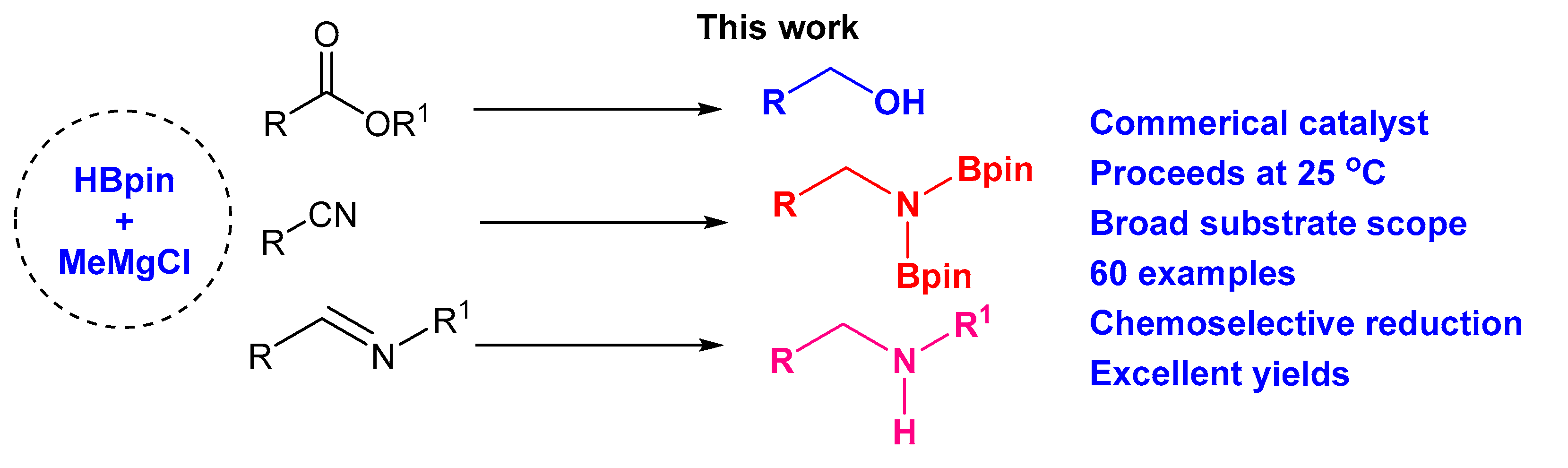
Grignard Reagent-Catalyzed Hydroboration of Esters, Nitriles, and Imines
Abstract
:1. Introduction
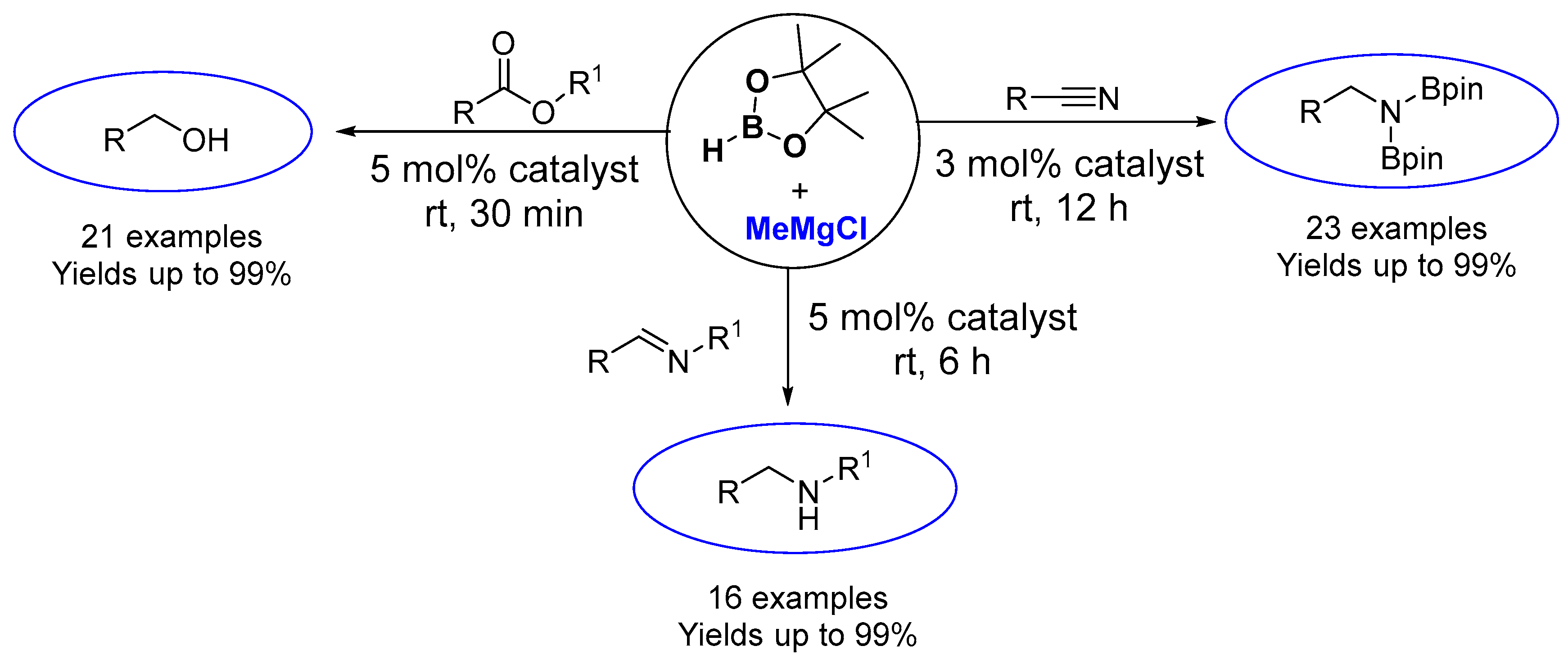
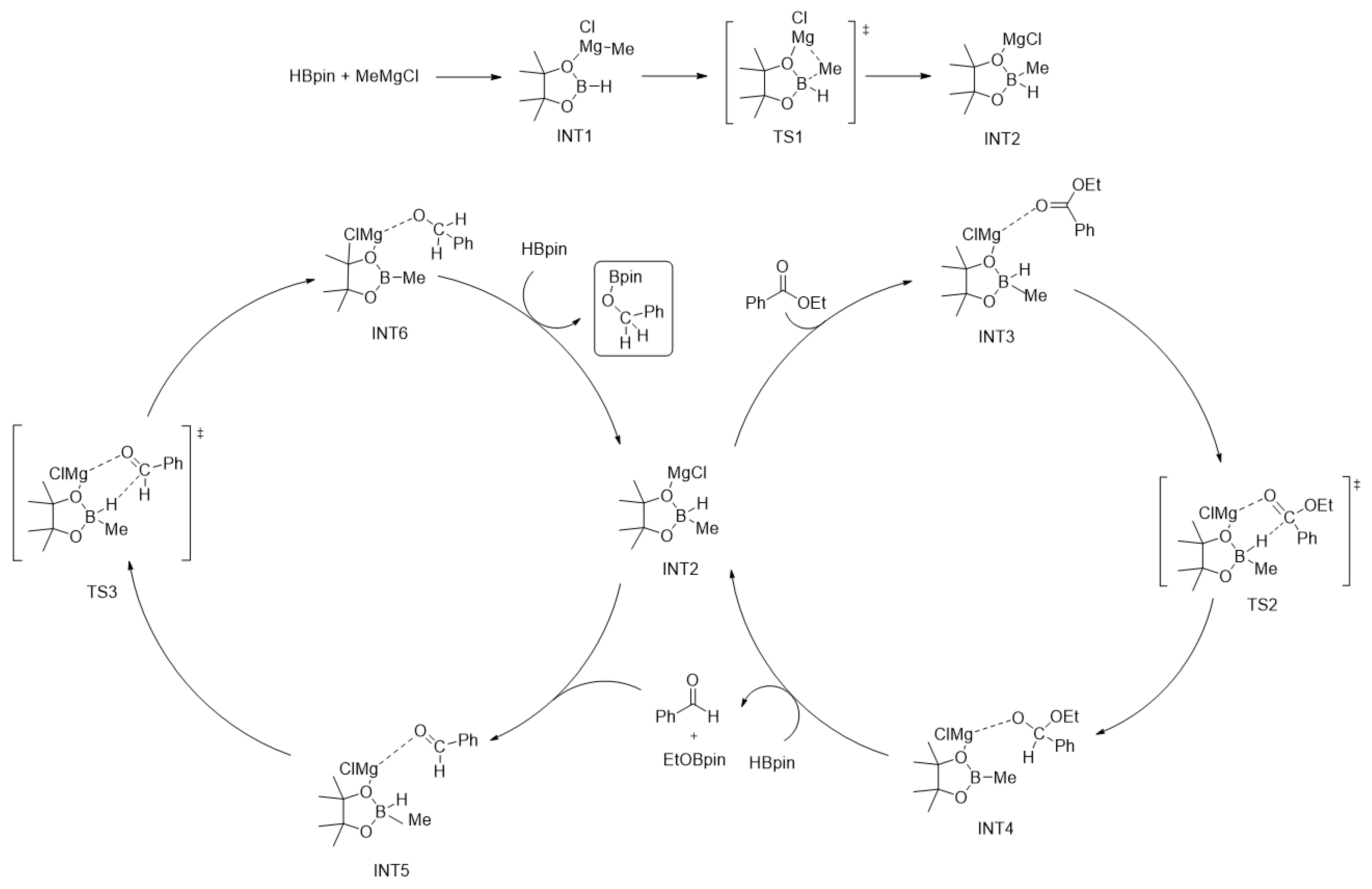
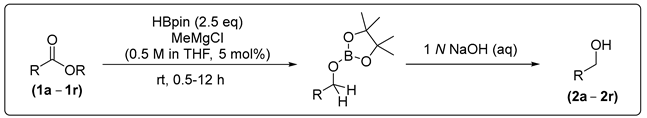
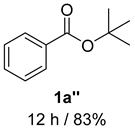
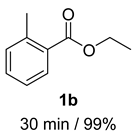
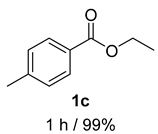
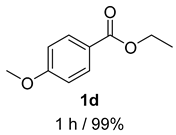
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure
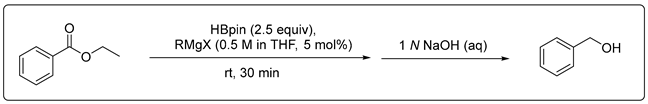
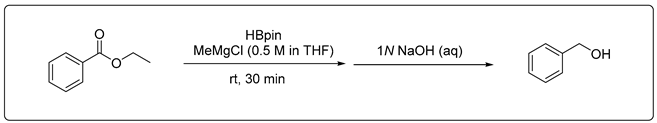
3.2.1. Catalytic Hydroboration of Ester
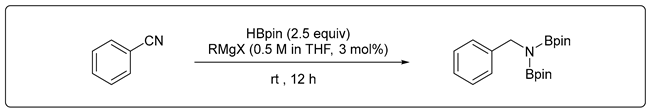
3.2.2. Catalytic Hydroboration of Nitrile
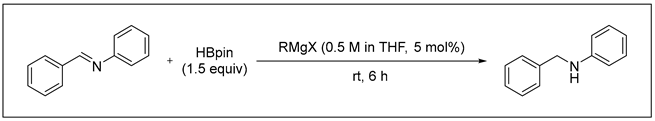
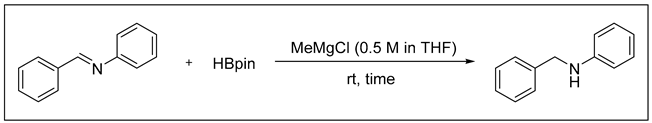
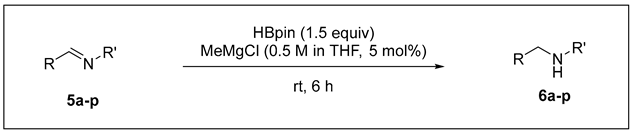
3.2.3. Catalytic Hydroboration of Imine
3.3. Characterization of Products
- Benzyl alcohol (2a) [57]: Colorless oil (53 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.36 (d, J = 4.3 Hz, 4H), 7.33–7.26 (m, 1H), 4.66 (d, J = 2.2 Hz, 2H), 2.06–1.86 (m, 1H); 13C NMR (100 MHz, Chloroform-d) δ 140.96, 128.67, 127.76, 127.11, 65.42.
- 2-Metylbenzyl alcohol (2b) [57]: White solid (61 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.34 (dd, J = 6.3, 2.6 Hz, 1H), 7.26–7.14 (m, 3H), 4.66 (s, 2H), 2.35 (s, 3H), 1.97–1.87 (m, 1H); 13C NMR (100 MHz, Chloroform-d) δ 138.80, 136.20, 130.42, 127.88, 127.63, 126.16, 63.54, 18.75.
- 4-Metylbenzyl alcohol (2c) [57]: White solid (61 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.24 (d, J = 8.0 Hz, 2H), 7.17 (d, J = 7.8 Hz, 2H), 4.62 (d, J = 2.6 Hz, 2H), 2.35 (s, 3H), 1.98–1.76 (m, 1H); 13C NMR (100 MHz, Chloroform-d) δ 138.02, 137.48, 129.34, 127.23, 65.30, 21.26.
- 4-Methoxybenzyl alcohol (2d) [57]: White solid (69 mg, 99% yield);1H NMR (400 MHz, Chloroform-d) δ 7.27 (d, J = 8.5 Hz, 2H), 6.88 (d, J = 8.6 Hz, 2H), 4.59 (s, 2H), 3.79 (s, 3H), 1.90–1.76 (m, 1H); 13C NMR (100 MHz, Chloroform-d) δ 159.27, 133.22, 128.76, 114.04, 65.08, 55.39.
- 4-Fluorobenzyl alcohol (2e) [57]: Colorless oil (54 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.37–7.28 (m, 2H), 7.07–6.99 (m, 2H), 4.64 (s, 2H), 1.86 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 162.40 (d, J C-F = 245.5 Hz), 136.66 (d, J C-F = 3.2 Hz), 128.86 (d, J C-F = 8.1 Hz), 115.48 (d, J C-F = 21.5 Hz), 64.72.
- 4-Chlorobenzyl alcohol (2f) [7]: White solid (71 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.29 (q, J = 8.6 Hz, 4H), 4.64 (s, 2H), 1.94 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 139.33, 133.44, 128.77, 128.38, 64.62.
- 2-Bromobenzyl alcohol (2g) [58]: White solid (94 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.54 (d, J = 7.9 Hz, 1H), 7.47 (d, J = 8.4 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H), 7.16 (td, J = 7.7, 1.8 Hz, 1H), 4.74 (s, 2H), 2.01 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 139.80, 132.70, 129.24, 129.02, 127.77, 122.69, 65.20.
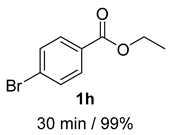
- 4-Bromobenzyl alcohol (2h) [7]: White solid (93 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.48 (d, J = 8.3 Hz, 2H), 7.23 (s, 2H), 4.65 (d, J = 5.9 Hz, 2H), 1.72–1.59 (m, 1H); 13C NMR (100 MHz, Chloroform-d) δ 139.83, 131.71, 128.69, 121.53, 64.58.
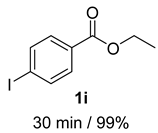
- 4-Iodobenzyl alcohol (2i) [58]: White solid (116 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.67 (d, J = 8.3 Hz, 2H), 7.09 (d, J = 8.1 Hz, 2H), 4.62 (s, 2H), 1.80 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 140.52, 137.69, 128.91, 93.11, 64.74.
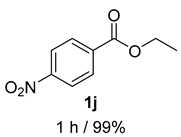
- 4-Nitrobenzyl alcohol (2j) [7]: Pale yellow solid (76 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 8.19 (d, J = 8.7 Hz, 2H), 7.51 (d, J = 8.8 Hz, 2H), 4.82 (s, 2H), 2.07 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 148.32, 147.33, 127.10, 123.83, 64.08.
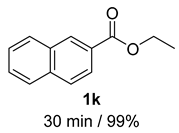
- 2-Naphthalenemethanol (2k) [57]: White solid (75 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.89–7.78 (m, 4H), 7.52–7.44 (m, 3H), 4.85 (s, 2H), 1.74 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 138.39, 133.46, 133.04, 128.46, 127.99, 127.82, 126.31, 126.02, 125.55, 125.27, 65.62.
- Cinnamyl alcohol (2l) [59]: White solid (67 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.38 (d, J = 7.3 Hz, 2H), 7.32 (t, J = 7.4 Hz, 2H), 7.24 (t, J = 7.2 Hz, 1H), 6.61 (d, J = 15.9 Hz, 1H), 6.36 (dt, J = 15.9, 5.7 Hz, 1H), 4.31 (dd, J = 5.7, 1.6 Hz, 2H), 1.92 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 136.78, 131.19, 128.72, 128.62, 127.81, 126.58, 63.77.
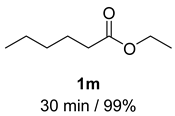
- Hexanol (2m) [59]: Colorless oil (50 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 3.62 (tq, J = 6.7, 1.4 Hz, 2H), 1.63–1.52 (m, 2H), 1.49–1.39 (m, 1H), 1.37–1.27 (m, 6H), 0.87 (td, J = 6.9, 2.2 Hz, 3H); 13C NMR (100 MHz, Chloroform-d) δ 63.16, 32.84, 31.72, 25.50, 22.72, 14.12.
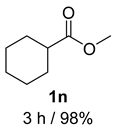
- Cyclohexylmethanol (2n) [59]: Colorless oil (57 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 3.42 (d, J = 6.7 Hz, 2H), 1.76–1.63 (m, 5H), 1.52–1.42 (m, 1H), 1.42–1.30 (m, 1H), 1.20 (dq, J = 24.5, 12.1, 11.7 Hz, 3H), 0.91 (q, J = 11.2, 10.5 Hz, 2H); 13C NMR (100 MHz, Chloroform-d) δ 68.86, 40.57, 29.64, 26.67, 25.92.
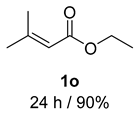
- 3-Methylbut-2-en-1-ol (2o) [60]: Colorless oil (38mg, 90% yield); 1H NMR (400 MHz, Chloroform-d) δ 5.40 (t, J = 7.3 Hz, 1H), 4.12 (d, J = 7.1 Hz, 2H), 1.73 (s, 3H), 1.67 (s, 3H), 1.16 (dt, J = 21.1, 8.5 Hz, 1H); 13C NMR (100 MHz, Chloroform-d) δ 123.67, 59.48, 25.84, 17.91.
- 4-Chlorobutan-1-ol (2p) [61]: 1H NMR (400 MHz, Chloroform-d) δ 3.66 (t, J = 6.3 Hz, 2H), 3.56 (t, J = 6.6 Hz, 2H), 1.86 (dt, J = 14.5, 6.7 Hz, 2H), 1.74–1.67 (m, 2H), 1.65 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 62.13, 45.01, 29.99, 29.10.
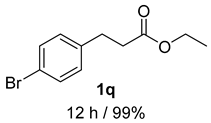
- 3-(4-Bromophenyl)propan-1-ol (2q) [62]: Colorless oil (107 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.39 (d, J = 8.3 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 3.65 (t, J = 6.4 Hz, 2H), 2.71–2.61 (m, 2H), 1.85 (dt, J = 13.6, 6.5 Hz, 2H), 1.29 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 140.85, 131.53, 130.30, 119.67, 62.09, 34.09, 31.53.
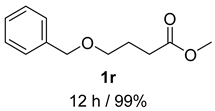
- 4-(benzyloxy)butan-1-ol (2r) [63]: Colorless oil (89 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.37–7.25 (m, 5H), 4.51 (s, 2H), 3.64 (q, J = 5.6 Hz, 2H), 3.51 (t, J = 5.6 Hz, 2H), 2.12 (t, J = 5.7 Hz, 1H), 1.69 (dq, J = 11.9, 6.2 Hz, 4H); 13C NMR (100 MHz, Chloroform-d) δ 138.21, 128.53, 127.83, 73.17, 70.43, 62.86, 30.29, 26.82.
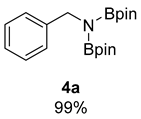
- N-Benzyl-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2 dioxaborolan-2-amine (4a) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.53 (d, J = 7.6 Hz, 2H), 7.19 (t, J = 7.6 Hz, 2H), 7.06 (t, J = 7.6 Hz, 1H), 4.56 (s, 2H), 0.97 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 143.49, 128.00, 126.37, 82.29, 47.62, 24.42.
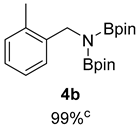
- 4,4,5,5-Tetramethyl-N-(2-methylbenzyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4b) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.61 (d, J = 7.7 Hz, 1H), 7.20 (t, J = 7.5 Hz, 1H), 7.02 (t, J = 7.4 Hz, 1H), 6.93 (d, J = 7.4 Hz, 1H), 4.56 (s, 2H), 2.07 (s, 3H), 0.97 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 140.89, 135.06, 129.86, 125.90, 125.72, 125.33, 82.29, 45.14, 24.35, 18.75.
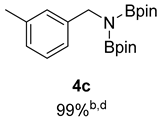
- 4,4,5,5-Tetramethyl-N-(3-methylbenzyl)-N-(1,4,4,5,5-pentamethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4c) [65]: 1H NMR (400 MHz, Benzene-d6) δ 7.33 (d, J = 7.7 Hz, 1H), 7.29 (s, 1H), 7.13 (d, J = 7.5 Hz, 1H), 6.88 (d, J = 7.5 Hz, 1H), 4.49 (s, 2H), 2.12 (s, 3H), 0.96 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 143.33, 137.12, 128.64, 127.05, 124.74, 82.21, 47.48, 24.42, 21.22.
- 4,4,5,5-Tetramethyl-N-(4-methylbenzyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4d) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.46 (d, J = 7.5 Hz, 2H), 7.01 (d, J = 7.7 Hz, 2H), 4.54 (s, 2H), 2.09 (s, 3H), 0.98 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 140.58, 135.46, 128.78, 82.23, 47.30, 24.44, 20.85.
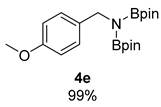
- N-(4-methoxybenzyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4e) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.47 (d, J = 8.5 Hz, 2H), 6.79 (d, J = 8.4 Hz, 2H), 4.48 (s, 2H), 3.30 (s, 3H), 0.97 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 158.66, 135.71, 129.14, 113.52, 82.20, 54.45, 46.96, 24.45.
- N-(4-(dimethylamino)benzyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine) (4f) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.57 (d, J = 8.5 Hz, 2H), 6.65 (d, J = 8.6 Hz, 2H), 4.58 (s, 2H), 2.50 (s, 6H), 1.01 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 149.65, 132.05, 128.99, 112.72, 82.16, 47.08, 40.29, 24.51.
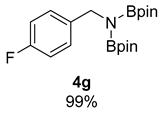
- N-(4-fluorobenzyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4g) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.36 (dd, J = 8.5, 5.6 Hz, 2H), 6.83 (t, J = 8.7 Hz, 2H), 4.42 (s, 2H), 0.96 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 161.95 (d, J C-F = 243.2 Hz), 139.24 (d, J C-F = 3.1 Hz), 129.50 (d, J C-F = 7.7 Hz), 114.71 (d, J C-F = 21.1 Hz).
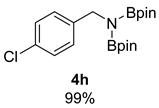
- N-(4-chlorobenzyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4h) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.29 (d, J = 8.1 Hz, 2H), 7.13 (d, J = 7.8 Hz, 2H), 4.39 (s, 2H), 0.96 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 141.96, 129.26, 128.00, 82.40, 46.88, 24.39.
- N-(4-bromobenzyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-S4 dioxaborolan-2-amine (4i) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.27 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 6.8 Hz, 2H), 4.36 (s, 2H), 0.95 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 142.42, 131.17, 129.63, 120.31, 82.40, 46.92, 24.39.
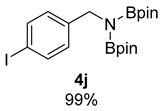
- N-(4-iodobenzyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4j) [64]:1H NMR (400 MHz, Benzene-d6) δ 7.46 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 7.8 Hz, 2H), 4.35 (s, 2H), 0.94 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 143.04, 137.17, 129.89, 91.76, 82.39, 46.99, 24.41.
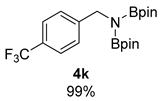
- 4,4,5,5-Tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-N-(4-(trifluoromethyl)benzyl)-1,3,2-dioxaborolan-2-amine (4k) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.36 (s, 4H), 4.43 (s, 2H), 0.95 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 147.39, 129.05, 129.01, 128.73, 128.42, 126.30, 125.04, 125.00, 124.96, 124.92, 123.60, 82.47, 47.14, 24.35.
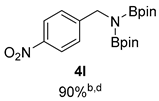
- 4,4,5,5-Tetramethyl-N-(4-nitrobenzyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4l) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.85 (d, J = 8.7 Hz, 2H), 7.20 (d, J = 8.5 Hz, 2H), 4.30 (s, 2H), 0.94 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 150.34, 146.83, 127.87, 123.45, 82.55, 46.99, 24.33.
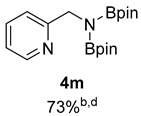
- 4,4,5,5-Tetramethyl-N-(pyridin-2-ylmethyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4m) [64]: 1H NMR (400 MHz, Benzene-d6) δ 8.42 (d, J = 5.0 Hz, 1H), 7.19–7.12 (m, 2H), 6.62–6.56 (m, 1H), 4.80 (s, 2H), 0.96 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 162.62, 148.98, 135.39, 120.79, 119.48, 82.25, 49.61, 24.38.
- 4,4,5,5-Tetramethyl-N-(pyridin-4-ylmethyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4n) [64]: 1H NMR (400 MHz, Benzene-d6) δ 8.55 (d, J = 5.8 Hz, 2H), 7.12 (d, J = 5.8 Hz, 2H), 4.35 (s, 2H), 0.94 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 151.43, 149.91, 122.13, 82.52, 46.68, 24.33.
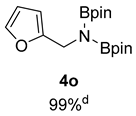
- N-(Furan-2-ylmethyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4o) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.07 (s, 1H), 6.18 (d, J = 3.2 Hz, 1H), 6.12–6.05 (m, 1H), 4.47 (s, 2H), 0.96 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 156.95, 140.98, 110.05, 105.43, 82.30, 40.97, 24.38.
- 4,4,5,5-Tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-N-(thiophen-2-ylmethyl)-1,3,2-dioxaborolan-2-amine (4p) [65]: 1H NMR (400 MHz, Benzene-d6) δ 7.05 (d, J = 3.5 Hz, 1H), 6.85 (d, J = 5.0 Hz, 1H), 6.74 (dd, J = 5.2, 3.5 Hz, 1H), 4.64 (s, 2H), 0.99 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 147.02, 126.33, 124.94, 123.85, 82.47, 42.40, 24.48.
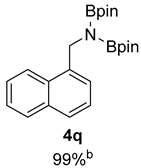
- 4,4,5,5-Tetramethyl-N-(naphthalen-1-ylmethyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4q) [64]: 1H NMR (400 MHz, Benzene-d6) δ 8.09–8.05 (m, 1H), 7.75 (d, J = 7.2 Hz, 1H), 7.62–7.58 (m, 1H), 7.53 (d, J = 8.2 Hz, 1H), 7.35 (t, J = 7.7 Hz, 1H), 7.20–7.15 (m, 2H), 5.08 (s, 2H), 0.97 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 138.51, 133.95, 131.59, 128.57, 126.88, 123.49, 123.03, 82.34, 45.12, 24.35.
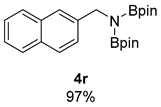
- 4,4,5,5-Tetramethyl-N-(naphthalen-2-ylmethyl)-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4r) [66,67]: 1H NMR (400 MHz, Benzene-d6) δ 7.98 (s, 1H), 7.72–7.57 (m, 4H), 7.21 (pd, J = 6.9, 1.5 Hz, 2H), 4.72 (s, 2H), 0.98 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 140.96, 133.93, 132.87, 126.63, 126.12, 125.74, 125.20, 82.63, 82.37, 47.74, 24.44.
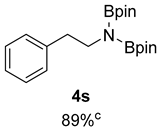
- 4,4,5,5-Tetramethyl-N-phenethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4s) [64]: 1H NMR (400 MHz, Benzene-d6) δ 7.23 (d, J = 7.5 Hz, 2H), 7.09 (d, J = 5.6 Hz, 2H), 7.00 (t, J = 7.5 Hz, 1H), 3.63 (t, J = 7.3 Hz, 2H), 2.95 (t, J = 7.3 Hz, 2H), 0.98 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 140.48, 129.47, 128.19, 125.81, 82.00, 45.77, 39.95, 24.44.
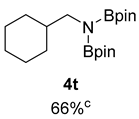
- N-(cyclohexylmethyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4t) [35]: 1H NMR (400 MHz, Benzene-d6) δ 3.27 (d, J = 7.4 Hz, 2H), 1.85 (d, J = 11.0 Hz, 2H), 1.76–1.63 (m, 4H), 1.54 (d, J = 1.3 Hz, 1H), 1.26–1.16 (m, 4H), 1.02 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 81.94, 50.21, 40.64, 30.89, 26.95, 26.35, 24.45.
- N-hexyl-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4u) [64]: 1H NMR (400 MHz, Benzene-d6) δ 3.42 (t, J = 7.1 Hz, 2H), 1.70 (q, J = 7.8 Hz, 2H), 1.36–1.21 (m, 6H), 1.02 (s, 24H), 0.81 (t, J = 3.6 Hz, 3H); 13C NMR (100 MHz, Benzene-d6) δ 81.95, 44.12, 33.69, 31.95, 26.72, 24.47, 22.84, 14.01.
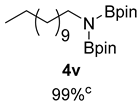
- N-dodecyl-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4v) [20,33]: 1H NMR (400 MHz, Benzene-d6) δ 3.37 (t, J = 7.2 Hz, 2H), 1.69 (p, J = 7.1 Hz, 2H), 1.23–1.16 (m, 18H), 1.01 (s, 24H), 0.84 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, Benzene-d6) δ 81.89, 44.05, 33.65, 32.02, 29.86, 29.50, 27.01, 24.45, 22.80, 14.06.
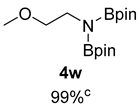
- N-(2-methoxyethyl)-4,4,5,5-tetramethyl-N-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1,3,2-dioxaborolan-2-amine (4w) [64]: 1H NMR (400 MHz, Benzene-d6) δ 3.43 (t, J = 7.2 Hz, 2H), 3.30 (t, J = 6.7 Hz, 2H), 3.06 (s, 3H), 0.98 (s, 24H); 13C NMR (100 MHz, Benzene-d6) δ 81.96, 70.75, 57.90, 41.37, 24.42.
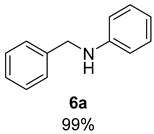
- N-Benzylaniline (6a) [68]: White solid (91 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.41–7.30 (m, 4H), 7.30–7.26 (m, 1H), 7.21–7.12 (m, 2H), 6.73 (d, J = 1.2 Hz, 1H), 6.63 (d, J = 7.7 Hz, 2H), 4.33 (s, 2H), 4.02 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 148.39, 139.70, 129.51, 128.88, 127.74, 127.46, 117.77, 113.07, 48.48.
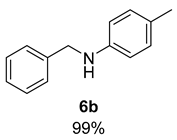
- N-Benzyl-4-methylaniline (6b) [68]: Pale yellow oil (98 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.39–7.30 (m, 4H), 7.29–7.25 (m, 1H), 6.98 (d, J = 8.5 Hz, 2H), 6.56 (d, J = 8.3 Hz, 2H), 4.30 (s, 2H), 3.90 (s, 1H), 2.23 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ 152.33, 142.65, 139.92, 128.78, 127.73, 127.35, 115.07, 114.27, 55.93, 49.35.
- N-Benzyl-4-methoxyaniline (6c) [68]: Pale yellow oil (106 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.39–7.30 (m, 4H), 7.29–7.25 (m, 1H), 6.82–6.72 (m, 2H), 6.60 (d, J = 8.8 Hz, 2H), 4.28 (s, 2H), 3.83 (s, 1H), 3.73 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ 152.32, 142.63, 139.89, 128.77, 127.72, 127.33, 115.06, 114.26, 55.93, 49.35.
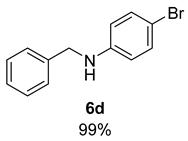
- N-Benzyl-4-bromoaniline (6d) [68]: Pale yellow solid (131 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.34 (d, J = 4.3 Hz, 4H), 7.31–7.25 (m, 1H), 7.25–7.20 (m, 2H), 6.57–6.45 (m, 2H), 4.29 (s, 2H), 4.07 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 147.29, 139.12, 132.14, 128.94, 127.62, 127.59, 114.66, 109.23, 48.35.
- N-(4-Methylbenzyl)aniline (6e) [68]: Pale yellow solid (98 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.26 (d, J = 8.6 Hz, 2H), 7.21–7.12 (m, 4H), 6.71 (t, J = 7.3 Hz, 1H), 6.63 (d, J = 8.4 Hz, 2H), 4.28 (s, 2H), 3.97 (s, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ 148.46, 137.06, 136.62, 129.54, 129.49, 127.75, 117.69, 113.06, 48.25, 21.35.
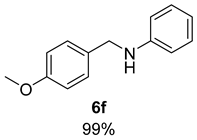
- N-(4-Methoxybenzyl)aniline (6f) [68]: Pale yellow solid (106 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.29 (d, J = 8.6 Hz, 2H), 7.23–7.11 (m, 2H), 6.87 (d, J = 8.6 Hz, 2H), 6.71 (t, J = 7.3 Hz, 1H), 6.63 (d, J = 7.7 Hz, 2H), 4.25 (s, 2H), 3.94 (s, 1H), 3.80 (s, 3H); 13C NMR (100 MHz, Chloroform-d) δ 159.05, 148.44, 131.65, 129.47, 129.01, 117.67, 114.22, 113.05, 55.46, 47.93.
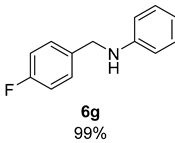
- N-(4-Fluorobenzyl)aniline (6g) [68]: Pale yellow oil (100 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.33 (dd, J = 8.3, 5.5 Hz, 2H), 7.17 (t, J = 7.7 Hz, 2H), 7.02 (t, J = 8.6 Hz, 2H), 6.72 (td, J = 7.3, 1.0 Hz, 1H), 6.61 (d, J = 8.4 Hz, 2H), 4.29 (s, 2H), 4.01 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 162.21 (d, J C-F= 245.0 Hz), 148.15, 135.33 (d, J C-F= 3.1 Hz), 129.49, 129.18 (d, J C-F = 8.1 Hz), 117.90, 115.61 (d, J C-F = 21.3 Hz), 113.07, 47.72.
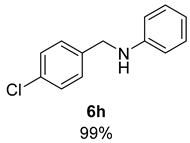
- N-(4-Chlorobenzyl)aniline (6h) [68]: Pale yellow solid (108 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.29 (s, 4H), 7.16 (ddd, J = 8.5, 7.4, 1.1 Hz, 2H), 6.71 (tt, J = 7.4, 1.1 Hz, 1H), 6.60 (dq, J = 7.5, 1.1 Hz, 2H), 4.31 (s, 2H), 4.05 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 148.07, 138.27, 133.01, 129.54, 128.95, 128.91, 117.98, 113.11, 47.73.
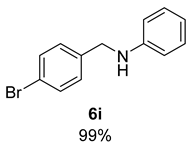
- N-(4-Bromobenzyl)aniline (6i) [68]: Pale yellow solid (131 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.45 (d, J = 8.3 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 7.16 (t, J = 7.7 Hz, 2H), 6.72 (t, J = 7.3 Hz, 1H), 6.60 (d, J = 8.4 Hz, 2H), 4.29 (s, 2H), 4.05 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 148.00, 138.78, 131.88, 129.52, 129.25, 121.09, 117.98, 113.09, 47.76.
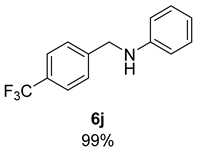
- N-(4-(Trifluoromethyl)benzyl)aniline (6j) [68]: Pale yellow oil (124 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.62–7.55 (m, 2H), 7.48 (d, J = 8.1 Hz, 2H), 7.17 (t, J = 7.7 Hz, 2H), 6.73 (t, J = 7.3 Hz, 1H), 6.60 (d, J = 8.4 Hz, 2H), 4.43–4.39 (m, 2H), 4.14 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 147.09, 140.07, 132.10, 127.19, 126.51, 121.98, 114.64, 109.36, 43.82.
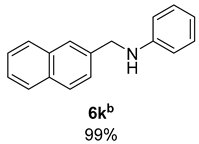
- N-(Naphthalen-2-ylmethyl)aniline (6k) [68]: Pale yellow solid (115 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.86–7.78 (m, 4H), 7.53–7.41 (m, 3H), 7.18 (tt, J = 7.4, 1.1 Hz, 2H), 6.77–6.69 (m, 2H), 6.67 (q, J = 1.0 Hz, 2H), 4.50 (s, 2H), 4.14 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 148.27, 137.04, 133.59, 132.86, 129.41, 128.49, 127.87, 127.81, 126.27, 126.02, 125.84, 117.75, 113.03, 48.61.
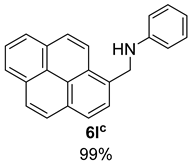
- N-(Pyren-1-ylmethyl)aniline (6l) [68]: Yellow solid (152 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 8.32 (d, J = 9.2 Hz, 1H), 8.20 (d, J = 8.0 Hz, 2H), 8.17–8.10 (m, 2H), 8.09–8.04 (m, 3H), 8.02 (t, J = 7.6 Hz, 1H), 7.25–7.19 (m, 2H), 6.81–6.72 (m, 3H), 4.99 (s, 2H), 4.10 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 148.38, 132.20, 131.40, 131.10, 130.91, 129.50, 129.07, 128.03, 127.55, 127.45, 126.80, 126.15, 125.42, 125.35, 124.95, 124.90, 123.10, 117.82, 112.93, 46.82.
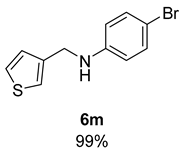
- 4-Bromo-N-(thiophen-3-ylmethyl)aniline (6m) [68]: Pale yellow solid (133 mg, 99% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.30 (dd, J = 4.9, 3.0 Hz, 1H), 7.24 (d, J = 8.3 Hz, 2H), 7.17 (dt, J = 2.9, 1.3 Hz, 1H), 7.05 (dd, J = 5.0, 1.4 Hz, 1H), 6.57–6.46 (m, 2H), 4.29 (d, J = 4.7 Hz, 2H), 4.01 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 147.12, 140.10, 132.12, 127.23, 126.53, 122.01, 114.67, 109.35, 43.82.
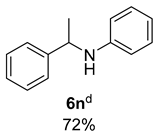
- N-(1-Phenylethyl)aniline (6n) [68]: Pale yellow oil (71 mg, 72% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.36 (d, J = 7.6 Hz, 2H), 7.31 (t, J = 7.6 Hz, 2H), 7.22 (t, J = 7.2 Hz, 1H), 7.09 (t, J = 7.9 Hz, 2H), 6.65 (t, J = 7.3 Hz, 1H), 6.53 (d, J = 8.0 Hz, 2H), 4.48 (q, J = 6.7 Hz, 1H), 1.52 (d, J = 6.7 Hz, 3H); 13C NMR (100 MHz, Chloroform-d) δ 147.37, 145.32, 129.20, 128.74, 126.96, 125.94, 117.31, 113.36, 53.54, 25.16.
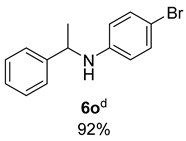
- 4-Bromo-N-(1-phenylethyl)aniline (6o) [68]: Pale yellow solid (128 mg, 92% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.34–7.28 (m, 4H), 7.24–7.18 (m, 1H), 7.14 (d, J = 8.7 Hz, 2H), 6.37 (d, J = 8.6 Hz, 2H), 4.42 (q, J = 6.8 Hz, 1H), 4.07 (s, 1H), 1.50 (d, J = 6.7 Hz, 3H); 13C NMR (100 MHz, Chloroform-d) δ 146.25, 144.70, 131.87, 128.83, 127.15, 125.84, 114.96, 108.73, 53.58, 25.09.
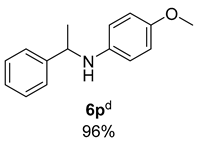
- 4-Methoxy-N-(1-phenylethyl)aniline (6p) [68]: Pale yellow soild (109 mg, 96% yield); 1H NMR (400 MHz, Chloroform-d) δ 7.37–7.28 (m, 4H), 7.24–7.18 (m, 1H), 6.70–6.66 (m, 2H), 6.49–6.44 (m, 2H), 4.40 (q, J = 6.7 Hz, 1H), 3.68 (s, 3H), 1.50 (s, 3H); 3H); 13C NMR (100 MHz, Chloroform-d) δ 151.99, 145.62, 141.69, 128.74, 126.94, 126.01, 114.86, 114.65, 55.84, 54.36, 25.28.
- 4-(Hydroxymethyl)benzonitrile (8a) [69]: 1H NMR (400 MHz, Chloroform-d) δ 7.65 (d, J = 8.0 Hz, 2H), 7.47 (d, J = 7.9 Hz, 2H), 4.78 (d, J = 5.6 Hz, 2H), 1.84 (t, J = 5.8 Hz, 1H); 13C NMR (100 MHz, Chloroform-d) δ 146.41, 132.41, 127.11, 118.99, 111.12, 64.24.
- (4-Vinylphenyl)methanol (8b) [70]: 1H NMR (400 MHz, Chloroform-d) δ 7.40 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 8.1 Hz, 2H), 6.71 (dd, J = 17.6, 10.9 Hz, 1H), 5.75 (d, J = 17.6 Hz, 1H), 5.24 (d, J = 10.9 Hz, 1H), 4.68 (s, 2H); 13C NMR (100 MHz, Chloroform-d) δ 140.55, 137.05, 136.60, 127.32, 126.48, 113.98, 65.02.
- (4-Ethynylphenyl)methanol (8c) [71]: 1H NMR (400 MHz, Chloroform-d) δ 7.48 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 4.69 (s, 2H), 3.06 (s, 1H), 1.74 (s, 1H); 13C NMR (100 MHz, Chloroform-d) δ 141.69, 132.37, 126.83, 121.28, 83.63, 77.40, 64.68.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Magano, J.; Dunetz, J.R. Large-Scale Carbonyl Reductions in the Pharmaceutical Industry. Org. Process Res. Dev. 2012, 16, 1156–1184. [Google Scholar] [CrossRef]
- Seyden-Penne, J. Reductions by Alumino- and Borohydrides in Organic Synthesis, 2nd ed.; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Brown, H.C.; Narasimhan, S.; Choi, Y.M. Selective Reductions. 30. Effect of cation and solvent on the reactivity of saline borohydrides for reduction of carboxylic esters. Improved procedures for the conversion of esters to alcohols by metal borohydrides. J. Org. Chem. 1982, 47, 4702–4708. [Google Scholar] [CrossRef]
- Pasumansky, L.; Haddenham, D.; Clary, J.W.; Fisher, G.B.; Goralski, C.T.; Singaram, B. Lithium Aminoborohydrides 16. Synthesis and Reactions of Monomeric and Dimeric Aminoboranes. J. Org. Chem. 2008, 73, 1898–1905. [Google Scholar] [CrossRef]
- Pouilloux, Y.; Autin, F.; Barrault, J. Selective hydrogenation of methyl oleate into unsaturated alcohols Relationships between catalytic properties and composition of cobalt–tin catalysts. Catal. Today 2000, 63, 87–100. [Google Scholar] [CrossRef]
- Corre, Y.; Rysak, V.; Trivelli, X.; Agbossou-Niedercorn, F.; Michon, C. A Versatile Iridium(III) Metallacycle Catalyst for the Effective Hydrosilylation of Carbonyl and Carboxylic Acid Derivatives. Eur. J. Org. Chem. 2017, 32, 4820–4826. [Google Scholar] [CrossRef]
- Kovalenko, O.O.; Adolfsson, H. Highly Efficient and Chemoselective Zinc-Catalyzed Hydrosilylation of Esters under Mild Conditions. Chem. Eur. J. 2015, 21, 2785–2788. [Google Scholar] [CrossRef]
- Behera, R.R.; Ghosh, R.; Panda, S.; Khamari, S.; Bagh, B. Hydrosilylation of Esters Catalyzed by Bisphosphine Manganese(I) Complex: Selective Transformation of Esters to Alcohols. Org. Lett. 2020, 22, 3642–3648. [Google Scholar] [CrossRef] [PubMed]
- Bézier, D.; Venkanna, G.T.; Castro, L.C.M.; Zheng, J.; Roisnel, T.; Sortais, J.; Darcel, C. Iron-Catalyzed Hydrosilylation of Esters. Adv. Synth. Catal. 2012, 354, 1879–1884. [Google Scholar] [CrossRef]
- Pehlivan, L.; Métay, E.; Laval, S.; Dayoub, W.; Delbrayelle, D.; Mignani, G.; Lemaire, M. Reduction of Aromatic and Aliphatic Esters Using the Reducing Systems MoO2(acac)2 or V(O)(OiPr)3 in Combination with 1,1,3,3-Tetramethyldisiloxane. Eur. J. Org. Chem. 2011, 36, 7400–7406. [Google Scholar] [CrossRef]
- Addis, D.; Das, S.; Junge, K.; Beller, M. Selective Reduction of Carboxylic Acid Derivatives by Catalytic Hydrosilylation. Angew. Chem. Int. Ed. 2011, 50, 6004–6011. [Google Scholar] [CrossRef]
- Clarke, J.A.; Est, A.; Nikonov, G.I. Base-Catalyzed Hydrosilylation of Nitriles to Amines and Esters to Alcohols. Eur. J. Org. Chem. 2021, 31, 4434–4439. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ellern, A.; Sadow, A.D. Magnesium-catalyzed hydroboration of esters: Evidence for a new zwitterionic mechanism. Chem. Sci. 2014, 5, 959–964. [Google Scholar] [CrossRef]
- Cao, X.; Wang, W.; Lu, K.; Yao, W.; Xue, F.; Ma, M. Magnesium-catalyzed hydroboration of organic carbonates, carbon dioxide and esters. Dalton Trans. 2020, 49, 2776–2780. [Google Scholar] [CrossRef] [PubMed]
- Barman, M.K.; Baishya, A.; Nembenna, S. Magnesium amide catalyzed selective hydroboration of esters. Dalton Trans. 2017, 46, 4152–4156. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Shirase, S.; Spaniol, T.P.; Mashima, K.; Okuda, J. Magnesium hydridotriphenylborate [Mg(thf)6][HBPh3]2: A versatile hydroboration catalyst. Chem. Commun. 2016, 52, 13155–13158. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kang, Z.; Yan, D.; Xue, M. La[N(SiMe3)2]3-Catalyzed Hydroboration of Esters and Other Challenging Unsaturated Groups. Chin. J. Chem. 2019, 37, 1142–1146. [Google Scholar] [CrossRef]
- Tamang, S.R.; Singh, A.; Bedi, D.; Bazkiaei, A.R.; Warner, A.A.; Glogau, K.; McDonald, C.; Unruh, D.K.; Findlater, M. Polynuclear lanthanide–diketonato clusters for the catalytic hydroboration of carboxamides and esters. Nat. Catal. 2020, 3, 154–162. [Google Scholar] [CrossRef]
- Patnaik, S.; Sadow, A.D. Interconverting Lanthanum Hydride and Borohydride Catalysts for C=O Reduction and C−O Bond Cleavage. Angew. Chem. Int. Ed. 2019, 131, 2527–2531. [Google Scholar] [CrossRef]
- Thenarukandiyil, R.; Satheesh, V.; Shimon, L.J.W.; Ruiter, G. Hydroboration of Nitriles, Esters, and Carbonates Catalyzed by Simple Earth-Abundant Metal Triflate Salts. Chem. Asian J. 2021, 16, 999–1006. [Google Scholar] [CrossRef]
- Makarov, K.; Kaushansky, A.; Eisen, M.S. Catalytic Hydroboration of Esters by Versatile Thorium and Uranium Amide Complexes. ACS Catal. 2022, 12, 273–284. [Google Scholar] [CrossRef]
- Yan, B.; Dutta, S.; Ma, X.; Ni, C.; Koley, D.; Yang, Z.; Roesky, H.W. Organoaluminum hydrides catalyzed hydroboration of carbonates, esters, carboxylic acids, and carbon dioxide. Dalton Trans. 2022, 51, 6756–6765. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Huang, Z.; Zhang, F.; Shao, Y. tBuOLi-Promoted Hydroboration of Esters and Epoxides. ACS Omega 2022, 7, 18876–18886. [Google Scholar] [CrossRef]
- Kumar, G.S.; Bhattacharjee, J.; Kumari, K.; Moorthy, S.; Bandyopadhyay, A.; Kumar Singh, S.; Panda, T.K. Hydroboration of nitriles, esters, and amides catalyzed by simple neosilyllithium. Polyhedron 2022, 219, 115784. [Google Scholar] [CrossRef]
- Du, Z.; Behera, B.; Kumar, A.; Ding, Y. Super hydride catalyzed ester and isocyanate hydroboration. J. Organomet. Chem. 2021, 950, 121982–121987. [Google Scholar] [CrossRef]
- Bisai, M.K.; Gour, K.; Das, T.; Vanka, K.; Sen, S.S. Readily available lithium compounds as catalysts for the hydroboration of carbodiimides and esters. J. Organomet. Chem. 2021, 949, 121924–121930. [Google Scholar] [CrossRef]
- Légaré Lavergne, J.; To, H.; Fontaine, F. Boric acid as a precatalyst for BH3-catalyzed hydroboration. RSC Adv. 2021, 11, 31941–31949. [Google Scholar] [CrossRef]
- Sarkar, N.; Kumar Sahoo, R.; Nembenna, S. Aluminium-Catalyzed Selective Hydroboration of Esters and Epoxides to Alcohols: C−O Bond Activation. Chem. Eur. J. 2023, 29, e202203023. [Google Scholar] [CrossRef] [PubMed]
- Nugent, T.C.; El-Shazly, M. Chiral Amine Synthesis—Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal. 2010, 352, 753–819. [Google Scholar] [CrossRef]
- Pubill-Ulldemolins, C.; Bonet, A.; Bo, C.; Gulyás, H.; Fernández, E. A new context for palladium mediated B-addition reaction: An open door to consecutive functionalization. Org. Biomol. Chem. 2010, 8, 2667–2682. [Google Scholar] [CrossRef]
- Chong, C.C.; Kinjo, R. Catalytic Hydroboration of Carbonyl Derivatives, Imines, and Carbon Dioxide. ACS Catal. 2015, 5, 3238–3259. [Google Scholar] [CrossRef]
- Lin, Y.; Hatzakis, E.; Mccarthy, S.M.; Reichl, K.D.; Lai, T.; Yennawar, H.P.; Radosevich, A.T. P–N Cooperative Borane Activation and Catalytic Hydroboration by a Distorted Phosphorous Triamide Platform. J. Am. Chem. Soc. 2017, 139, 6008–6016. [Google Scholar] [CrossRef]
- Kaithal, A.; Chatterjee, B.; Gunanathan, C. Ruthenium-Catalyzed Selective Hydroboration of Nitriles and Imines. J. Org. Chem. 2016, 81, 11153–11161. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.; Cheng, J.; Zheng, S.; Golen, J.A.; Manke, D.R.; Zhang, G. Cobalt (II) Coordination Polymer as a Precatalyst for Selective Hydroboration of Aldehydes, Ketones, and Imines. J. Org. Chem. 2018, 83, 9442–9448. [Google Scholar] [CrossRef]
- Saha, S.; Eisen, M.S. Catalytic Recycling of a Th–H Bond via Single or Double Hydroboration of Inactivated Imines or Nitriles. ACS Catal. 2019, 9, 5947–5956. [Google Scholar] [CrossRef]
- Jaladi, A.K.; Kim, H.; Lee, J.H.; Shin, W.K.; Hwang, H.; An, D.K. Lithium diisobutyl-t-butoxyaluminum hydride (LDBBA) catalyzed hydroboration of alkynes and imines with pinacolborane. New J. Chem. 2019, 43, 16524–16529. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Hill, M.S.; Kociok-Köhn, G. Magnesium Catalysis of Imine Hydroboration. Chem. Eur. J. 2013, 19, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Han, Y.; Wang, C.; Cheng, X. Rare-earth metal-catalyzed hydroboration of unsaturated compounds. Appl. Organomet. Chem. 2022, 36, e6570. [Google Scholar] [CrossRef]
- Geier, S.J.; Vogels, C.M.; Melanson, J.A.; Westcott, S.A. The transition metal-catalysed hydroboration reaction. Chem. Soc. Rev. 2022, 51, 8877–8922. [Google Scholar] [CrossRef]
- Shegavi, M.L.; Bose, S.K. Recent advances in the catalytic hydroboration of carbonyl compounds. Catal. Sci. Technol. 2019, 9, 3307–3336. [Google Scholar] [CrossRef]
- Roy, M.M.D.; Omaña, A.A.; Wilson, A.S.S.; Hill, M.S.; Aldridge, S.; Rivard, E. Molecular Main Group Metal Hydrides. Chem. Rev. 2021, 121, 12784–12965. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, F.; Yu, C.; Luo, Y. Reduction of Amides to Amines with Pinacolborane Catalyzed by Heterogeneous Lanthanum Catalyst La(CH2C6H4NMe2-o)3@SBA-15. Inorg. Chem. 2021, 60, 13122–13135. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Eisen, M.S. Organo-f-Complexes for Efficient and Selective Hydroborations. Synthesis 2020, 52, 629–644. [Google Scholar] [CrossRef]
- Sadow, A.D. Alkali and Alkaline Earth Element-Catalyzed Hydroboration Reactions. In Early Main Group Metal Catalysis; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Tamang, S.R.; Findlater, M. Emergence and Applications of Base Metals (Fe, Co, and Ni) in Hydroboration and Hydrosilylation. Molecules 2019, 24, 3194. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Bhattacharya, P.; Dai, H.; Guan, H. Nickel and Iron Pincer Complexes as Catalysts for the Reduction of Carbonyl Compounds. Acc. Chem. Res. 2015, 48, 1995–2003. [Google Scholar] [CrossRef]
- Magre, M.; Szewczyk, M.; Rueping, M. s-Block Metal Catalysts for the Hydroboration of Unsaturated Bonds. Chem. Rev. 2022, 122, 8261–8312. [Google Scholar] [CrossRef]
- Banerjee, I.; Panda, T.K. Recent developments in the reduction of unsaturated bonds by magnesium precursors. Appl. Organomet. Chem. 2021, 35, e6333. [Google Scholar] [CrossRef]
- Arrowsmith, M.; Hill, M.S.; Hadlington, T.; Kociok-Köhn, G.; Weetman, C. Magnesium-Catalyzed Hydroboration of Pyridines. Organometallics 2011, 30, 5556–5559. [Google Scholar] [CrossRef]
- Weetman, C.; Anker, M.D.; Arrowsmith, M.; Hill, M.S.; Kociok-Köhn, G.; Liptrot, D.J.; Mahon, M.F. Magnesium-catalysed nitrile hydroboration. Chem. Sci. 2016, 7, 628–641. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Sheng, X.; Hua, H.; Yao, W.; Pullarkat, S.A.; Xu, L.; Ma, M. Unsymmetrical β-diketiminate magnesium(I) complexes: Syntheses and application in catalytic hydroboration of alkyne, nitrile and carbonyl compounds. Org. Chem. Front. 2018, 5, 3538–3547. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.; Qin, Y.; Yao, W.; Yuan, D.; Pullarkat, S.A.; Xu, L.; Ma, M. Grignard reagents-catalyzed hydroboration of aldehydes and ketones. Tetrahedron 2020, 76, 131145–131150. [Google Scholar] [CrossRef]
- Han, H.J.; Kim, H.T.; Kim, J.H.; Jaladi, A.K.; An, D.K. Magnesium-mediated hydroboration under ambient condition: A reduction of esters, aldehydes, and ketones. Tetrahedron 2023, 142, 133500–133505. [Google Scholar] [CrossRef]
- Jang, Y.K.; Magre, M.; Rueping, M. Chemoselective Luche-Type Reduction of α,β-Unsaturated Ketones by Magnesium Catalysis. Org. Lett. 2019, 21, 8349–8352. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Magre, M.; Zubar, V.; Rueping, M. Reduction of Cyclic and Linear Organic Carbonates Using a Readily Available Magnesium Catalyst. ACS Catal. 2019, 9, 11634–11639. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Bhattacharya, P.; Krause, J.A.; Guan, H. Iron Hydride Complexes Bearing Phosphinite-Based Pincer Ligands: Synthesis, Reactivity, and Catalytic Application in Hydrosilylation Reactions. Organometallics 2011, 30, 4720–4729. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Zhang, C.; Ma, J.; Zhao, D. Highly Efficient System for Reduction of Carboxylic Acids and Their Derivatives to Alcohols by HfCl4/KBH4. Synth. Commun. 2009, 39, 1640–1654. [Google Scholar] [CrossRef]
- Kim, H.; Shin, H.L.; Yi, J.; Choi, H.S.; Lee, J.H.; Hwang, H.; An, D.K. Lithium Bromide/HBpin: A Mild and Effective Catalytic System for the Selective Hydroboration of Aldehydes and Ketones. Bull. Korean Chem. Soc. 2020, 41, 1009–1018. [Google Scholar] [CrossRef]
- Cano, I.; Martínez-Prieto, L.M.; Vendier, L.; van Leeuwen, P.W.N.M. An iridium–SPO complex as bifunctional catalyst for the highly selective hydrogenation of aldehydes. Catal. Sci. Technol. 2018, 8, 221–228. [Google Scholar] [CrossRef]
- Barry, C.N.; Evans, S.A., Jr. Triphenylphosphine-tetrachloromethane-promoted chlorination and cyclodehydration of simple diols. J. Org. Chem. 1981, 46, 3361–3364. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Wan, H.; Wang, W.; Zhang, S. Stereoselective organocatalytic oxidation of alcohols to enals: A homologation method to prepare polyenes. Chem. Commun. 2016, 52, 3532–3535. [Google Scholar] [CrossRef]
- Avuluri, S.; Bujaranipalli, S.; Das, S.; Yadav, J.S. Stereoselective synthesis of 5′-hydroxyzearalenone. Tetrahedron Lett. 2018, 59, 3547–3549. [Google Scholar] [CrossRef]
- Seok, J.E.; Kim, H.T.; Kim, J.; Lee, J.H.; Jaladi, A.K.; Hwang, H.; An, D.K. Effective Magnesium-catalyzed Hydroboration of Nitriles and Imines. Asian J. Org. Chem. 2022, 11, e2022004. [Google Scholar] [CrossRef]
- Ghosh, P.; Jacobi von Wangelin, A. Manganese-Catalyzed Hydroborations with Broad Scope. Chem. Int. Ed. 2021, 60, 16035–16043. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, S.; Zhu, X.; Yuan, Q.; Wei, Y.; Zhou, S.; Mu, X. Well-Defined Amidate-Functionalized N-Heterocyclic Carbene -Supported Rare-Earth Metal Complexes as Catalysts for Efficient Hydroboration of Unactivated Imines and Nitriles. Inorg. Chem. 2018, 57, 15069–15078. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharjee, J.; Panda, T.K. An imidazolin-2-iminato ligand organozinc complex as a catalyst for hydroboration of organic nitriles. New J. Chem. 2019, 43, 16812–16818. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.T.; Lee, J.H.; Hwang, H.; An, D.K. Lithium bromide: An inexpensive and efficient catalyst for imine hydroboration with pinacolborane at room temperature. RSC Adv. 2020, 10, 34421–34427. [Google Scholar] [CrossRef] [PubMed]
- Boobalan, R.; Liu, K.; Chao, J.; Chen, C. Synthesis and biological assay of erlotinib analogues and BSA-conjugated erlotinib analogue. Bioorg. Med. Chem. 2017, 27, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Du, X.; Cui, Z.; Zeng, Y.; Liu, Y.; Yang, T.; Wen, J.; Zhang, X. Homogeneous Hydrogenation with a Cobalt/Tetraphosphine Catalyst: A Superior Hydride Donor for Polar Double Bonds and N-Heteroarenes. J. Am. Chem. Soc. 2019, 141, 20424–20433. [Google Scholar] [CrossRef]
- Vestberg, R.; Westlund, R.; Eriksson, A.; Lopes, C.; Carlsson, M.; Eliasson, B.; Glimsdal, E.; Lindgren, M.; Malmström, E. Dendron Decorated Platinum (II) Acetylides for Optical Power Limiting. Macromolecules 2006, 39, 2238–2246. [Google Scholar] [CrossRef]


 | ||
|---|---|---|
| Entry | RMgX | Conversion a (%) |
| 1 | MeMgCl | 99 |
| 2 | MeMgBr | 99 |
| 3 | MeMgI | 75 |
| 4 | BuMgCl | 93 |
| 5 | t-BuMgCl | 96 |
| 6 | i-PrMgCl | 82 |
| 7 | PhMgCl | 89 |
 | |||
|---|---|---|---|
| Entry | HBpin (Equiv) | MeMgCl (mol%) | Conversion a (%) |
| 1 | 2.0 | 5 | 94 |
| 2 | 2.2 | 5 | 96 |
| 3 | 2.4 | 5 | 98 |
| 4 | 2.5 | 5 | 99 |
| 5 | 2.5 | 3 | 63 |
| 6 | 2.5 | 4 | 82 |
 | ||
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 | ||
|---|---|---|
| Entry | RMgX | Conversion a (%) |
| 1 | MeMgCl | 99 |
| 2 | MeMgBr | 58 |
| 3 | MeMgI | 57 |
| 4 | BuMgCl | 94 |
| 5 | i-PrMgCl | 99 |
| 6 | t-BuMgCl | 99 |
| 7 | PhMgCl | 92 |
| 8 | PhMgBr | 70 |
 | ||||
|---|---|---|---|---|
| Entry | HBpin (Equiv) | MeMgCl (mol%) | Time (h) | Conversion a (%) |
| 1 | 3.0 | 3.0 | 6 | 97 |
| 2 | 3.0 | 3.0 | 12 | 99 |
| 3 | 3.0 | 2.0 | 12 | 45 |
| 4 | 2.5 | 3.0 | 12 | 99 |
| 5 | 2.5 | 2.0 | 12 | 83 |
| 6 | 2.5 | 2.0 | 24 | 99 |
| 7 | 2.2 | 3.0 | 12 | 98 |
| 8 | 2.2 | 3.0 | 24 | 99 |
 | |||
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  | |
 | ||
|---|---|---|
| Entry | RMgX | Conversion a (%) |
| 1 | MeMgCl | 99 |
| 2 | MeMgBr | 91 |
| 3 | MeMgI | 78 |
| 4 | i-PrMgCl | 83 |
| 5 | BuMgCl | 97 |
| 6 | t-BuMgCl | 99 |
| 7 | PhMgCl | 83 |
 | ||||
|---|---|---|---|---|
| Entry | MeMgCl (mol%) | HBpin (Equiv) | Time (h) | Conversion a (%) |
| 1 | 5 | 2.0 | 3 | 77 |
| 2 | 5 | 1.5 | 6 | 99 |
| 3 | 5 | 1.5 | 3 | 52 |
| 4 | 5 | 1.3 | 6 | 64 |
| 5 | 5 | 1.3 | 12 | 89 |
| 6 | 3 | 1.5 | 6 | 21 |
| 7 | 3 | 2.0 | 12 | 53 |
 | ||
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 |  |  |
 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.J.; Park, S.Y.; Jeon, S.E.; Kwak, J.S.; Lee, J.H.; Jaladi, A.K.; Hwang, H.; An, D.K. Grignard Reagent-Catalyzed Hydroboration of Esters, Nitriles, and Imines. Molecules 2023, 28, 7090. https://doi.org/10.3390/molecules28207090
Han HJ, Park SY, Jeon SE, Kwak JS, Lee JH, Jaladi AK, Hwang H, An DK. Grignard Reagent-Catalyzed Hydroboration of Esters, Nitriles, and Imines. Molecules. 2023; 28(20):7090. https://doi.org/10.3390/molecules28207090
Chicago/Turabian StyleHan, Hyun Ji, Suh Youn Park, So Eun Jeon, Jae Seok Kwak, Ji Hye Lee, Ashok Kumar Jaladi, Hyonseok Hwang, and Duk Keun An. 2023. "Grignard Reagent-Catalyzed Hydroboration of Esters, Nitriles, and Imines" Molecules 28, no. 20: 7090. https://doi.org/10.3390/molecules28207090
APA StyleHan, H. J., Park, S. Y., Jeon, S. E., Kwak, J. S., Lee, J. H., Jaladi, A. K., Hwang, H., & An, D. K. (2023). Grignard Reagent-Catalyzed Hydroboration of Esters, Nitriles, and Imines. Molecules, 28(20), 7090. https://doi.org/10.3390/molecules28207090






