and Evaluation of Their Effect in Cultured Mouse Neurons Submitted to Oxidative Stress. Oxidative Med. Cell Longev. 2020, 2020, 5417024. [Google Scholar] [CrossRef]
- Almeida, G.M.; Rafique, J.; Saba, S.; Siminski, T.; Mota, N.S.; Wilhelm Filho, D.; Ourique, F. Novel selenylated imidazo[1,2- a ]pyridines for breast cancer chemotherapy: Inhibition of cell proliferation by Akt-mediated regulation, DNA cleavage and apoptosis. Biochem. Biophys. Res. Commun. 2018, 503, 1291–1297. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Rafique, J.; Saba, S.; Almeida, G.M.; Siminski, T.; Pádua, C.; Ourique, F. Apoptosis oxidative damage-mediated and antiproliferative effect of selenylated imidazo[1,2-a]pyridines on hepatocellular carcinoma HepG2 cells and in vivo. J. Biochem. Mol. Toxicol. 2021, 35, e22663. [Google Scholar] [CrossRef]
- Veloso, I.C.; Delanogare, E.; Machado, A.E.; Braga, S.P.; Rosa, G.K.; De Bem, A.F.; Rafique, J.; Saba, S.; Trindade, R.N.; Galetto, F.Z.; et al. A selanylimidazopyridine (3-SePh-IP) reverses the prodepressant- and anxiogenic-like effects of a high-fat/high-fructose diet in mice. J. Pharm. Pharmacol. 2021, 73, 673–681. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, R.A.; Semreen, M.H.; Saber-Ayad, M.M.; Cyprian, F.; Menon, V.; Al-Tel, T.H. Modulation of DNA damage response and induction of apoptosis mediates synergism between doxorubicin and a new imidazopyridine derivative in breast and lung cancer cells. DNA Repair 2016, 37, 1–11. [Google Scholar] [CrossRef]
- Battram, A.M.; Bachiller, M.; Martín-Antonio, B. Senescence in the Development and Response to Cancer with Immunotherapy: A Double-Edged Sword. Int. J. Mol. Sci. 2020, 21, 4346. [Google Scholar] [CrossRef] [PubMed]
- Felipe, K.; Benites, J.; Glorieux, C.; Sid, B.; Valenzuela, M.; Kviecinski, M.; Pedrosa, R.; Valderrama, J.; Levêque, P.; Gallez, B.; et al. Antiproliferative effects of phenylaminonaphthoquinones are increased by ascorbate and associated with the appearance of a senescent phenotype in human bladder cancer cells. Biochem. Biophys. Res. Commun. 2013, 433, 573–578. [Google Scholar] [CrossRef]
- Galant, L.S.; Rafique, J.; Braga, A.L.; Braga, F.C.; Saba, S.; Radi, R.; da Rocha, J.B.T.; Santi, C.; Monsalve, M.; Farina, M.; et al. The Thiol-Modifier Effects of Organoselenium Compounds and Their Cytoprotective Actions in Neuronal Cells. Neurochem. Res. 2021, 46, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Saba, S.; Rafique, J.; Braga, A.L. KIO4-mediated Selective Hydroxymethylation/Methylenation of Imidazo-Heteroarenes: A Greener Approach. Angew. Chem. Int. Ed. Engl. 2021, 60, 18454–18460. [Google Scholar] [CrossRef]
- Saba, S.; dos Santos, C.R.; Zavarise, B.R.; Naujorks, A.A.S.; Franco, M.S.; Schneider, A.R.; Scheide, M.R.; Affeldt, R.F.; Rafique, J.; Braga, A.L. Photoinduced, Direct C(sp2)−H Bond Azo Coupling of Imidazoheteroarenes and Imidazoanilines with Aryl Diazonium Salts Catalyzed by Eosin Y. Chem. Eur. J. 2020, 26, 4461–4466. [Google Scholar] [CrossRef]
- Peterle, M.M.; Scheide, M.R.; Silva, L.T.; Saba, S.; Rafique, J.; Braga, A.L. Copper-Catalyzed Three-Component Reaction of Oxadiazoles, Elemental Se/S and Aryl Iodides: Synthesis of Chalcogenyl (Se/S)-Oxadiazoles. ChemsitrySelect 2018, 3, 13191–13196. [Google Scholar] [CrossRef]
- Meirinho, A.G.; Pereira, V.F.; Martins, G.M.; Saba, S.; Rafique, J.; Braga, A.L.; Mendes, S.R. Electrochemical Oxidative C(sp2)–H Bond Selenylation of Activated Arenes. Eur. J. Org. Chem. 2019, 2019, 6465–6469. [Google Scholar] [CrossRef]
- Botteselle, G.; Elias, W.; Bettanin, L.; Canto, R.; Salin, D.; Barbosa, F.; Saba, S.; Gallardo, H.; Ciancaleoni, G.; Domingos, J.; et al. Catalytic Antioxidant Activity of Bis-Aniline-Derived Diselenides as GPx Mimics. Molecules 2021, 26, 4446. [Google Scholar] [CrossRef] [PubMed]
- Rafique, J.; Farias, G.; Saba, S.; Zapp, E.; Bellettini, I.C.; Salla, C.A.M.; Bechtold, I.H.; Scheide, M.R.; Neto, J.S.S.; Junior, D.M.D.S.; et al. Selenylated-oxadiazoles as promising DNA intercalators: Synthesis, electronic structure, DNA interaction and cleavage. Dye. Pigment. 2020, 180, 108519–108519. [Google Scholar] [CrossRef] [PubMed]
- Scheide, M.R.; Peterle, M.M.; Saba, S.; Neto, J.S.S.; Lenz, G.F.; Cezar, R.D.; Felix, J.F.; Botteselle, G.V.; Schneider, R.; Rafique, J.; et al. Borophosphate glass as an active media for CuO nanoparticle growth: An efficient catalyst for selenylation of oxadiazoles and application in redox reactions. Sci. Rep. 2020, 10, 15233. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.L.; Townsend, D.M.; Tew, K.D. The impact of redox and thiol status on the bone marrow: Pharmacological intervention strategies. Pharmacol. Ther. 2011, 129, 172–184. [Google Scholar] [CrossRef]
- Davies, K.J. The broad spectrum of responses to oxidants in proliferating cells: A new paradigm for oxidative stress. IUBMB Life 1999, 48, 41–47. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Rafique, J.; Saba, S.; Grinevicius, V.M.; Zamoner, A.; Braga, A.L.; Ourique, F. IP-Se-06, a Selenylated Imidazo [1, 2-a] pyridine, Modulates Intracellular Redox State and Causes Akt/mTOR/HIF-1α and MAPK Signaling Inhibition, Promoting Antiproliferative Effect and Apoptosis in Glioblastoma Cells. Oxid. Med. Cell Longev. 2022, 2022, 3710449. [Google Scholar] [CrossRef]
- Pérez-Mancera, P.A.; Young, A.R.J.; Narita, M. Inside and out: The activities of senescence in cancer. Nat. Rev. Cancer 2014, 14, 547–558. [Google Scholar] [CrossRef]
- Lee, S.; Schmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef]
- Guillon, J.; Petit, C.; Toutain, B.; Guette, C.; Lelièvre, E.; Coqueret, O. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity. Cell Cycle 2019, 18, 2385–2397. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Guo, Y.-L.; Chakraborty, S.; Rajan, S.S.; Wang, R.; Huang, F. Effects of Oxidative Stress on Mouse Embryonic Stem Cell Proliferation, Apoptosis, Senescence, and Self-Renewal. Stem Cells Dev. 2010, 19, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Qin, S.; Townsend, D.; Schulte, B.A.; Tew, K.D.; Wang, G.Y. Oxidative stress induces senescence in breast cancer stem cells. Biochem. Biophys. Res. Commun. 2019, 514, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Kapor, S.; Čokić, V.; Santibanez, J.F. Mechanisms of Hydroxyurea-Induced Cellular Senescence: An Oxidative Stress Connection? Oxidative Med. Cell Longev. 2021, 2021, 7753857. [Google Scholar] [CrossRef]
- Dong, C.M.; Wang, X.L.; Wang, G.M.; Zhang, W.J.; Zhu, L.; Gao, S.; Xu, J. A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis. 2014, 5, e1116. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wei, R.; Liu, J.; Wang, H.; Cai, W.; Zhao, M.; Hu, Y.; Wang, S.; Yang, T.; Liu, X.; et al. Drug-induced premature senescence model in human dental follicle stem cells. Oncotarget 2017, 8, 7276–7293. [Google Scholar] [CrossRef]
- Geng, K.; Fu, N.; Yang, X.; Xia, W. Adjudin delays cellular senescence through Sirt3-mediated attenuation of ROS production. Int. J. Mol. Med. 2018, 42, 3522–3529. [Google Scholar] [CrossRef]
- Sieben, C.J.; Sturmlechner, I.; van de Sluis, B.; van Deursen, J.M. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018, 28, 723–737. [Google Scholar] [CrossRef]
- Lowe, S.W.; Cepero, E.; Evan, G. Intrinsic tumour suppression. Nature 2004, 432, 307–315. [Google Scholar] [CrossRef]
- Baraibar, M.A.; Hyzewicz, J.; Rogowska-Wrzesinska, A.; Bulteau, A.L.; Prip-Buus, C.; Butler-Browne, G.; Friguet, B. Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes. Free Radic. Biol. Med. 2014, 75, S23. [Google Scholar] [CrossRef] [PubMed]
- Parisotto, E.B.; Vidal, V.; García-Cerro, S.; Lantigua, S.; Filho, D.W.; Sanchez-Barceló, E.J.; Martínez-Cué, C.; Rueda, N. Chronic Melatonin Administration Reduced Oxidative Damage and Cellular Senescence in the Hippocampus of a Mouse Model of Down Syndrome. Neurochem. Res. 2016, 41, 2904–2913. [Google Scholar] [CrossRef]
- Kona, S.; Ravi, R.S.; Chava, V.N.R.; Perali, R.S. A Convenient Synthesis of C-3-Aryloxymethyl Imidazo[1,2-a]Pyridine Derivatives. J. Chem. 2013, 2013, 296792. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Rosario, A.R.; Braga, A.L. Braga. Regioselective, Solvent- and Metal-Free Chalcogenation of Imidazo[1,2-a ]pyridines by Employing I2/DMSO as the Catalytic Oxidation System. Chem. Eur. J. 2016, 22, 11854–11862. [Google Scholar] [CrossRef]
- Bettanin, L.; Saba, S.; Doerner, C.V.; Franco, M.S.; Godoi, M.; Rafique, J.; Braga, A.L. NH4I-catalyzed chalcogen(S/Se)-functionalization of 5-membered N-heteroaryls under metal-free conditions. Tetrahedron 2018, 74, 3971–3980. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Franco, M.S.; Bettanin, L.; Schneider, A.R.; Silva, L.T.; Braga, A.L. Direct, Metal-free C(sp2)−H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO3. Chem. Eur. J. 2018, 24, 4173–4180. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espíndola, L.; Silva, D.O.; Braga, A.L. Rose Bengal catalysed photo-induced selenylation of indoles, imidazoles and arenes: A metal free approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and Practical Applications of 2′,7′-Dichlorodihydrofluorescein in Redox Assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- De Novais, L.M.; Ferreira, L.F.; de Sousa, P.T., Jr.; Ribeiro, T.A.; Jacinto, M.J.; Dos Santos, C.H.; de Carvalho, M.G.; Torquato, H.F.V.; Parede-Gamero, E.J.; Silva, V.C.P. Eglerisine, a Novel Sesquiterpenoid Tropolone from Dulacia egleri with Antiproliferative Effect against an Acute Myeloid Leukemia Lineage. Planta Med. 2020, 86, 55–60. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Gomes, G.B.; Zubieta, C.S.; Weber, S.S.; de Lima, D.P.; Reddy, T.N.; Guerrero, A.T.G.; Matos, M.D.F.C.; Parisotto, E.B.; Perdomo, R.T. Thiopyrimidine derivatives induce cytotoxicity, cell cycle arrest and oxidative stress in breast cancer 3D-spheroids. Chem. Pap. 2020, 75, 1211–1220. [Google Scholar] [CrossRef]
- Bird, R.P.; Draper, A.H. Comparative studies on different methods of malondyhaldehyde determination. Methods Enzymol. 1984, 90, 105–110. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]


| Compound | MRK-107 | MRK-113 | MRK-115 | MRK-116 |
|---|---|---|---|---|
| mLogP | 3.37 | 3.76 | 2.54 | 4.61 |
| MW (g/mol) | 393.34 | 363.31 | 208.26 | 316.42 |
| n° of violations | 0 | 0 | 0 | 1 (mLogP > 4.15) |
| Mutagenicity | - | - | - | - |
| Tumorigenicity | - | - | - | - |
| Irritability | - | - | - | - |
| Effects on reproduction | Yes | Yes | Yes | Yes |
| Absorption in the GIT | High | High | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkner, G.T.; Dias, D.A.; Souza, K.F.S.d.; Araújo, A.J.P.d.; Basilio, D.C.L.S.; Jacobsen, F.T.; Moraes, A.C.R.d.; Silva-Filho, S.E.; Cavalcante, M.F.d.O.; Moraes, C.A.d.O.; et al. Selenylated Imidazo[1,2-a]pyridine Induces Cell Senescence and Oxidative Stress in Chronic Myeloid Leukemia Cells. Molecules 2023, 28, 893. https://doi.org/10.3390/molecules28020893
Burkner GT, Dias DA, Souza KFSd, Araújo AJPd, Basilio DCLS, Jacobsen FT, Moraes ACRd, Silva-Filho SE, Cavalcante MFdO, Moraes CAdO, et al. Selenylated Imidazo[1,2-a]pyridine Induces Cell Senescence and Oxidative Stress in Chronic Myeloid Leukemia Cells. Molecules. 2023; 28(2):893. https://doi.org/10.3390/molecules28020893
Chicago/Turabian StyleBurkner, Gabriella Teles, Dhébora Albuquerque Dias, Kamylla Fernanda Souza de Souza, Anna Júlia Papa de Araújo, Denise Caroline Luiz Soares Basilio, Fernanda Tondello Jacobsen, Ana Carolina Rabello de Moraes, Saulo Euclides Silva-Filho, Marcos Filipe de Oliveira Cavalcante, Cassio Augusto de Oliveira Moraes, and et al. 2023. "Selenylated Imidazo[1,2-a]pyridine Induces Cell Senescence and Oxidative Stress in Chronic Myeloid Leukemia Cells" Molecules 28, no. 2: 893. https://doi.org/10.3390/molecules28020893
APA StyleBurkner, G. T., Dias, D. A., Souza, K. F. S. d., Araújo, A. J. P. d., Basilio, D. C. L. S., Jacobsen, F. T., Moraes, A. C. R. d., Silva-Filho, S. E., Cavalcante, M. F. d. O., Moraes, C. A. d. O., Saba, S., Macedo, M. L. R., Paredes-Gamero, E. J., Rafique, J., & Parisotto, E. B. (2023). Selenylated Imidazo[1,2-a]pyridine Induces Cell Senescence and Oxidative Stress in Chronic Myeloid Leukemia Cells. Molecules, 28(2), 893. https://doi.org/10.3390/molecules28020893