Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review
Abstract
:1. Introduction
2. The Role of Hydrogels with Different Gel-Forming Mechanisms in Promoting Bone Repair
2.1. Physically Cross-Linked Hydrogels
2.2. Injectable Hydrogels
2.3. Self-Healing Hydrogel
2.4. Photocurable Hydrogels
2.5. Temperature-Sensitive Hydrogels
2.6. Stimuli-Responsive Hydrogels
3. Role of Different Material Matrix Hydrogels in Promoting Bone Repair
3.1. Hydroxyapatite
3.2. Polysaccharide Compounds
3.3. Silk Fibroin
4. Hydrogels Loaded with Natural Product Nanoparticles for Bone Repair Applications
5. Discussion and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, J.; Zheng, K.; Huang, X.; Liu, J.; Liu, H.; Boccaccini, A.R.; Wan, Y.; Guo, X.; Shao, Z. Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomater. 2019, 91, 60–71. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sajadi-Javan, Z.S.; Varshosaz, J.; Mirian, M.; Manshaei, M.; Aminzadeh, A. Thermo-responsive hydrogels based on methylcellulose/persian gum loaded with taxifolin enhance bone regeneration: An in vitro/in vivo study. Cellulose 2022, 29, 21. [Google Scholar] [CrossRef]
- Zhang, M.; Matinlinna, J.P.; Tsoi, J.; Liu, W.W.; Cui, X.; Lu, W.; Pan, H. Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview. J. Orthop. Transl. 2020, 22, 26–33. [Google Scholar] [CrossRef]
- Song, L.; Xie, X.; Lv, C.; Khan, A.U.R.; Sun, Y.; Li, R.; Yao, J.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; et al. Electrospun biodegradable nanofibers loaded with epigallocatechin gallate for guided bone regeneration. Compos. Pt. B-Eng. 2022, 238, 12. [Google Scholar] [CrossRef]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2018, 21, 362–376. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Gao, F.; Zheng, P.; Liu, W. Repair of volumetric bone defects with a high strength bmp-loaded-mineralized hydrogel tubular scaffold. J. Mater. Chem. B 2017, 5, 5588–5596. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Z.; Liu, W.; Shi, Y.; Cui, L.; Cao, Y. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J. Craniofac. Surg. 2001, 12, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ding, L.; Deng, D. Research progress of hydrogel combined with mesenchymal stem cells in the treatment of spinal cord injury. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2021, 38, 805–811. [Google Scholar]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in hydrogels for skin wound repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Huang, K.; Luo, Y.; Zhang, L.; Kuang, T.; Chen, Z.; Liao, G. Double network hydrogel for tissue engineering. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2018, 10, e1520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Yang, M. Application of hydrogels in cartilage tissue engineering. Curr. Stem Cell Res. Ther. 2018, 13, 497–516. [Google Scholar] [CrossRef]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite hydrogels for bone regeneration. Materials 2016, 9, 267. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Chen, W.; Hu, Y.; Geng, Z.; Su, J. Bone/cartilage targeted hydrogel: Strategies and applications. Bioact. Mater. 2023, 23, 156–169. [Google Scholar] [CrossRef]
- Ding, Q.; Ding, C.; Liu, X.; Zheng, Y.; Zhao, Y.; Zhang, S.; Sun, S.; Peng, Z.; Liu, W. Preparation of nanocomposite membranes loaded with taxifolin liposome and its mechanism of wound healing in diabetic mice. Int. J. Biol. Macromol. 2023, 241, 124537. [Google Scholar] [CrossRef]
- Hao, M.; Ding, C.; Sun, S.; Peng, X.; Liu, W. Chitosan/sodium alginate/velvet antler blood peptides hydrogel promotes diabetic wound healing via regulating angiogenesis, inflammatory response and skin flora. J. Inflamm. Res. 2022, 15, 4921–4938. [Google Scholar] [CrossRef]
- Peng, X.; Ding, C.; Zhao, Y.; Hao, M.; Liu, W.; Yang, M.; Xiao, F.; Zheng, Y. Poloxamer 407 and hyaluronic acid thermosensitive hydrogel-encapsulated ginsenoside rg3 to promote skin wound healing. Front. Bioeng. Biotechnol. 2022, 10, 831007. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Hong, B.; Ma, L.; Zhao, Y.; Zhang, S.; Sun, S.; Ding, Q.; Wang, Y.; Liu, W.; et al. Dihydroquercetin composite nanofibrous membrane prevents uva radiation-mediated inflammation, apoptosis and oxidative stress by modulating mapks/nrf2 signaling in human epidermal keratinocytes. Biomed. Pharmacother. 2022, 155, 113727. [Google Scholar] [CrossRef]
- Huang, X.; Ma, C.; Xu, Y.; Cao, J.; Li, J.; Li, J.; Shi, S.Q.; Gao, Q. A tannin-functionalized soy protein-based adhesive hydrogel as a wound dressing. Ind. Crop. Prod. 2022, 182, 11. [Google Scholar] [CrossRef]
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of gelatin-hyaluronic acid composite hydrogels for accelerating wound healing. J. Biomater. Appl. 2017, 31, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Kong, Y.; Shao, C.; Cheng, Y.; Lu, J.; Tao, Y.; Du, J.; Wang, H. Chitosan-based multifunctional flexible hemostatic bio-hydrogel. Acta Biomater. 2021, 136, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Giraudier, S.; Hellio, D.; Djabourov, M.; Larreta-Garde, V. Influence of weak and covalent bonds on formation and hydrolysis of gelatin networks. Biomacromolecules 2004, 5, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.J.; Panitch, A. Interplay between covalent and physical interactions within environment sensitive hydrogels. Biomacromolecules 2009, 10, 1090–1099. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Ji, X.; Hu, D.; Pan, M.; Zhang, L.; Qian, Z. Current research progress of photopolymerized hydrogels in tissue engineering. Chin. Chem. Lett. 2021, 32, 2117–2126. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.; Tang, W.; Du, S.; Yu, M.; Lu, H.; Tan, H.; Xing, X. A facile injectable carbon dot/oxidative polysaccharide hydrogel with potent self-healing and high antibacterial activity. Carbohydr. Polym. 2021, 251, 117040. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Slita, A.; Backman, O.; Gounani, Z.; Rosqvist, E.; Peltonen, J.; Willfor, S.; Xu, C.; Rosenholm, J.M.; et al. Digital light processing (dlp) 3d-fabricated antimicrobial hydrogel with a sustainable resin of methacrylated woody polysaccharides and hybrid silver-lignin nanospheres. Green Chem. 2022, 24, 2129–2145. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-sensitive injectable hydrogels based on polysaccharides and their biomedical applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Nabavi, M.A.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar]
- Dimitriou, R.; Jones, E.; Mcgonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, C.; Wang, J.; He, Y. Lithium-incorporated deproteinized bovine bone substitute improves osteogenesis in critical-sized bone defect repair. J. Biomater. Appl. 2018, 32, 1421–1434. [Google Scholar] [CrossRef]
- Zheng, L.; Tu, Q.; Meng, S.; Zhang, L.; Yu, L.; Song, J.; Hu, Y.; Sui, L.; Zhang, J.; Dard, M.; et al. Runx2/dicer/mirna pathway in regulating osteogenesis. J. Cell. Physiol. 2017, 232, 182–191. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.S.; Silva, C.L.D.; Vashishth, D. Synergistic effect of extracellularly supplemented osteopontin and osteocalcin on stem cell proliferation, osteogenic differentiation, and angiogenic properties. J. Cell. Biochem. 2019, 120, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Pan, Y.; Li, Y.; Xu, L.; Zhong, Y.; Wang, W.; Zuo, J.; Yu, H.; Lv, Z.; et al. Long-term induction of endogenous bmps growth factor from antibacterial dual network hydrogels for fast large bone defect repair. J. Colloid Interface Sci. 2022, 607, 1500–1515. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Li, X.; Zhang, Z.; Zhao, X.; Wang, C.; Wei, F. Microrna-21 promotes bone reconstruction in maxillary bone defects. J. Oral Rehabil. 2020, 47, 4–11. [Google Scholar] [CrossRef]
- Han, X.; Du, J.; Liu, D.; Liu, H.; Amizuka, N.; Li, M. Histochemical examination of systemic administration of eldecalcitol combined with guided bone regeneration for bone defect restoration in rats. J. Mol. Histol. 2017, 48, 41–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, Y.; Wang, M.; Huang, Y.; Xu, H.; Su, Y.; Zhao, Y.; Shang, Y. Supramolecular hydrogel based on an osteogenic growth peptide promotes bone defect repair. ACS Omega 2022, 7, 11395–11404. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, W.; Chen, D.; Zhang, C.; Wang, L.; Zhang, H.; Qin, N.; Sun, Y. Asperosaponin vi stimulates osteogenic differentiation of rat adipose-derived stem cells. Regen. Ther. 2019, 11, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, J.; Zhang, J.; Pan, Z.; Liu, Y.; Zhou, F.; Hong, Y.; Hu, Y.; Gu, Y.; Ouyang, H.; et al. An interleukin-4-loaded bi-layer 3d printed scaffold promotes osteochondral regeneration. Acta Biomater. 2020, 117, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Bai, X.; Wang, C.; Ren, L.; Ma, D. A simple polysaccharide based injectable hydrogel compositing nano-hydroxyapatite for bone tissue engineering. Mater. Lett. 2021, 293, 129755. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and tough hydrogel through physical cross-linking and molecular alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef]
- Erikci, S.; Mundinger, P.; Boehm, H. Small physical cross-linker facilitates hyaluronan hydrogels. Molecules 2020, 15, 4166. [Google Scholar] [CrossRef]
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-flexibility, high-toughness double-cross-linked chitin hydrogels by sequential chemical and physical cross-linkings. Adv. Mater. 2016, 28, 5844–5849. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, S.Y.; Xiao, R.; Yin, J.; Wu, Z.L.; Zheng, Q.; Qian, J. Constitutive behaviors of tough physical hydrogels with dynamic metal-coordinated bonds. J. Mech. Phys. Solids 2020, 139, 103935. [Google Scholar] [CrossRef]
- Hanif, W.; Hardiansyah, A.; Randy, A.; Asri, L. Physically crosslinked pva/graphene-based materials/aloe vera hydrogel with antibacterial activity. RSC Adv. 2021, 11, 29029–29041. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Samadi, N.; Sabzi, M.; Babaahmadi, M. Self-healing and tough hydrogels with physically cross-linked triple networks based on agar/pva/graphene. Int. J. Biol. Macromol. 2018, 107 Pt B, 2291–2297. [Google Scholar] [CrossRef]
- Schweizer, S.; Monteiro, I.; Oliveira, A.S.; Nolasco, P.; Colaço, R.; Serro, A.P. Physically crosslinked polyvinyl alcohol hydrogels as synthetic cartilage materials. Ann. Med. 2021, 53, S25–S26. [Google Scholar] [CrossRef]
- Yan, K.; Xiong, Y.; Wu, S.; Bentley, W.E.; Deng, H.; Du, Y.; Payne, G.F.; Shi, X.W. Electro-molecular assembly: Electrical writing of information into an erasable polysaccharide medium. ACS Appl. Mater. Interfaces 2016, 8, 19780–19786. [Google Scholar] [CrossRef]
- Bi, S.; Wang, P.; Hu, S.; Li, S.; Pang, J.; Zhou, Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S.; et al. Construction of physical-crosslink chitosan/pva double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019, 224, 115176. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Marie, P.J. Fgf signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002, 16, 1446–1465. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.; van der Jagt, O.P.; Koevoet, W.; Kops, N.; van Manen, C.J.; Hellingman, C.A.; Jahr, H.; O’Brien, F.J.; Verhaar, J.; Weinans, H.; et al. Chondrogenic priming of human bone marrow stromal cells: A better route to bone repair? Tissue Eng. Part C Methods 2009, 15, 285–295. [Google Scholar] [CrossRef]
- Lan, W.; Xu, M.; Qin, M.; Cheng, Y.; Zhao, Y.; Huang, D.; Wei, X.; Guo, Y.; Chen, W. Physicochemical properties and biocompatibility of the bi-layer polyvinyl alcohol-based hydrogel for osteochondral tissue engineering. Mater. Des. 2021, 204, 109652. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Lee, M. Supramolecular hydrogels based on nanoclay and guanidine-rich chitosan: Injectable and moldable osteoinductive carriers. ACS Appl. Mater. Interfaces 2020, 12, 16088–16096. [Google Scholar] [CrossRef]
- Li, W.; Kang, J.; Yuan, Y.; Xiao, F.; Yao, H.; Liu, S.; Lu, J.; Wang, Y.; Wang, Z.; Ren, L. Preparation and characterization of pva-peek/pva-β-tcp bilayered hydrogels for articular cartilage tissue repair. Compos. Sci. Technol. 2016, 128, 58–64. [Google Scholar] [CrossRef]
- Bichara, D.A.; Bodugoz-Sentruk, H.; Ling, D.; Malchau, E.; Bragdon, C.R.; Muratoglu, O.K. Osteochondral defect repair using a polyvinyl alcohol-polyacrylic acid (pva-paac) hydrogel. Biomed. Mater. 2014, 9, 45012. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, W.; Zhao, R.; Lu, W.; Chen, L.; Su, W.; Zeng, M.; Hu, Y. Fabrication and characterization of microstructure-controllable col-ha-pva hydrogels for cartilage repair. J. Mater. Sci. Mater. Med. 2021, 32, 100. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gao, H.; Li, Q.; Lin, Z.; Feng, Q.; Yu, C.; Zhang, X.; Dong, H.; Chen, D.; Cao, X. Engineered macroporous hydrogel scaffolds via pickering emulsions stabilized by mgo nanoparticles promote bone regeneration. J. Mater. Chem. B 2020, 8, 6100–6114. [Google Scholar] [CrossRef]
- Chen, R.; Chen, H.; Xue, P.; Yang, W.; Luo, L.; Tong, M.; Zhong, B.; Xu, H.; Zhao, Y.; Yuan, J. Ha/mgo nanocrystal-based hybrid hydrogel with high mechanical strength and osteoinductive potential for bone reconstruction in diabetic rats. J. Mater. Chem. B 2021, 9, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.S.; Rodrigues, M.A.; Silva, T.I.; Lopes, M.A.; Santos, M.; Santos, J.D.; Botelho, C.M. Development and characterization of novel alginate-based hydrogels as vehicles for bone substitutes. Carbohydr. Polym. 2013, 95, 134–142. [Google Scholar] [CrossRef]
- Phogat, K.; Ghosh, S.B.; Ghosh, S.B. Recent advances on injectable nanocomposite hydrogels towards bone tissue rehabilitation. J. Appl. Polym. Sci. 2023, 140, e53362. [Google Scholar] [CrossRef]
- Bar, A.; Ruvinov, E.; Cohen, S. Live imaging flow bioreactor for the simulation of articular cartilage regeneration after treatment with bioactive hydrogel. Biotechnol. Bioeng. 2018, 115, 2205–2216. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Fathi, A.; Razak, N.; Kadri, N.A.; Sheikhi, A.; Baino, F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021, 136, 1–36. [Google Scholar] [CrossRef]
- Mo, C.; Xiang, L.; Chen, Y. Advances in injectable and self-healing polysaccharide hydrogel based on the schiff base reaction. Rapid Commun. 2021, 42, e2100025. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, S.; Chen, X. Injectable hydrogels for tendon and ligament tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1333–1348. [Google Scholar] [CrossRef]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Gan, D.; Li, Z.; Shen, J.; Tang, P.; Luo, S.; Li, P.; Lu, X.; Zheng, W. The characteristics of mussel-inspired nha/osa injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater. Res. Part B 2020, 108, 1814–1825. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G.; et al. Mussel-inspired bisphosphonated injectable nanocomposite hydrogels with adhesive, self-healing, and osteogenic properties for bone regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef]
- Chen, M.; Tan, H.; Xu, W.; Wang, Z.; Zhang, J.; Li, S.; Zhou, T.; Li, J.; Niu, X. A self-healing, magnetic and injectable biopolymer hydrogel generated by dual cross-linking for drug delivery and bone repair. Acta Biomater. 2022, 153, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Cao, Y.; Wang, J.; Liu, M.; Pei, R. Injectable hydrogels from enzyme-catalyzed crosslinking as bmscs-laden scaffold for bone repair and regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 96, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-based pdlsc-ecm screening for in vivo repair of alveolar bone defect using cell-laden; injectable and photocrosslinkable hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef]
- Yuan, T.; Li, Z.; Zhang, Y.; Shen, K.; Zhang, X.; Xie, R.; Liu, F.; Fan, W. Injectable ultrasonication-induced silk fibroin hydrogel for cartilage repair and regeneration. Tissue Eng. Part A 2021, 27, 1213–1224. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Wang, P.; Ma, J.; Wang, P.; Han, X.; Fan, Y.; Bai, D.; Sun, Y.; Zhang, X. Cell-mediated injectable blend hydrogel-bcp ceramic scaffold for in situ condylar osteochondral repair. Acta Biomater. 2021, 123, 364–378. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Xiao, K.; Li, Z.; Ma, Q.; Li, W.; Shen, S.; Weng, X. Self-healing and injectable hybrid hydrogel for bone regeneration of femoral head necrosis and defect. Biochem. Biophys. Res. Commun. 2019, 508, 25–30. [Google Scholar] [CrossRef]
- Qiao, M.; Xu, Z.; Pei, X.; Liu, Y.; Wang, J.; Chen, J.; Zhu, Z.; Wan, Q. Nano sim@zif-8 modified injectable high-intensity biohydrogel with bidirectional regulation of osteogenesis and anti-adipogenesis for bone repair. Chem. Eng. J. 2022, 434, 134583. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, F.; Li, X.; Liang, Q.; Zhuo, Z.; Huang, J.; Duan, L.; Xiong, J.; Wang, D. Repair of osteochondral defects using injectable chitosan-based hydrogel encapsulated synovial fluid-derived mesenchymal stem cells in a rabbit model. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 99, 541–551. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Lancucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid–based injectable hydrogels for tissue engineering applications-design, physicochemical and biological characterization. Colloid Surf. B-Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, C.; Toh, W.S.; Wan, A.C.A.; Gao, S.J.; Kurisawa, M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 2014, 35, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, M.; Geng, Z.; Liu, Y. Functional hydrogels for the repair and regeneration of tissue defects. Front. Bioeng. Biotechnol. 2023, 11, 1190171. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, J.; Jiang, Y.; Li, M.; Wei, J.; Qin, J.; Peng, W.; Lasaosa, F.L.; He, Y.; Mao, H.; et al. Injectable adhesive self-healing multicross-linked double-network hydrogel facilitates full-thickness skin wound healing. ACS Appl. Mater. Interfaces 2020, 12, 57782–57797. [Google Scholar] [CrossRef]
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-healing, expansion-contraction, and shape-memory properties of a preorganized supramolecular hydrogel through host-guest interactions. Angew. Chem.-Int. Ed. 2015, 54, 8984–8987. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.N.; Xiong, X.M.; Rong, M.Z.; Zhang, M.Q.; Kong, F. Self-healing of polymer in acidic water toward strength restoration through the synergistic effect of hydrophilic and hydrophobic interactions. ACS Appl. Mater. Interfaces 2017, 9, 37300–37309. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A new self-healing hydrogel containing hucmsc-derived exosomes promotes bone regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Biocomposite scaffolds for bone regeneration: Role of chitosan and hydroxyapatite within poly-3-hydroxybutyrate-co-3-hydroxyvalerate on mechanical properties and in vitro evaluation. J. Mech. Behav. Biomed. Mater. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Zhang, X.; Tao, L.; Li, S.; Wei, Y. Facilely prepared inexpensive and biocompatible self-healing hydrogel: A new injectable cell therapy carrier. Polym. Chem. 2012, 3, 3235. [Google Scholar] [CrossRef]
- Taylor, D.L.; In, H.P.M. Self-healing hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Lee, C.S.; Hwang, H.S.; Kim, S.; Fan, J.; Aghaloo, T.; Lee, M. Inspired by nature: Facile design of nanoclay–organic hydrogel bone sealant with multifunctional properties for robust bone regeneration. Adv. Funct. Mater. 2020, 30, 2003717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Kong, M.; Li, Z.; Yang, T.; Wang, Q.; Teng, W. Self-healing hybrid hydrogels with sustained bioactive components release for guided bone regeneration. J. Nanobiotechnol. 2023, 21, 62. [Google Scholar] [CrossRef]
- Chen, L.; Yu, C.; Xiong, Y.; Chen, K.; Liu, P.; Panayi, A.C.; Xiao, X.; Feng, Q.; Mi, B.; Liu, G. Multifunctional hydrogel enhances bone regeneration through sustained release of stromal cell-derived factor-1alpha and exosomes. Bioact. Mater. 2023, 25, 460–471. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Huang, X.; Hang, R.; Zhang, X.; Wang, Y.; Yao, X. Dlp printing photocurable chitosan to build bio-constructs for tissue engineering. Carbohydr. Polym. 2020, 235, 115970. [Google Scholar] [CrossRef]
- Hao, Y.; Shih, H.; Munoz, Z.; Kemp, A.; Lin, C.C. Visible light cured thiol-vinyl hydrogels with tunable degradation for 3d cell culture. Acta Biomater. 2014, 10, 104–114. [Google Scholar] [CrossRef]
- Hughes, T.; Simon, G.P.; Saito, K. Chemistries and capabilities of photo-formable and photoreversible crosslinked polymer networks. Mater. Horizons 2019, 6, 1762–1773. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Chen, Y.; Liu, X.; Guo, S.; Zhu, L.; Wang, Y. An in situ phototriggered-imine-crosslink composite hydrogel for bone defect repair. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Su, J.; Liu, Y.; Lin, H.; Wang, Y.; Cheng, H. A novel visible light-curing chitosan-based hydrogel membrane for guided tissue regeneration. Colloid Surf. B-Biointerfaces 2022, 218, 112760. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.; Zhu, Y.; Chen, F.; Chen, Z.; Guo, L.; Wu, P.; Li, G.; Zhang, C.; Wei, R.; et al. Mild photothermal-stimulation based on injectable and photocurable hydrogels orchestrates immunomodulation and osteogenesis for high-performance bone regeneration. Small 2023, 16, e2300111. [Google Scholar] [CrossRef]
- Bhuiyan, M.H.; Clarkson, A.N.; Ali, M.A. Optimization of thermoresponsive chitosan/beta-glycerophosphate hydrogels for injectable neural tissue engineering application. Colloid Surf. B-Biointerfaces 2023, 224, 113193. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Wang, C.; Zhang, Q.; Qu, X.; Liang, C.; Si, C.; Wang, L. Treatment of periodontal inflammation in diabetic rats with il-1ra thermosensitive hydrogel. Int. J. Mol. Sci. 2022, 23, 13939. [Google Scholar] [CrossRef]
- Rocker, A.J.; Cavasin, M.; Johnson, N.R.; Shandas, R.; Park, D. Sulfonated thermoresponsive injectable gel for sequential release of therapeutic proteins to protect cardiac function after myocardial infarction. ACS Biomater. Sci. Eng. 2022, 8, 3883–3898. [Google Scholar] [CrossRef]
- Shefa, A.A.; Park, M.; Gwon, J.; Lee, B. Alpha tocopherol-nanocellulose loaded alginate membranes and pluronic hydrogels for diabetic wound healing. Mater. Des. 2022, 224, 111404. [Google Scholar] [CrossRef]
- Xu, T.; Hua, Y.; Mei, P.; Zeng, D.; Jiang, S.; Liao, C. Black phosphorus thermosensitive hydrogels loaded with bone marrow mesenchymal stem cell-derived exosomes synergistically promote bone tissue defect repair. J. Mater. Chem. B 2023, 11, 4396–4407. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef]
- Ghanta, P.; Winschel, T.; Hessel, E.; Oyewumi, O.; Czech, T.; Oyewumi, M.O. Efficacy assessment of methylcellulose-based thermoresponsive hydrogels loaded with gallium acetylacetonate in osteoclastic bone resorption. Drug Deliv. Transl. Res. 2023, 13, 2533–2549. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Huo, H.; Ji, C.; Sun, M.; Nie, L. Injectable temperature-sensitive hydrogel with vegf loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018, 229, 138–141. [Google Scholar] [CrossRef]
- Sheridan, W.S.; Grant, O.B.; Duffy, G.P.; Murphy, B.P. The application of a thermoresponsive chitosan/beta-gp gel to enhance cell repopulation of decellularized vascular scaffolds. J. Biomed. Mater. Res. Part B 2014, 102, 1700–1710. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, Z.; Zhang, L.; Lu, S.; Liang, F.; Li, S. Chitosan-based thermo-sensitive hydrogel loading oyster peptides for hemostasis application. Materials 2020, 13, 5038. [Google Scholar] [CrossRef]
- Jeong, S.; Jeon, H.J.; Jang, K.J.; Park, S.; Choi, H.S.; Chung, J.H. Injectable thermosensitive chitosan solution with beta-glycerophosphate as an optimal submucosal fluid cushion for endoscopic submucosal dissection. Polymers 2021, 13, 1696. [Google Scholar] [CrossRef]
- Herron, C.; Hastings, C.L.; Herron-Rice, C.; Kelly, H.M.; O’Dwyer, J.; Duffy, G.P. A thermoresponsive chitosan/beta-glycerophosphate hydrogel for minimally invasive treatment of critical limb ischaemia. Polymers 2021, 13, 3568. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of chitosan hydrogel for sustained delivery of vegf for odontogenic differentiation of dental pulp stem cells. Stem Cells Int. 2019, 2019, 1515040. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Liang, C.; Qu, X.; Zou, X.; Du, S.; Zhang, Q.; Wang, L. Role of berberine thermosensitive hydrogel in periodontitis via pi3k/akt pathway in vitro. Int. J. Mol. Sci. 2023, 24, 6364. [Google Scholar] [CrossRef]
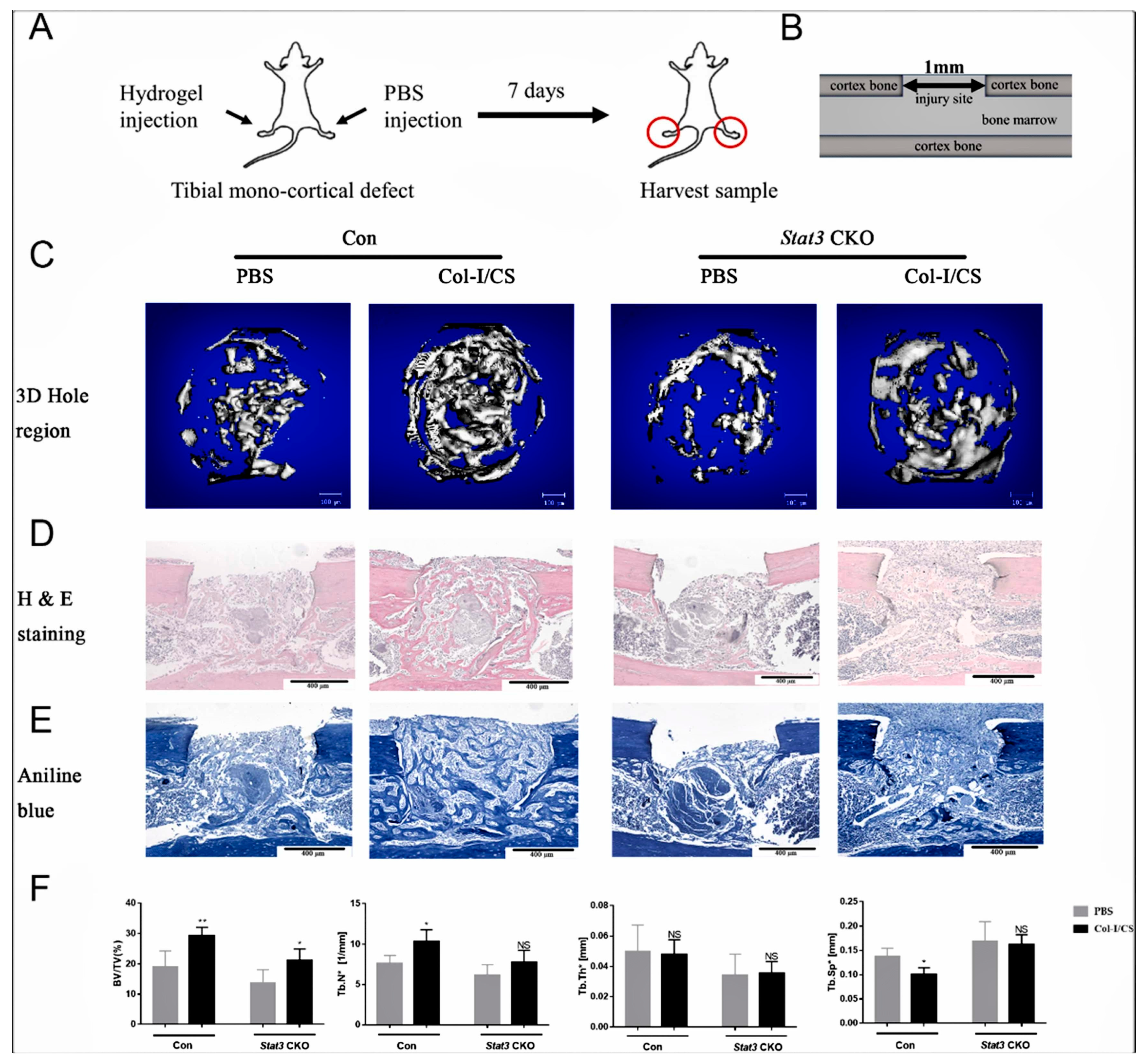
- Lu, J.; Xu, R.; Chen, Y.; Chan, L.; Feng, X.; Lin, L.; Yao, Y.; Hu, X.; Zhang, X. Injectable col-i/cs hydrogel enhances bone regeneration in mice tibial mono-cortical defect with impaired osteogenesis. Mater. Today Commun. 2022, 32, 104070. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, J.; Feng, X.; Chan, L.; Lu, J.; Lei, L.; Huang, Z.; Zhang, X. Loss of signal transducer and activator of transcription 3 impaired the osteogenesis of mesenchymal progenitor cells in vivo and in vitro. Cell Biosci. 2021, 11, 172. [Google Scholar] [CrossRef]
- Borges, R.; Kai, K.C.; Lima, C.A.; Zezell, D.M.; de Araujo, D.R.; Marchi, J. Bioactive glass/poloxamer 407 hydrogel composite as a drug delivery system: The interplay between glass dissolution and drug release kinetics. Colloid Surf. B-Biointerfaces 2021, 206, 111934. [Google Scholar] [CrossRef]
- Leroy, A.; Nottelet, B.; Bony, C.; Pinese, C.; Charlot, B.; Garric, X.; Noel, D.; Coudane, J. Pla-poloxamer/poloxamine copolymers for ligament tissue engineering: Sound macromolecular design for degradable scaffolds and msc differentiation. Biomater. Sci. 2015, 3, 617–626. [Google Scholar] [CrossRef]
- Liu, J.; Fang, Q.; Lin, H.; Yu, X.; Zheng, H.; Wan, Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr. Polym. 2020, 247, 116593. [Google Scholar] [CrossRef]
- Fu, X.; Tan, J.; Sun, C.G.; Leng, H.J.; Xu, Y.S.; Song, C.L. Intraosseous injection of simvastatin in poloxamer 407 hydrogel improves pedicle-screw fixation in ovariectomized minipigs. J. Bone Jt. Surg. Am. 2016, 98, 1924–1932. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and ph-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 111, 110862. [Google Scholar] [CrossRef]
- Hafeez, S.; Islam, A.; Durrani, A.K.; Butt, M.T.Z.; Rehmat, S.; Khurshid, A.; Khan, S.M. Fabrication of pectin-based stimuli responsive hydrogel for the controlled release of ceftriaxone. Chem. Pap. 2023, 77, 1809–1819. [Google Scholar] [CrossRef]
- Ni, X.; Xing, X.; Deng, Y.; Li, Z. Applications of stimuli-responsive hydrogels in bone and cartilage regeneration. Pharmaceutics 2023, 15, 982. [Google Scholar] [CrossRef]
- Coletta, D.J.; Ibanez-Fonseca, A.; Missana, L.R.; Jammal, M.V.; Vitelli, E.; Aimone, M.; Zabalza, F.; Issa, J.P.M.; Alonso, M.; Rodriguez-Cabello, J.C.; et al. Bone regeneration mediated by a bioactive and biodegradable extracellular matrix-like hydrogel based on elastin-like recombinamers. Tissue Eng. Part A 2017, 23, 1361–1371. [Google Scholar] [CrossRef]
- Bertoni, S.; Liu, Z.; Correia, A.; Martins, J.P.; Rahikkala, A.; Fontana, F.; Kemell, M.; Liu, D.; Albertini, B.; Passerini, N.; et al. Ph and reactive oxygen species-sequential responsive nano-in-micro composite for targeted therapy of inflammatory bowel disease. Adv. Funct. Mater. 2018, 28, 1806175. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Ressler, A.; Rodenas-Rochina, J.; Ivankovic, M.; Ivankovic, H.; Rogina, A.; Gallego, F.G. Injectable chitosan-hydroxyapatite hydrogels promote the osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 2018, 197, 469–477. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Shi, Y.; Wang, C.; Ye, L. Targeting reactive oxygen species in stem cells for bone therapy. Drug Discov. Today 2021, 26, 1226–1244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, K.; He, Y.; Tao, B.; Li, K.; Lin, C.; Hu, J.; Wu, J.; Wu, Y.; Liu, S.; et al. Ros-responsive hydrogel coating modified titanium promotes vascularization and osteointegration of bone defects by orchestrating immunomodulation. Biomaterials 2022, 287, 121683. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Jia, Z.; Chen, J.; Duan, L.; Liu, W.; Zhu, F.; Liang, Q.; Zhu, W.; You, W.; et al. Development of magnetic nanocomposite hydrogel with potential cartilage tissue engineering. ACS Omega 2018, 3, 6182–6189. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Electrical behavior of a natural polyelectrolyte hydrogel: Chitosan/carboxymethylcellulose hydrogel. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Z.; Zhang, L.; Li, J.; Wang, H.; Li, X. Advance of electroconductive hydrogels for biomedical applications in orthopedics. Adv. Mater. Sci. Eng. 2021, 2021, 6668209. [Google Scholar] [CrossRef]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv. Funct. Mater. 2020, 30, 2003759. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef]
- Ha, S.; Jang, H.L.; Nam, K.T.; Beck, G.J. Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 2015, 65, 32–42. [Google Scholar] [CrossRef]
- Liang, K.; Zhao, C.; Song, C.; Zhao, L.; Qiu, P.; Wang, S.; Zhu, J.; Gong, Z.; Liu, Z.; Tang, R.; et al. In situ biomimetic mineralization of bone-like hydroxyapatite in hydrogel for the acceleration of bone regeneration. ACS Appl. Mater. Interfaces 2023, 15, 292–308. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef]
- Khan, M.; Razak, S.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium alginate-f-go composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef]
- Luo, J.W.; Liu, C.; Wu, J.H.; Zhao, D.H.; Lin, L.X.; Fan, H.M.; Sun, Y.L. In situ forming gelatin/hyaluronic acid hydrogel for tissue sealing and hemostasis. J. Biomed. Mater. Res. Part B 2020, 108, 790–797. [Google Scholar] [CrossRef]
- Bush, J.R.; Liang, H.; Dickinson, M.; Botchwey, E.A. Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym. Adv. Technol. 2016, 27, 1050–1055. [Google Scholar] [CrossRef]
- Chen, F.; Ni, Y.; Liu, B.; Zhou, T.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y. Self-crosslinking and injectable hyaluronic acid/rgd-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017, 166, 31–44. [Google Scholar] [CrossRef]
- Liu, C.; Qin, W.; Wang, Y.; Ma, J.; Liu, J.; Wu, S.; Zhao, H. 3d printed gelatin/sodium alginate hydrogel scaffolds doped with nano-attapulgite for bone tissue repair. Int. J. Nanomed. 2021, 16, 8417–8432. [Google Scholar] [CrossRef]
- Lisman, A.; Butruk, B.; Wasiak, I.; Ciach, T. Dextran/albumin hydrogel sealant for dacron (r) vascular prosthesis. J. Biomater. Appl. 2014, 28, 1386–1396. [Google Scholar] [CrossRef]
- Ritz, U.; Kogler, P.; Hofer, I.; Frank, P.; Klees, S.; Gebhard, S.; Brendel, C.; Kaufmann, K.; Hofmann, A.; Rommens, P.; et al. Photocrosslinkable polysaccharide hydrogel composites based on dextran or pullulan-amylose blends with cytokines for a human co-culture model of human osteoblasts and endothelial cells. J. Mater. Chem. B 2016, 4, 6552–6564. [Google Scholar] [CrossRef]
- Montaseri, Z.; Abolmaali, S.S.; Tamaddon, A.M.; Farvadi, F. Composite silk fibroin hydrogel scaffolds for cartilage tissue regeneration. J. Drug Deliv. Sci. Technol. 2023, 79, 104018. [Google Scholar] [CrossRef]
- Shimada, K.; Honda, T.; Kato, K.; Hori, R.; Ujike, N.; Uemura, A.; Murakami, T.; Kitpipatkun, P.; Nakazawa, Y.; Tanaka, R. Silk fibroin-based vascular repairing sheet with angiogenic-promoting activity of svvyglr peptide regenerated the damaged vascular in rats. J. Biomater. Appl. 2022, 37, 3–11. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Lv, L.; Zhou, Y. Nir light-assisted phototherapies for bone-related diseases and bone tissue regeneration: A systematic review. Theranostics 2020, 10, 11837–11861. [Google Scholar] [CrossRef]
- Chang, L.; Huang, S.; Zhao, X.; Hu, Y.; Ren, X.; Mei, X.; Chen, Z. Preparation of ros active and photothermal responsive hydroxyapatite nanoplatforms for anticancer therapy. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 125, 112098. [Google Scholar] [CrossRef]
- Miao, H.; Shen, R.; Zhang, W.; Lin, Z.; Wang, H.; Yang, L.; Liu, X.; Lin, N. Near-infrared light triggered silk fibroin scaffold for photothermal therapy and tissue repair of bone tumors. Adv. Funct. Mater. 2021, 31, 2007188. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S.; Jiang, Y.; Shen, W.; Zhang, S.; et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Li, H.; Dai, Y.; Zhou, G.; Zhou, Z.; Xia, H.; Liu, H. Tanshinone iia delivery silk fibroin scaffolds significantly enhance articular cartilage defect repairing via promoting cartilage regeneration. ACS Appl. Mater. Interfaces 2020, 12, 21470–21480. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Chen, Y.; Gu, G.; Gao, R.; Peng, B.; Wang, Y.; Li, A.; Guo, J.; Xu, X.; et al. Updated pharmacological effects; molecular mechanisms and therapeutic potential of natural product geniposide. Molecules 2022, 27, 3319. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Cao, D.; Ma, X. Pharmacological basis of tanshinone and new insights into tanshinone as a multitarget natural product for multifaceted diseases. Biomed. Pharmacother. 2020, 130, 110599. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Chen, R.; Gao, S.; Xu, Z.; Li, N. Cell death regulation: A new way for natural products to treat osteoporosis. Pharmacol. Res. 2023, 187, 106635. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Guo, S.; Liu, Z.; Zhao, L.; Qiao, R.; Li, C. Rutin-loaded stimuli-responsive hydrogel for anti-inflammation. ACS Appl. Mater. Interfaces 2022, 164, 26327–26337. [Google Scholar] [CrossRef]
- Wan, J.; Liang, Y.; Wei, X.; Liang, H.; Chen, X.L. Chitosan-based double network hydrogel loading herbal small molecule for accelerating wound healing. Int. J. Biol. Macromol. 2023, 246, 125610. [Google Scholar] [CrossRef]
- Camont, L.; Cottart, C.H.; Rhayem, Y.; Nivet-Antoine, V.; Djelidi, R.; Collin, F.; Beaudeux, J.L.; Bonnefont-Rousselot, D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta 2009, 634, 121–128. [Google Scholar] [CrossRef]
- Penalva, R.; Morales, J.; Gonzalez-Navarro, C.J.; Larraneta, E.; Quincoces, G.; Penuelas, I.; Irache, J.M. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Shi, L. Controlled drug delivery systems in eradicating bacterial biofilm-associated infections. J. Control. Release 2021, 329, 1102–1116. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Wu, T.; Huang, L.; Sun, J.; Sun, J.; Yan, Q.; Duan, B.; Zhang, L.; Shi, B. Multifunctional chitin-based barrier membrane with antibacterial and osteogenic activities for the treatment of periodontal disease. Carbohydr. Polym. 2021, 269, 118276. [Google Scholar] [CrossRef]
- Dong, Z.; Lin, Y.; Xu, S.; Chang, L.; Zhao, X.; Mei, X.; Gao, X. Nir-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration. Mater. Des. 2023, 225, 111487. [Google Scholar] [CrossRef]
- Cho, J.K.; Park, J.W.; Song, S.C. Injectable and biodegradable poly(organophosphazene) gel containing silibinin: Its physicochemical properties and anticancer activity. J. Pharm. Sci. 2012, 101, 2382–2391. [Google Scholar] [CrossRef]
- Leena, R.S.; Vairamani, M.; Selvamurugan, N. Alginate/gelatin scaffolds incorporated with silibinin-loaded chitosan nanoparticles for bone formation in vitro. Colloid Surf. B-Biointerfaces 2017, 158, 308–318. [Google Scholar] [CrossRef]




| Hydrogel Matrix | Preparation Methods | Applications | Ref. |
|---|---|---|---|
| Nanoclay and guanidine radicalization chitosan | Self-assembly | Promoting osteogenic differentiation of MSCs | [59] |
| Polyetheretherketone/polyvinyl alcohol/β-tricalcium phosphate | Repeated freezing and thawing | To promote the repair of knee joint defects in rabbits | [60] |
| Polyvinyl alcohol/polyacrylic acid | Repeated freezing and thawing | Promoting the repair of medial condylar bone defects in rabbits | [61] |
| Hydroxyapatite/collagen/polyvinyl alcohol | Repeated freezing and thawing | Promoting the repair of femoral defects in goats | [62] |
| Methacryl gelatin/magnesium oxide | Sulfhydryl-ene click reaction | Promoting cranial bone repair in rats | [63] |
| Magnesium oxide/hydroxyapatite/cysteine modified γ-polyglutamic acid | The mixture was homogenized by ultrasound | Promoting tibial repair in diabetic rats | [64] |
| Alginate/hyaluronic acid/hydroxyapatite | Ion cross-link | It is a potential bone repair material with a good degradation rate and swelling | [65] |
| Hydrogel Matrix | Methods of Preparation | Mode of Crosslinking | Applications | Ref. |
|---|---|---|---|---|
| Sulfhydrylated hyaluronic acid/type I collagen | Disulfide bond crosslinking | Promotes the regeneration of cartilage | [78] | |
| Bisphosphonate modified hyaluronic acid | Non-covalent crosslinking | Promoting the repair of femoral head necrosis in rabbits | [79] | |
| Polyethylene glycol diacrylate/sodium alginate | Photocross-link | Repair of irregular bone defects in hyperlipidemic rats | [80] | |
| Ethylene glycol chitosan/benzaldehyde terminated polyethylene oxide derivatives | Benzoic acid-imine linkage | Promoting repair of cartilage defects in the rabbit knee | [81] | |
| N-succinyl-chitosan/hyaluronic acid | The precursor matrix was dissolved and mixed | Schiff base reaction | Promote the survival of articular chondrocytes | [82] |
| Collagen/chitosan/hyaluronic acid/silica | The precursor solution was mixed | Genipin cross-linking | Promote the osteogenic differentiation of bone marrow stromal cells | [83] |
| Gelatin methacrylate/self-adhesive polymer | Microfluidic devices | Optical crosslinking | It has a significant therapeutic effect on the development of osteoarthritis | [84] |
| Gelatin-hydroxyphenylpropionic acid | The precursor matrix was dissolved and mixed | Enzyme crosslinking | To promote the repair of osteochondral defects in rabbits | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Zhang, S.; Liu, X.; Zhao, Y.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Liu, W.; Ding, C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules 2023, 28, 7039. https://doi.org/10.3390/molecules28207039
Ding Q, Zhang S, Liu X, Zhao Y, Yang J, Chai G, Wang N, Ma S, Liu W, Ding C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules. 2023; 28(20):7039. https://doi.org/10.3390/molecules28207039
Chicago/Turabian StyleDing, Qiteng, Shuai Zhang, Xinglong Liu, Yingchun Zhao, Jiali Yang, Guodong Chai, Ning Wang, Shuang Ma, Wencong Liu, and Chuanbo Ding. 2023. "Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review" Molecules 28, no. 20: 7039. https://doi.org/10.3390/molecules28207039
APA StyleDing, Q., Zhang, S., Liu, X., Zhao, Y., Yang, J., Chai, G., Wang, N., Ma, S., Liu, W., & Ding, C. (2023). Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules, 28(20), 7039. https://doi.org/10.3390/molecules28207039





