Ionic Liquids Hybridization for Carbon Dioxide Capture: A Review
Abstract
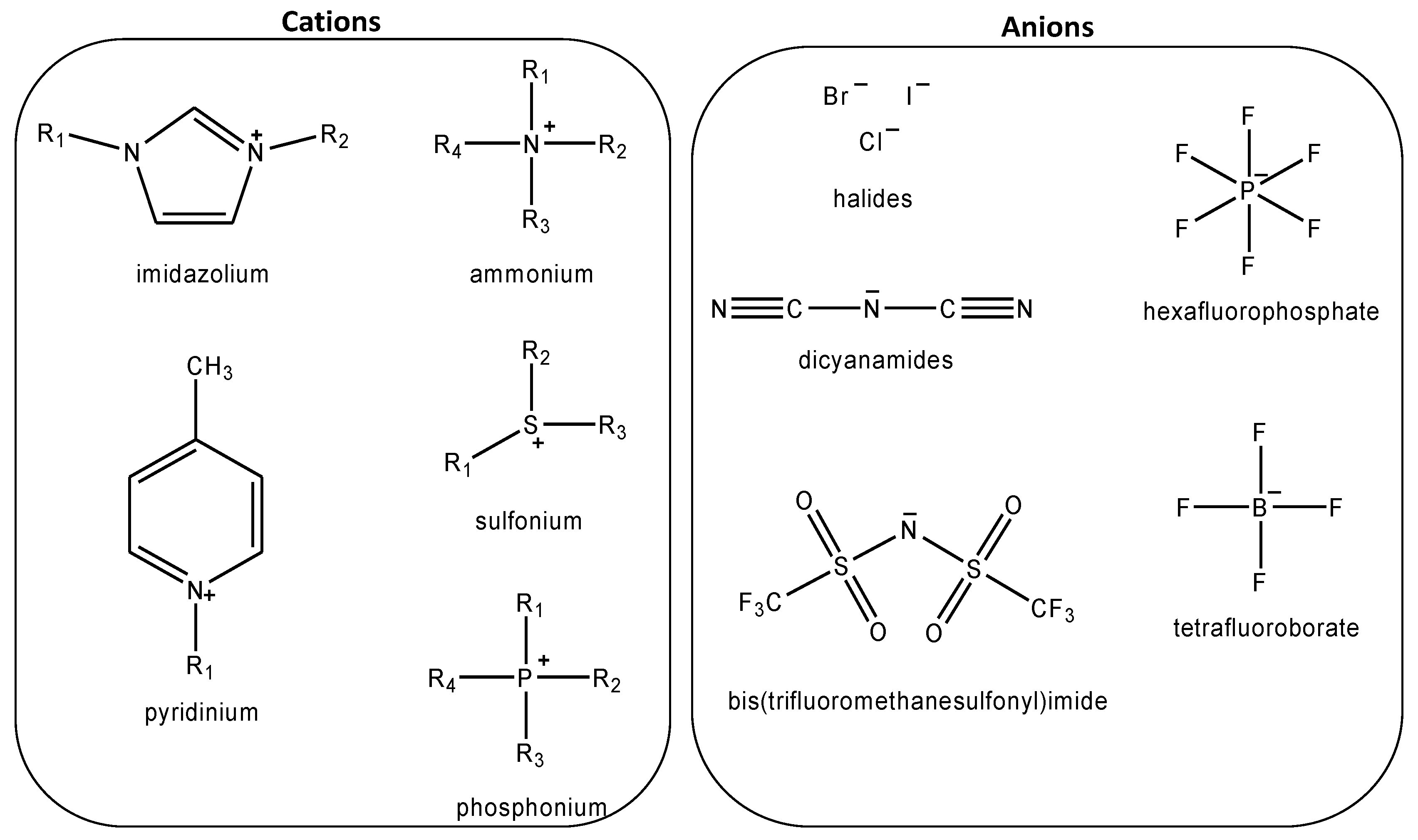
:1. Introduction
2. ILs Utilization for CO2 Capture
Challenges in ILs Viscosity towards CO2 Capture
3. ILs Hybridization Strategy
3.1. Methods in ILs Hybridization
3.1.1. Blending/Mixing
3.1.2. Immobilization
3.1.3. Wet Impregnation
3.1.4. Polymerization Technique
3.1.5. Ionothermal Synthesis
4. Application of ILs Hybrid Material in Carbon Dioxide
4.1. ILs/Amines
4.2. ILs/Activated Carbon
4.3. ILs/Mesoporous Silica
4.4. ILs/MOF
4.5. ILs/Cellulose
5. Challenges in IL-Hybrid Materials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| MDEA | Methyldiethanolamine |
| DEA | Diethanolamine |
| MEA | Monoethanolamine |
| EDA | Ethanoldiamine |
| DETA | Dietylenetriamine |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| [Cho][Gly] | Choline glycinate |
| [BMIM][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| [EMMIM][Tf2N] | 2,3-dimethyl-1-ethylimidazolium bistrifluoromethyl(sulfonyl)imide |
| [EMIM][Tf2N] | 1-ethyl-3-methylimidazolium bistrifluoromethyl(sulfonyl)imide |
| [2-AEmim][Tf2N] | 1-(3-aminoethyl)-3-methylimidazolium bistrifluoromethyl(sulfonyl)imide |
| [PVBIM][BF4] | Poly 1-(4-vinylbenzyl)-3-butylimidazolium tetrafluoroborate |
| [BMIM][BF4] | 1-butyl-3-methylimidazolium tetrafluoroborate |
| [PVBIM][PF6] | Poly 1-(4-vinylbenzyl)-3-butylimidazolium hexafluorophosphate |
| [PBIMT][BF4] | Poly 2-(1-butylimidazolium-3-yl)ethyl methacrylate tetrafluoroborate |
| [MDAP][Tf2N]-PDC | Methyl-diaminopyridinium bis(trifluoromethanesulfonyl)imide-(2,6-pyridinedicarbonyl chloride) |
| [P(C4)4][Gly] | Tetrabutylphosphonium glycinate |
| [P66614][Pro] | Trihexyl(tetradecyl)phosphonium prolinate |
| [P4444][Pro] | Tetrabutylphoshonium prolinate |
| [B4MPyr][Arg] | 1-butyl-4-methyl pyridinium arginate |
| [C1ImPA][Gly] | 1-(2-aminopropyl)-3-methylimidazolium glycinate |
| [TEPA][Im] | Tetraehylnepentamine imidazolate |
| [TETAH][Pz] | Triethylenetetramine 1,2,4-triazolate |
| [TMGH][Pyrr] | Tetramethylgunidinium pyrrole |
| [TMGH][Im] | Tetramethylgunidinium imidazole |
| [TBP][MeSO3] | Tetrabutylphsphonium methanesulfonate |
| [TETAH][Ly] | triethylenetetramine L-lysine |
| [VEIM][Br] | 1-vinyl-3-ethylimidazolium bromide |
| [EMIM][Br] | 1-ethyl-3-methylimidazolium bromide |
| [BMIM][OTF] | 1-butyl-3-methylimidazolium trifluoromethanesulfonate |
| [ASBI[TfO] | 3-allyl-1- (4-sulphobutyl)imidazolium triflate |
| [ASBI][HSO4] | 3-allyl-1- (4-sulphobutyl)imidazolium sulphate |
| [EMIM][OAc] | 1-ethyl-3-methylimidazolium acetate |
| [BEIM][BF4] | 1-butyl-2-ethylimidazolium tetrafluoroborate |
| [DMAPAH][OAc] | 3-dimethylamino-1-propylamine acetate |
| [BMIM][OAc] | 1-butyl-3-methylimidazolium acetate |
| [EMIM][OcSO4] | 1-butyl-3-methylimidazolium octylsulphate |
| [HMIM][BF4] | 1-hexyl-3-methylimidazolium tetrafluoroborate |
| [EMIM][Gly] | 1-ethyl-3-methylimidazolium glycinate |
| [P8883][Tf2N] | Phosphonium bis-trifluoromethanesulfonimide |
| [VBTMA][Arg] | 4-(vinylbenzyltrimethyl)ammonium argininate |
| [VBTMA][Lys] | 4-(vinylbenzyltrimethyl)ammonium lysinate |
| [VBTMA][Gly] | 4-(vinylbenzyltrimethyl)ammonium glycinate |
| [BMIM][Cl] | 1-butyl-3-methylimidazolium chloride |
| [TEPA][NO3] | Tetraethylenepentammonium nitrate |
| [i-C5mim][Tf2N] | 1-isopentyl-3-methylimidazolium bis-trifluoromethanesulfonimide |
| [EMIM][Phe] | 1-ethyl-3-methylimidazolium phenolate |
| [HMIM][DCN] | 1-hexyl-3-methylimidazolium dicynamide |
| [Celmim][Cl] | Cellulosic-6-imidazolium chloride |
| [CelEt3N][Cl] | Cellulosic-6-triethylammonium chloride |
| [Celmim][Tf2N] | Cellulosic-6-imidazolium bis-trifluoromethanesulfonimide |
| [Celmim][BF4] | Cellulosic-6-imidazolium tetrafluoroborate |
| [Celmim][PF6] | Cellulosic-6-imidazolium hexafluorophosphate |
| [CelEt3N][BF4] | Cellulosic-6-triethylammonium tetrafluoroborate |
| [CelEt3N][PF6] | Cellulosic-6-triethylammonium hexafluorophosphate |
| [N444][Oac] | Tetrabutylammonium acetate |
| [N1888][Oac] | Tetraoctylmethylammonium acetate |
| [N888][Br] | Tetraoctylammonium bromide |
| P[DADMA][Cl] | Poly(diallyldimethylammonium) acetate |
| P[DADMA][OAc] | Poly(diallyldimethylammonium) acetate |
| P[VBA][Cl] | Poly(p-vinylbenzyltriethylammonium) chloride |
| P[VBMPyr][Cl] | Poly(1-methyl-1-(4′-vinylbenzyl)pyrrolidinium) chloride |
| [P2228][6BrInda] | triethyl(octyl)phosphonium 6-bromoindazolide |
| [DMAPAH][4F-PhO] | N,N-dimethyl-1,3-propane diamine 3-fluorophenolate |
References
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Chapter 3—Basic Concepts of Natural Gas Processing. In Handbook of Natural Gas Transmission and Processing, 3rd ed.; Poe, W.A., Ed.; Gulf Profesional Publishing: Boston, MA, USA, 2015; pp. 123–135. [Google Scholar]
- Wasiu, A.B.; Heikal, M.R. The effect of carbon dioxide content-natural gas on the performance characteristics of engines: A review. J. Appl. Sci. 2012, 12, 2350. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for carbon dioxide capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Karame, I., Shaya, J., Srour, H., Eds.; IntechOpen Limited: London, UK, 2018; pp. 142–163. [Google Scholar]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013—A Review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Raksajati, A.; Ho, M.; Wiley, D. Solvent development for post-combustion CO2 capture: Recent development and opportunities. In Proceedings of the 24th Regional Symposium on Chemical Engineering (RSCE 2017), Semarang, Indonesia, 15–16 November 2018. [Google Scholar]
- Kafi, M.; Sanaeepur, H.; Ebadi Amooghin, A. Grand Challenges in CO2 Capture and Conversion. J. Res. Recov. 2023, 1, 1007. [Google Scholar] [CrossRef]
- Salim, S. Treatment of amine wastes generated in industrial processes. In Proceedings of the 2nd International Conference on Innovative Technology, Engineering and Sciences (iCITES 2020), Pahang, Malaysia, 22–23 December 2020. [Google Scholar]
- Heggset, E.B.; Syverud, K.; Øyaas, K. Novel pretreatment pathways for dissolution of lignocellulosic biomass based on ionic liquid and low temperature alkaline treatment. Biomass Bioenergy 2016, 93, 194–200. [Google Scholar] [CrossRef]
- Goutham, R.; Rohit, P.; Vigneshwar, S.S.; Swetha, A.; Arun, J.; Gopinath, K.P.; Pugazhendhi, A. Ionic liquids in wastewater treatment: A review on pollutant removal and degradation, recovery of ionic liquids, economics and future perspectives. J. Mol. Liq. 2022, 349, 118150. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.; Silvestre, A.J.; Freire, M.G. Ionic Liquids in Drug Delivery. Encyclopedia 2021, 1, 324–339. [Google Scholar] [CrossRef]
- Casiello, M.; Catucci, L.; Fracassi, F.; Fusco, C.; Laurenza, A.G.; Di Bitonto, L.; Pastore, C.; D’Accolti, L.; Nacci, A. ZnO/Ionic liquid catalyzed biodiesel production from renewable and waste lipids as feedstocks. Catalysts 2019, 9, 71. [Google Scholar] [CrossRef]
- Song, J. Research progress of ionic liquids as lubricants. ACS Omega 2021, 6, 29345–29349. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Brandl, P.; Hallett, J.P.; Mac Dowell, N. Challenges and opportunities for the utilisation of ionic liquids as solvents for CO2 capture. Mol. Syst. Des. Eng. 2018, 3, 560–571. [Google Scholar] [CrossRef]
- Lian, S.; Song, C.; Liu, Q.; Duan, E.; Ren, H.; Kitamura, Y. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, Y.; Ji, X.; Yang, Z.; Lu, X. Experimental study of CO2 absorption in aqueous cholinium-based ionic liquids. Fluid Phase Equilibria 2017, 445, 14–24. [Google Scholar] [CrossRef]
- Karadas, F.; Köz, B.; Jacquemin, J.; Deniz, E.; Rooney, D.; Thompson, J.; Yavuz, C.T.; Khraisheh, M.; Aparicio, S.; Atihan, M. High pressure CO2 absorption studies on imidazolium-based ionic liquids: Experimental and simulation approaches. Fluid Phase Equilibria 2013, 351, 74–86. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Gu, Z.; Brennecke, J.F. High-pressure phase behavior of ionic liquid/CO2 systems. J. Phys. Chem. B 2001, 105, 2437–2444. [Google Scholar] [CrossRef]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Morrow, T.I.; Brennecke, J.F.; Maginn, E.J. Why is CO2 so soluble in imidazolium-based ionic liquids? J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef]
- Orhan, O.Y. Effects of various anions and cations in ionic liquids on CO2 capture. J. Mol. Liq. 2021, 333, 115981. [Google Scholar] [CrossRef]
- Jia, X.; Hu, X.; Su, K.; Wang, W.; Du, C. Molecular screening of ionic liquids for CO2 absorption and molecular dynamic simulation. Open Chem. 2022, 20, 379–387. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Aki, S.N.; Mellein, B.R.; Saurer, E.M.; Brennecke, J.F. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J. Phys. Chem. B 2004, 108, 20355–20365. [Google Scholar] [CrossRef]
- Sharma, P.; Do Park, S.; Park, K.T.; Nam, S.C.; Jeong, S.K.; Yoon, Y.I.; Baek, I.H. Solubility of carbon dioxide in amine-functionalized ionic liquids: Role of the anions. Chem. Eng. J 2012, 193, 267–275. [Google Scholar] [CrossRef]
- Hussain, S.; Dong, H.; Zeng, S.; Ahmad, M.U.; Shehzad, F.K.; Wu, H.; Zhang, Y. Investigation uncovered the impact of anions on CO2 absorption by low viscous ether functionalized pyridinium ionic liquids. J. Mol. Liq. 2021, 336, 116362. [Google Scholar] [CrossRef]
- Zhang, R.; Ke, Q.; Zhang, Z.; Zhou, B.; Cui, G.; Lu, H. Tuning Functionalized Ionic Liquids for CO2 Capture. Int. J. Mol. Sci. 2022, 23, 11401. [Google Scholar] [CrossRef]
- Keller, A.N.; Bentley, C.L.; Morales-Collazo, O.; Brennecke, J.F. Design and characterization of aprotic N-heterocyclic anion ionic liquids for carbon capture. J. Chem. Eng. Data 2022, 67, 375–384. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Tu, Z.; Wu, Y.; Hu, X. Low-viscous diamino protic ionic liquids with fluorine-substituted phenolic anions for improving CO2 reversible capture. J. Mol. Liq. 2018, 268, 617–624. [Google Scholar] [CrossRef]
- Tang, J.; Sun, W.L.; Tang, H.; Radosz, M.; Shen, Y. Enhanced CO2 Absorption of Poly(ionic liquid)s. Macromolecules 2005, 38, 2037–2039. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Mantione, D.; El Tall, O.; Ruipérez, F.; Sarwar, M.I.; Rothenberger, A.; Mecerreyes, D. Pyridinium containing amide based polymeric ionic liquids for CO2/CH4 separation. ACS Sustain. Chem. Eng. 2019, 7, 10241–10247. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, J. Benzimidazole-based hyper-cross-linked poly (ionic liquid) s for efficient CO2 capture and conversion. Chem. Eng. J. 2020, 385, 123973. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported absorption of CO2 by tetrabutylphosphonium amino acid ionic liquids. Chem. Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, M.; Izadyar, M.; Nakhaei Pour, A. Carbon Dioxide Absorption by the Imidazolium–Amino Acid Ionic Liquids, Kinetics, and Mechanism Approach. J. Phys. Chem. A 2018, 122, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Noorani, N.; Mehrdad, A.; Ahadzadeh, I. CO2 absorption in amino acid-based ionic liquids: Experimental and theoretical studies. Fluid Phase Equilibria 2021, 547, 113185. [Google Scholar] [CrossRef]
- Kang, S.; Chung, Y.G.; Kang, J.H.; Song, H. CO2 absorption characteristics of amino group functionalized imidazolium-based amino acid ionic liquids. J. Mol. Liq. 2020, 297, 111825. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Bao, Z.; Zhang, Z.; Yang, Y.; Ren, Q.; Xing, H.; Dai, S. New insights into CO2 absorption mechanisms with amino-acid ionic liquids. ChemSusChem 2016, 9, 806–812. [Google Scholar] [CrossRef]
- Onofri, S.; Bodo, E. CO2 Capture in Biocompatible Amino Acid Ionic Liquids: Exploring the Reaction Mechanisms for Bimolecular Absorption Processes. J. Phys. Chem. B 2021, 125, 5611–5619. [Google Scholar] [CrossRef]
- Cui, G.; Wang, J.; Zhang, S. Active chemisorption sites in functionalized ionic liquids for carbon capture. Chem. Soc. Rev. 2016, 45, 4307–4339. [Google Scholar] [CrossRef]
- Lv, B.; Jing, G.; Qian, Y.; Zhou, Z. An efficient absorbent of amine-based amino acid-functionalized ionic liquids for CO2 capture: High capacity and regeneration ability. Chem. Eng. J. 2016, 289, 212–218. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, S.; Hou, Y.; Zhang, K.; Wu, W. Highly efficient absorption of NO by dual functional ionic liquids with low viscosity. Ind. Eng. Chem. Res. 2019, 58, 13313–13320. [Google Scholar] [CrossRef]
- Tiwari, S.C.; Pant, K.K.; Upadhyayula, S. Efficient CO2 absorption in aqueous dual functionalized cyclic ionic liquids. J. CO2 Util. 2021, 45, 101416. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shah, F.U. Ether functionalized choline tethered amino acid ionic liquids for enhanced CO2 capture. ACS Sustain. Chem. Eng. 2016, 4, 5441–5449. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, F.; Huang, K.; Ma, J.-W.; Wu, Y.-T.; Zhang, Z.-B. Absorption of CO2 in amino acid ionic liquid (AAIL) activated MDEA solutions. Int. J. Greenh. Gas Control 2013, 19, 379–386. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Jiang, D.e.; Li, H.; Dai, S. Carbon dioxide capture by superbase-derived protic ionic liquids. Angew. Chem. 2010, 122, 6114–6117. [Google Scholar] [CrossRef]
- Li, F.; Bai, Y.; Zeng, S.; Liang, X.; Wang, H.; Huo, F.; Zhang, X. Protic ionic liquids with low viscosity for efficient and reversible capture of carbon dioxide. Int. J. Greenh. Gas Control 2019, 90, 102801. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Kunov-Kruse, A.J.; Fehrmann, R.; Riisager, A. Amine-functionalized amino acid-based ionic liquids as efficient and high-capacity absorbents for CO2. ChemSusChem 2014, 7, 897–902. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Process analysis overview of ionic liquids on CO2 chemical capture. Chem. Eng. J. 2020, 390, 124509. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, H.; Gao, H.; Olson, W.; Liang, Z. CO2 capture with hybrid absorbents of low viscosity imidazolium-based ionic liquids and amine. Appl. Energy 2019, 235, 311–319. [Google Scholar] [CrossRef]
- Qazi, S.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Younas, M.; Sanchez-Marcano, J. Mathematical modeling of CO2 absorption with ionic liquids in a membrane contactor, study of absorption kinetics and influence of temperature. J. Chem. Technol. Biotechnol. 2020, 95, 1844–1857. [Google Scholar] [CrossRef]
- Ramdin, M.; Amplianitis, A.; Bazhenov, S.; Volkov, A.; Volkov, V.; Vlugt, T.J.; de Loos, T.W. Solubility of CO2 and CH4 in ionic liquids: Ideal CO2/CH4 selectivity. Ind. Eng. Chem. Res. 2014, 53, 15427–15435. [Google Scholar] [CrossRef]
- Latini, G.; Signorile, M.; Rosso, F.; Fin, A.; d’Amora, M.; Giordani, S.; Pirri, F.; Crocella, V.; Bordiga, S.; Bocchini, S. Efficient and reversible CO2 capture in bio-based ionic liquids solutions. J. CO2 Util. 2022, 55, 101815. [Google Scholar] [CrossRef]
- Luo, X.Y.; Fan, X.; Shi, G.L.; Li, H.R.; Wang, C.M. Decreasing the viscosity in CO2 capture by amino-functionalized ionic liquids through the formation of intramolecular hydrogen bond. J. Phys. Chem. B 2016, 120, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Seo, S.; Quiroz-Guzman, M.; DeSilva, M.A.; Lee, T.B.; Huang, Y.; Goodrich, B.F.; Schneider, W.F.; Brennecke, J.F. Chemically tunable ionic liquids with aprotic heterocyclic anion (AHA) for CO2 capture. J. Phys. Chem. B 2014, 118, 5740–5751. [Google Scholar] [CrossRef]
- Luo, Q.-x.; An, B.-w.; Ji, M.; Zhang, J. Hybridization of metal–organic frameworks and task-specific ionic liquids: Fundamentals and challenges. Mater. Chem. Front. 2018, 2, 219–234. [Google Scholar] [CrossRef]
- Xie, Z.L.; Su, D.S. Ionic liquid based approaches to carbon materials synthesis. Eur. J. Inorg. Chem. 2015, 2015, 1137–1147. [Google Scholar] [CrossRef]
- Kim, T.; Tung, T.T.; Lee, T.; Kim, J.; Suh, K.S. Poly (ionic liquid)-mediated hybridization of single-walled carbon nanotubes and conducting polymers. Chem. Asian J. 2010, 5, 256–260. [Google Scholar] [CrossRef]
- Chen, N. Functional Ionic Liquids and Related Porous Materials for Gas Capture and Catalysis. Ph.D. Thesis, University of Tennessee, Knoxxville, TN, USA, May 2018. [Google Scholar]
- Roh, E.J.; Lee, J.K.; Park, M.J.; Park, J.H.; Lee, B.S.; Lee, E. Investigation of Anion Effects in Ionic Liquid-Nano Hybrid Materials; Korea Institute of Science and Technology (Korea South) Medical Chemistry: Seoul, Republic of Korea, 2006. [Google Scholar]
- Huang, Q.; Luo, Q.; Wang, Y.; Pentzer, E.; Gurkan, B. Hybrid ionic liquid capsules for rapid CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 10503–10509. [Google Scholar] [CrossRef]
- Haider, J.; Saeed, S.; Qyyum, M.A.; Kazmi, B.; Ahmad, R.; Muhammad, A.; Lee, M. Simultaneous capture of acid gases from natural gas adopting ionic liquids: Challenges, recent developments, and prospects. Renew. Sustain. Energy Rev. 2020, 123, 109771. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Z.; Dai, F.; Ji, X. Ionic Liquids/Deep Eutectic Solvents-Based Hybrid Solvents for CO2 Capture. Crystals 2020, 10, 978. [Google Scholar] [CrossRef]
- Zainul Anuar, M.a.U.; Taha, M.F.; Md Yunus, N.M.; Mat Ghani, S.M.; Idris, A. An optimization study of carbon dioxide absorption into the aqueous solution of monoethanolamine and tetrabutylphosphonium methanesulfonate hybrid solvent using RSM-CCD methodology. Processes 2021, 9, 1186. [Google Scholar] [CrossRef]
- Huang, Q.; Jing, G.; Zhou, X.; Lv, B.; Zhou, Z. A novel biphasic solvent of amino-functionalized ionic liquid for CO2 capture: High efficiency and regenerability. J. CO2 Util. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. RSC Adv. 2012, 2, 12525–12551. [Google Scholar] [CrossRef]
- Luo, Q.; Pentzer, E. Encapsulation of ionic liquids for tailored applications. ACS Appl. Mater. Interfaces 2019, 12, 5169–5176. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Jiang, H.-L. Incorporation of imidazolium-based poly (ionic liquid) s into a metal–organic framework for CO2 capture and conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Vaid, T.P.; Kelley, S.P.; Rogers, R.D. Structure-directing effects of ionic liquids in the ionothermal synthesis of metal–organic frameworks. IUCrJ 2017, 4, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.-T. CO2 capture using amine solution mixed with ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Othman, Z.S.; Koketsu, M.; Abd Karim, N.H.; Irwan Zubairi, S.; Hassan, N. Interaction study of binary solvent systems ionic liquid and deep eutectic solvent with rotenone. Sains Malays. 2018, 47, 1473–1482. [Google Scholar] [CrossRef]
- Park, Y.B.; Kasinathan, P. SiO2 Immobilized Imidazolium Ionic Liquid as Acid Catalysts for Diacetins and Triacetins Synthesis in Glycerol Esterification. J. Thermodyn. Catal. 2022, 13, 1–8. [Google Scholar]
- Akopyan, A.V.; Shlenova, A.O.; Cherednichenko, K.A.; Polikarpova, P.D. Immobilized multifunctional ionic liquids for highly efficient oxidation of sulfur-containing compounds in model fuels. Energy Fuels 2021, 35, 6755–6764. [Google Scholar] [CrossRef]
- Sietsma, J.R.; Van Dillen, A.J.; De Jongh, P.E.; De Jong, K.P. Application of ordered mesoporous materials as model supports to study catalyst preparation by impregnation and drying. In Studies in Surface Science and Catalysis; Gaigneaux, E.M., Devillers, M., DeVos, D.E., Hermans, S., Jacobs, P.A., Martens, J.A., Ruiz, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 162, pp. 95–102. [Google Scholar]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Investigation of CO2 capture using acetate-based ionic liquids incorporated into exceptionally porous metal–organic frameworks. Adsorption 2019, 25, 675–692. [Google Scholar] [CrossRef]
- Cota, I.; Martinez, F.F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption. Coord. Chem. Rev. 2017, 351, 189–204. [Google Scholar] [CrossRef]
- Jaffar, Z.; Yunus, N.M.; Shaharun, M.S.; Allim, M.F.; Rahim, A.H.A. Incorporated Metal–Organic Framework Hybrid Materials for Gas Separation, Catalysis and Wastewater Treatment. Processes 2022, 10, 2368. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. Room Temperature CO2 Adsorption Studies Using Pure and Ionic Liquid Immobilized Zeolites. J. Chem. Eng. Data 2022, 67, 3503–3515. [Google Scholar] [CrossRef]
- Santiago, R.; Lemus, J.; Hospital-Benito, D.; Moya, C.; Bedia, J.; Alonso-Morales, N.; Rodriguez, J.J.; Palomar, J. CO2 capture by supported ionic liquid phase: Highlighting the role of the particle size. ACS Sustain. Chem. Eng. 2019, 7, 13089–13097. [Google Scholar] [CrossRef]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. CO2 Adsorption Performance on Surface-Functionalized Activated Carbon Impregnated with Pyrrolidinium-Based Ionic Liquid. Processes 2022, 10, 2372. [Google Scholar] [CrossRef]
- Perumal, M.; Jayaraman, D. Amine-Ionic Liquid Blends in CO2 Capture Process for Sustainable Energy and Environment. Energy Environ. 2023, 34, 517–532. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Li, C.; Zhao, T.; Yang, A.; Liu, F. Highly efficient absorption of CO2 by protic ionic liquids-amine blends at high temperatures. ACS Omega 2021, 6, 34027–34034. [Google Scholar] [CrossRef]
- Zalewski, M.; Krawczyk, T.; Siewniak, A.; Sobolewski, A. Carbon dioxide capture using water-imidazolium ionic liquids-amines ternary systems. Int. J. Greenh. Gas Control 2021, 105, 103210. [Google Scholar] [CrossRef]
- Giraldo, L.; Vargas, D.P.; Moreno-Piraján, J.C. Study of CO2 adsorption on chemically modified activated carbon with nitric acid and ammonium aqueous. Front. Chem. 2020, 8, 543452. [Google Scholar] [CrossRef] [PubMed]
- Pellerano, M.; Pré, P.; Kacem, M.; Delebarre, A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia 2009, 1, 647–653. [Google Scholar] [CrossRef]
- Sun, N.; Tang, Z.; Wei, W.; Snape, C.E.; Sun, Y. Solid adsorbents for low-temperature CO2 capture with low-energy penalties leading to more effective integrated solutions for power generation and industrial processes. Front. Energy Res. 2015, 3, 9. [Google Scholar] [CrossRef]
- He, X.; Zhu, J.; Wang, H.; Zhou, M.; Zhang, S. Surface functionalization of activated carbon with phosphonium ionic liquid for CO2 adsorption. Coatings 2019, 9, 590. [Google Scholar] [CrossRef]
- Erto, A.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F.; Balsamo, M.; Lancia, A.; Montagnaro, F. Carbon-supported ionic liquids as innovative adsorbents for CO2 separation from synthetic flue-gas. J. Colloid. Interface Sci. 2015, 448, 41–50. [Google Scholar] [CrossRef]
- Shahrom, M.S.R.; Nordin, A.R.; Wilfred, C.D. The improvement of activated carbon as CO2 adsorbent with supported amine functionalized ionic liquids. J. Environ. Chem. Eng. 2019, 7, 103319. [Google Scholar] [CrossRef]
- Ravutsov, M.; Mitrev, Y.; Shestakova, P.; Lazarova, H.; Simeonov, S.; Popova, M. CO2 Adsorption on Modified Mesoporous Silicas: The Role of the Adsorption Sites. Nanomaterials 2021, 11, 2831. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Aparicio, S.; Canlier, A.; Yavuz, C.T. Insights of CO2 adsorption performance of amine impregnated mesoporous silica (SBA-15) at wide range pressure and temperature conditions. Int. J. Greenh. Gas Control 2015, 43, 22–32. [Google Scholar] [CrossRef]
- Polesso, B.B.; Duczinski, R.; Bernard, F.L.; Ferrari, H.Z.; Luz, M.d.; Vecchia, F.D.; Menezes, S.M.C.d.; Einloft, S. Imidazolium-based ionic liquids impregnated in silica and alumina supports for CO2 capture. Mater. Res. 2019, 22, e20180810. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, E.; Li, Y.; Bernards, M.T.; He, Y.; Shi, Y. CO2 capture with polyamine-based protic ionic liquid functionalized mesoporous silica. J. CO2 Util. 2019, 34, 606–615. [Google Scholar] [CrossRef]
- Duczinski, R.; Polesso, B.B.; Bernard, F.L.; Ferrari, H.Z.; Almeida, P.L.; Corvo, M.C.; Cabrita, E.J.; Menezes, S.; Einloft, S. Enhancement of CO2/N2 selectivity and CO2 uptake by tuning concentration and chemical structure of imidazolium-based ILs immobilized in mesoporous silica. J. Environ. Chem. Eng. 2020, 8, 103740. [Google Scholar] [CrossRef]
- Yaghi, O.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.-S.; Di Serio, M. Applications of metal organic frameworks in wastewater treatment: A review on adsorption and photodegradation. Front. Chem. Eng. 2020, 2, 581487. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–organic frameworks for drug delivery: A design perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Alhumaimess, M.S. Metal–organic frameworks and their catalytic applications. J. Saudi Chem. Soc. 2020, 24, 461–473. [Google Scholar] [CrossRef]
- Abid, H.R.; Rada, Z.H.; Shang, J.; Wang, S. Synthesis, characterization, and CO2 adsorption of three metal-organic frameworks (MOFs): MIL-53, MIL-96, and amino-MIL-53. Polyhedron 2016, 120, 103–111. [Google Scholar] [CrossRef]
- Usman, M.; Helal, A.; Abdelnaby, M.M.; Alloush, A.M.; Zeama, M.; Yamani, Z.H. Trends and Prospects in UiO-66 Metal-Organic Framework for CO2 Capture, Separation, and Conversion. Chem. Rec. 2021, 21, 1771–1791. [Google Scholar] [CrossRef]
- Choe, J.H.; Kim, H.; Hong, C.S. MOF-74 type variants for CO2 capture. Mater. Chem. Front. 2021, 5, 5172–5185. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.-D.; Yazaydin, A.O. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, X.; Ruan, L.-W.; Liu, B.; Wang, Y.-M.; Zhu, J.-F.; Qiu, L.-G. Post-combustion CO2 capture with the HKUST-1 and MIL-101 (Cr) metal–organic frameworks: Adsorption, separation and regeneration investigations. Micropor. Mesopor. Mater. 2013, 179, 191–197. [Google Scholar] [CrossRef]
- Polat, H.M.; Zeeshan, M.; Uzun, A.; Keskin, S. Unlocking CO2 separation performance of ionic liquid/CuBTC composites: Combining experiments with molecular simulations. Chem. Eng. J. 2019, 373, 1179–1189. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Ribeiro, R.P.; Mota, J.P.; Rebelo, L.P.; Esperança, J.M.; Esteves, I.A. Ionic Liquid-Impregnated Metal–Organic Frameworks for CO2/CH4 Separation. ACS Appl. Nano Mater. 2019, 2, 7933–7950. [Google Scholar] [CrossRef]
- Bernard, F.L.; Rodrigues, D.; Polesso, B.B.; Chaban, V.V.; Serefin, M.; Dalla Vecchia, F.; Einloft, S. Development of inexpensive cellulose-based sorbents for carbon dioxide. Braz. J. Chem. Eng. 2019, 36, 511–521. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, H.; Pudukudy, M.; Zhi, Y.; Zhao, W.; Shan, S.; Jia, Q.; Ni, Y. CO2 capture performance and characterization of cellulose aerogels synthesized from old corrugated containers. Carbohydr. Polym. 2020, 227, 115380. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Zaman, A.; Huang, F.; Jiang, M.; Wei, W.; Zhou, Z. Preparation, properties, and applications of natural cellulosic aerogels: A review. Energy Built Environ. 2020, 1, 60–76. [Google Scholar] [CrossRef]
- Rodríguez-Fabià, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobization of lignocellulosic materials part II: Chemical modification. Cellulose 2022, 29, 8957–8995. [Google Scholar] [CrossRef]
- Bernard, F.L.; Duczinski, R.B.; Rojas, M.F.; Fialho, M.C.C.; Carreno, L.A.; Chaban, V.V.; Dalla Vecchia, F.; Einloft, S. Cellulose based poly (ionic liquids): Tuning cation-anion interaction to improve carbon dioxide sorption. Fuel 2018, 211, 76–86. [Google Scholar] [CrossRef]
- Reed, D.G.; Dowson, G.R.; Styring, P. Cellulose-supported ionic liquids for low-cost pressure swing CO2 capture. Front. Energy Res. 2017, 5, 13. [Google Scholar] [CrossRef]
- Barrulas, R.V.; López-Iglesias, C.; Zanatta, M.; Casimiro, T.; Mármol, G.; Carrott, M.R.; García-González, C.A.; Corvo, M.C. The AEROPILs Generation: Novel Poly (Ionic Liquid)-Based Aerogels for CO2 Capture. Int. J. Mol. Sci. 2022, 23, 200. [Google Scholar] [CrossRef] [PubMed]
| Conditions | CO2 Absorption Capacity | |||||||
|---|---|---|---|---|---|---|---|---|
| Class of ILs | ILs | T (°C) | P (bar) | t (min) | Mole Fraction | molCO2/mol IL | wt% | Ref. |
| Conventional RTILs | [BMIM][PF6] | ___e | 80 | ___e | 0.600 | ___e | ___e | [18] |
| [BMIM][PF6] | 25 | 13 | ___e | ~0.210 | ___e | ___e | [23] | |
| [BMIM][PF6] | 40 | 93 | ___e | 0.720 | ___e | ___e | [22] | |
| [BMMIM][PF6] | 25 | 13 | ___e | ~0.190 | ___e | ___e | [23] | |
| [EMIM][Tf2N] | 25 | 13 | ___e | ~0.280 | ___e | ___e | [23] | |
| [EMMIM][Tf2N] | 25 | 13 | ___e | ~0.260 | ___e | ___e | [23] | |
| [HMIM][Tf2N] | 40 | 83.7 | ___e | 0.720 | ___e | ___e | [27] | |
| [OMIM][Tf2N] | 40 | 83.7 | ___e | 0.763 | ___e | ___e | [27] | |
| TSILs | [AEmim][Tf2N] | 30 | 1.6 | ___e | ___e | 0.490 | ___e | [28] |
| [AEmim][BF4] | 30 | 1.6 | ___e | ___e | 0.425 | ___e | [28] | |
| [AEmim][DCA] | 30 | 1.6 | ___e | ___e | 0.435 | ___e | [28] | |
| [P2228][6BrInda] | 58.9 | 0.83 | ___e | ___e | ~1.00 | ___e | [31] | |
| [DMAPAH][4F-PhO] | 30 | 1.5 | ___e | ___e | 0.86 | ___e | [32] | |
| [E1Py][C(CN)3] | 30 | 20 | ___e | 0.473 | ___e | ___e | [29] | |
| [E1Py][N(CN)2] | 30 | 20 | ___e | 0.382 | ___e | ___e | [29] | |
| [E1Py][SCN] | 30 | 20 | ___e | 0.184 | ___e | ___e | [29] | |
| aq-[APmim][Br] | 30 | ___e | ___e | ___e | 0.65 | ___e | [43] | |
| Polymer-ILs | [MDAP][TF2N]-PDC | 0 | 1.0 | ___e | ___e | ___e | 1.390 | [34] |
| [PBIMT][BF4] | 22 | 0.80 | 600 | ___e | ___e | 0.241 | [33] | |
| [PVBIM][PF6] | 22 | 0.80 | 600 | ___e | ___e | 0.322 | [33] | |
| [PVBIM][BF4] | 22 | 0.80 | 600 | ___e | ___e | 0.305 | [33] | |
| HPIL-Cl (1) | 25 | 1.0 | ___e | ___e | ___e | 5.30 | [35] | |
| AAILs | [P66614][Pro] | 25 | 1.01 | ___e | ___e | 0.91 | ___e | [40] |
| [P66614][Met] | 25 | 1.01 | ___e | ___e | 0.99 | ___e | [40] | |
| [P66614][Gly] | 25 | 1.01 | ___e | ___e | 0.97 | ___e | [40] | |
| [B4Pyr][Arg] | 25 | 6.0 | ___e | 0.613 | ___e | ___e | [38] | |
| [B4Pyr][Gly] | 25 | 6.0 | ___e | 0.475 | ___e | ___e | [38] | |
| [N66614][Lys] | 25 | 1.0 | 1440 | ___e | 2.1 | 13.1 | [50] | |
| [N66614][Lys] | 80 | 1.0 | 1440 | ___e | 1.0 | ___e | [50] | |
| Dual-functionalized ILs | aq-[APmim][Gly] | 30 | ___e | ___e | ___e | 1.23 | ___e | [43] |
| [N1,1,6,2O4][Lys] | 20 | 1.0 | 200 | ___e | 1.62 | 19.02 | [46] | |
| PILs | [MTBDH][HFPD2] | 23 | 1.0 | 60 | ___e | 2.04 | ___e | [48] |
| [TMGH][Pyrr] | 40 | 1.0 | ~80 | ___e | 0.66 | ___e | [49] | |
| [TMGH][Im] | 40 | 1.0 | ~80 | ___e | 0.64 | ___e | [49] | |
| Methods | Advantages | Disadvantages | Examples of IL-Hybrid Materials | Ref. |
|---|---|---|---|---|
| Blending |
|
| EDA/[DEAPOAc], ChoCl: 1, 4-butanediol (1: 5)/[BMIMOTF] | [73,74] |
| Immobilization |
|
| SiO2/[ASBI][TfO], SiO2/[ASBI][HSO4] and SBA-15/multifunctional Ils, | [75,76] |
| Wet impregnation |
|
| MIL-101/[EMIM][Oac], MOF-177/[EMIM][Oac] | [77,78] |
| Polymerization |
|
| polyILs@MIL-101 | [71] |
| Ionothermal synthesis |
|
| Cu3(tpt)4(BF4)3.(tpt)2/3.5H2O [tpt = 2,4,6-tris(4-pyridyl)-1,3,5-triazine], MOF-5 (IL), [Co2Na(bptc)2][EMIm]3 | [79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ab Rahim, A.H.; Yunus, N.M.; Bustam, M.A. Ionic Liquids Hybridization for Carbon Dioxide Capture: A Review. Molecules 2023, 28, 7091. https://doi.org/10.3390/molecules28207091
Ab Rahim AH, Yunus NM, Bustam MA. Ionic Liquids Hybridization for Carbon Dioxide Capture: A Review. Molecules. 2023; 28(20):7091. https://doi.org/10.3390/molecules28207091
Chicago/Turabian StyleAb Rahim, Asyraf Hanim, Normawati M. Yunus, and Mohamad Azmi Bustam. 2023. "Ionic Liquids Hybridization for Carbon Dioxide Capture: A Review" Molecules 28, no. 20: 7091. https://doi.org/10.3390/molecules28207091
APA StyleAb Rahim, A. H., Yunus, N. M., & Bustam, M. A. (2023). Ionic Liquids Hybridization for Carbon Dioxide Capture: A Review. Molecules, 28(20), 7091. https://doi.org/10.3390/molecules28207091








