Chemical Profiling and Biological Activity of Psydrax dicoccos Gaertn
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis
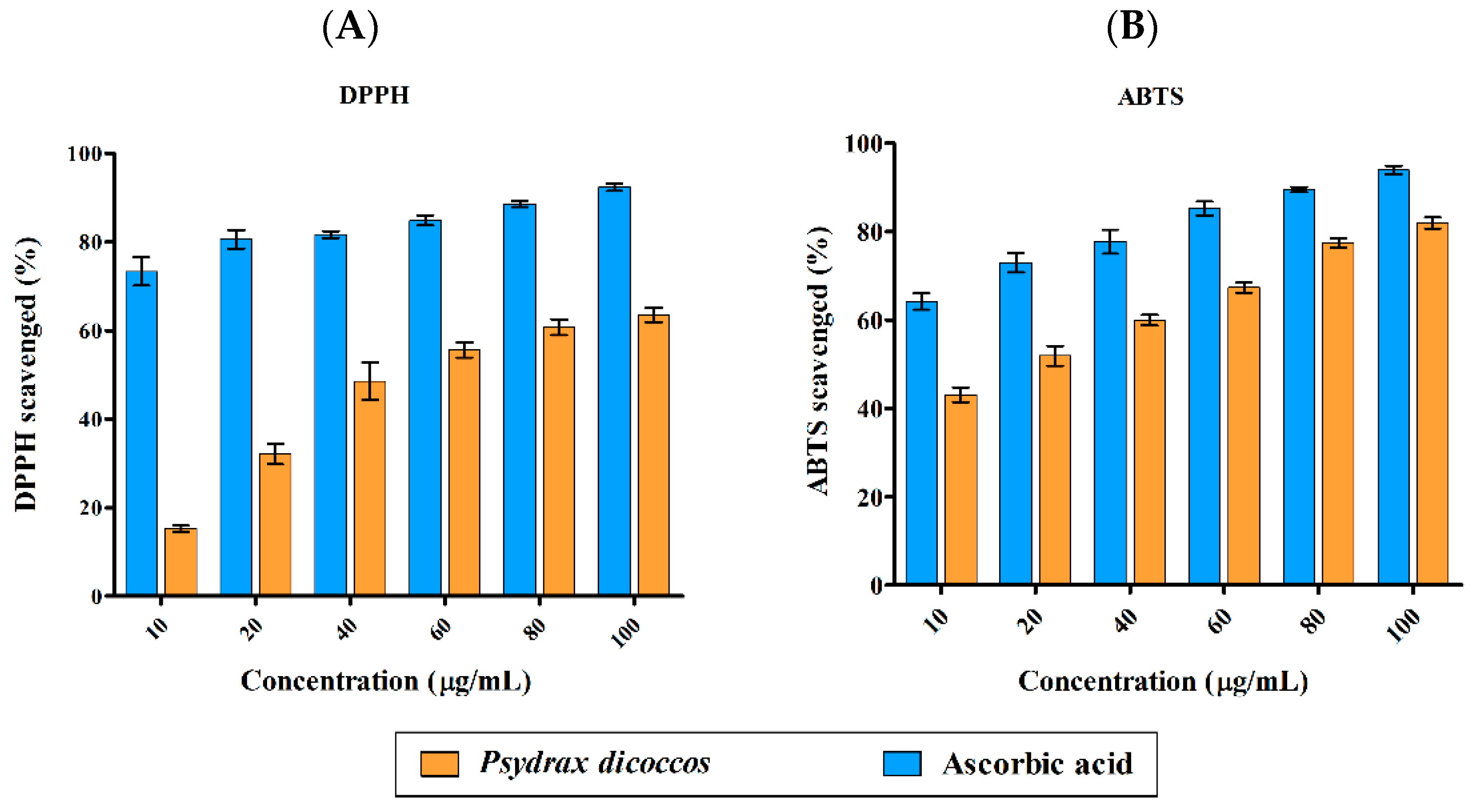
2.2. Antioxidant Activity of P. dicoccos Leaf Extract
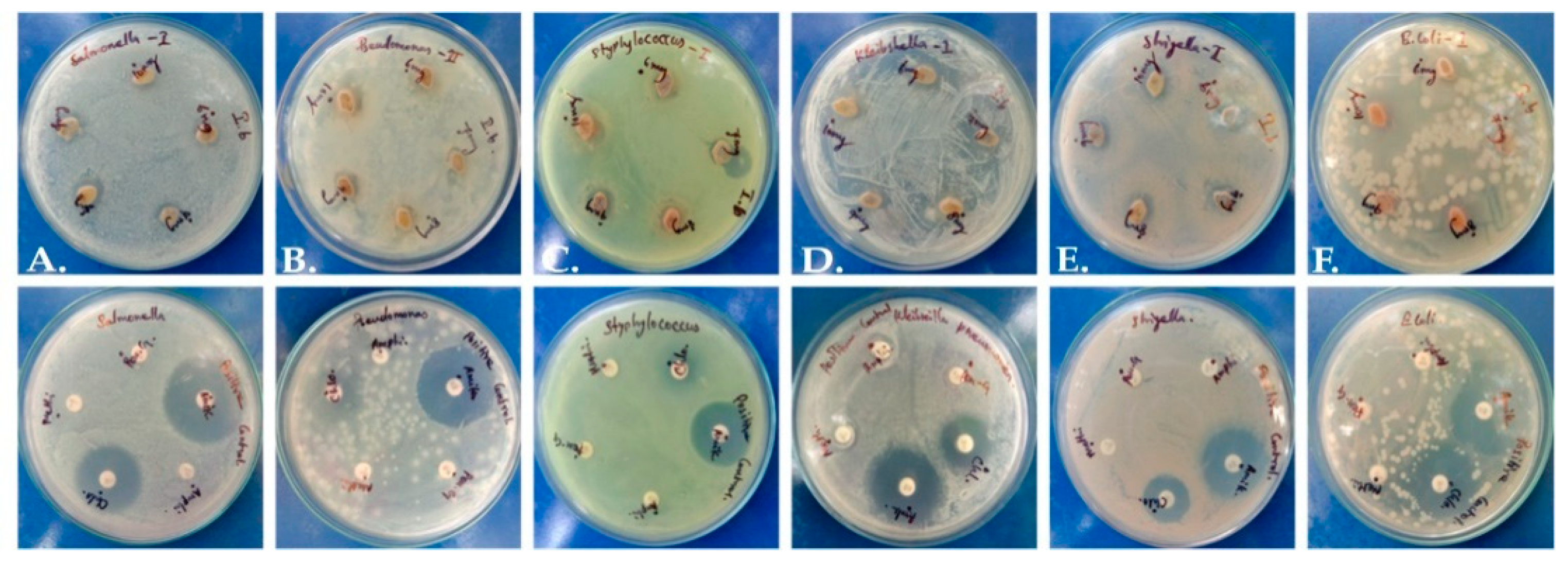
2.3. Anti-Bacterial Activity of P. dicoccos Leaf Extract
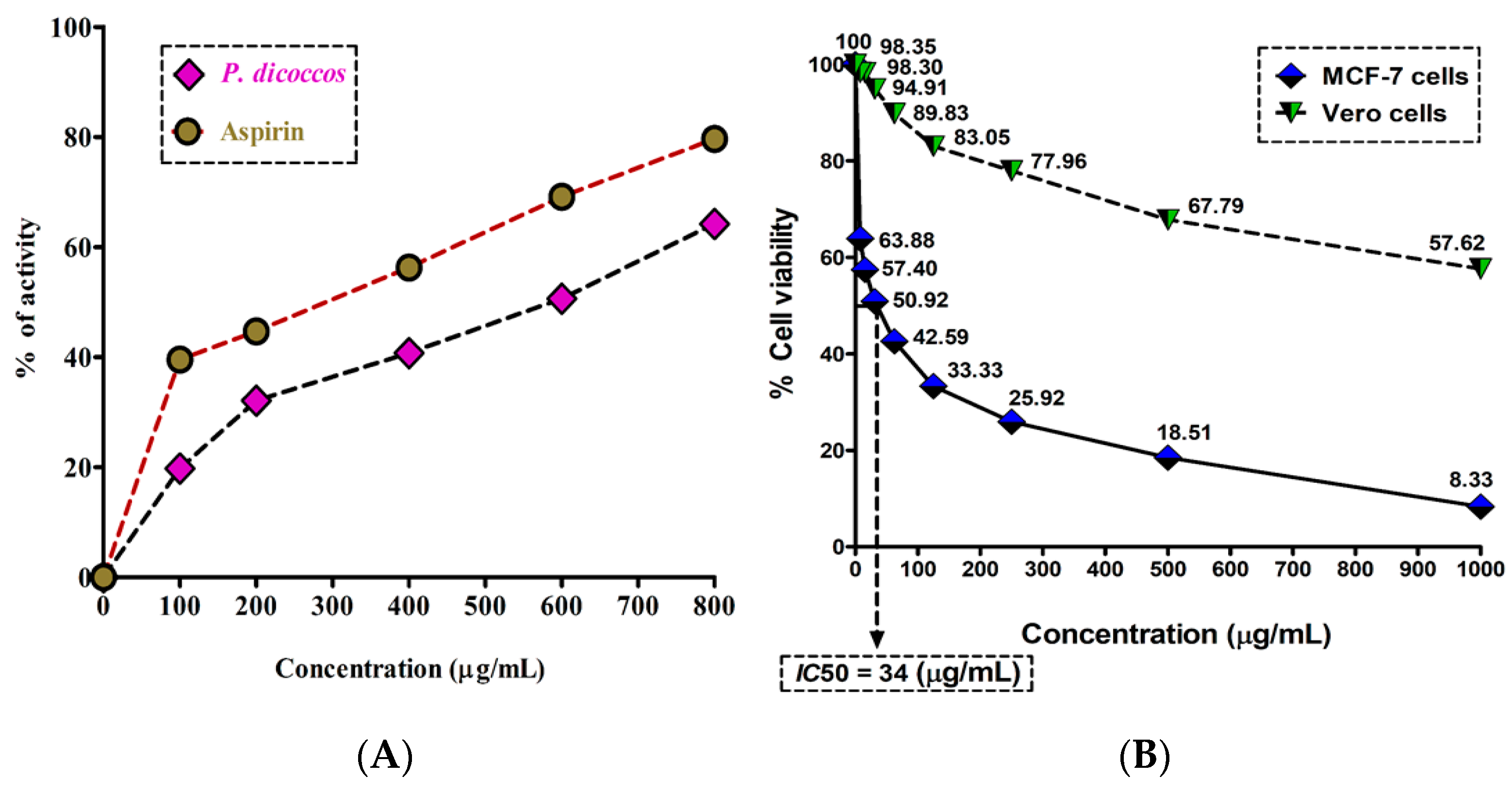
2.4. Anti-Inflammatory Activity of P. dicoccos Leaf Extract
2.5. Cytotoxicity of P. dicoccos Leaf Extract
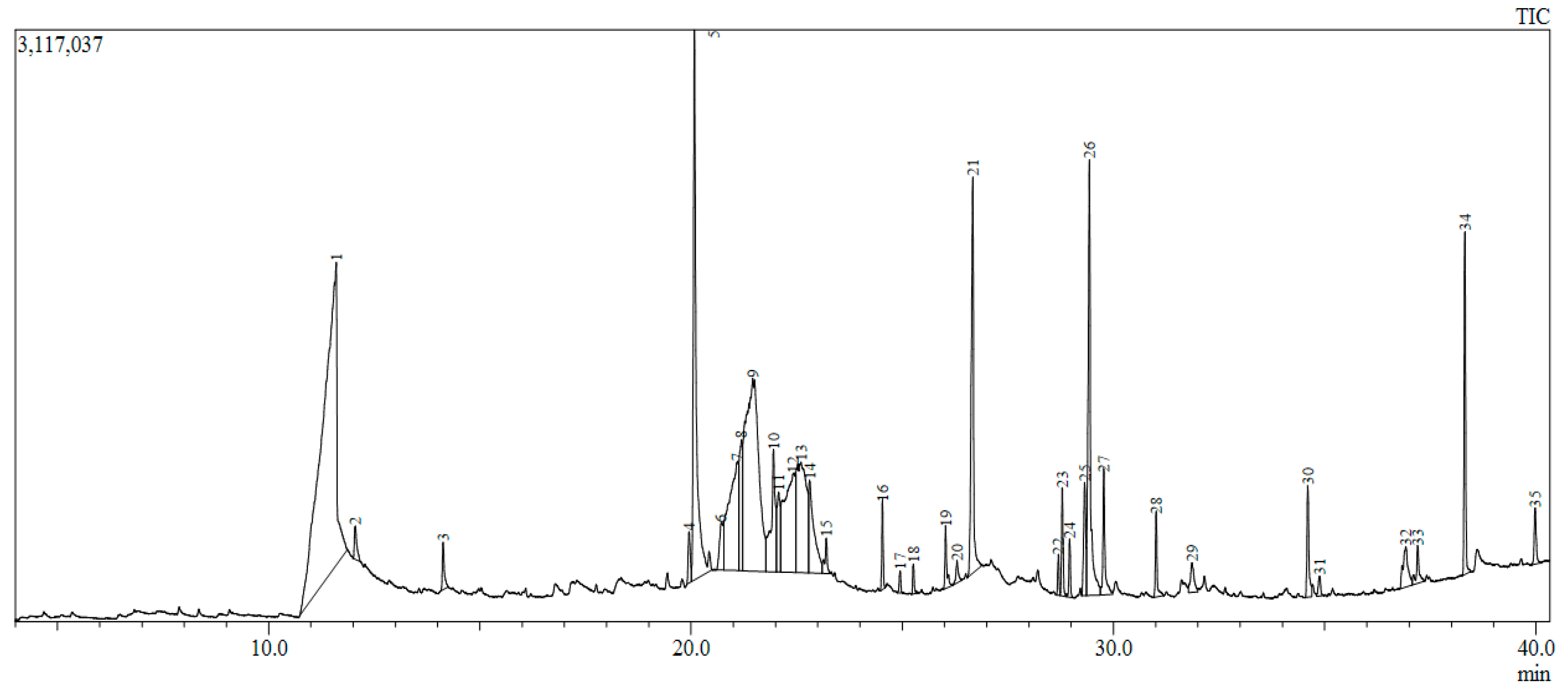
2.6. GC-MS and LC-MS Analyses of P. dicoccos Leaf Extract
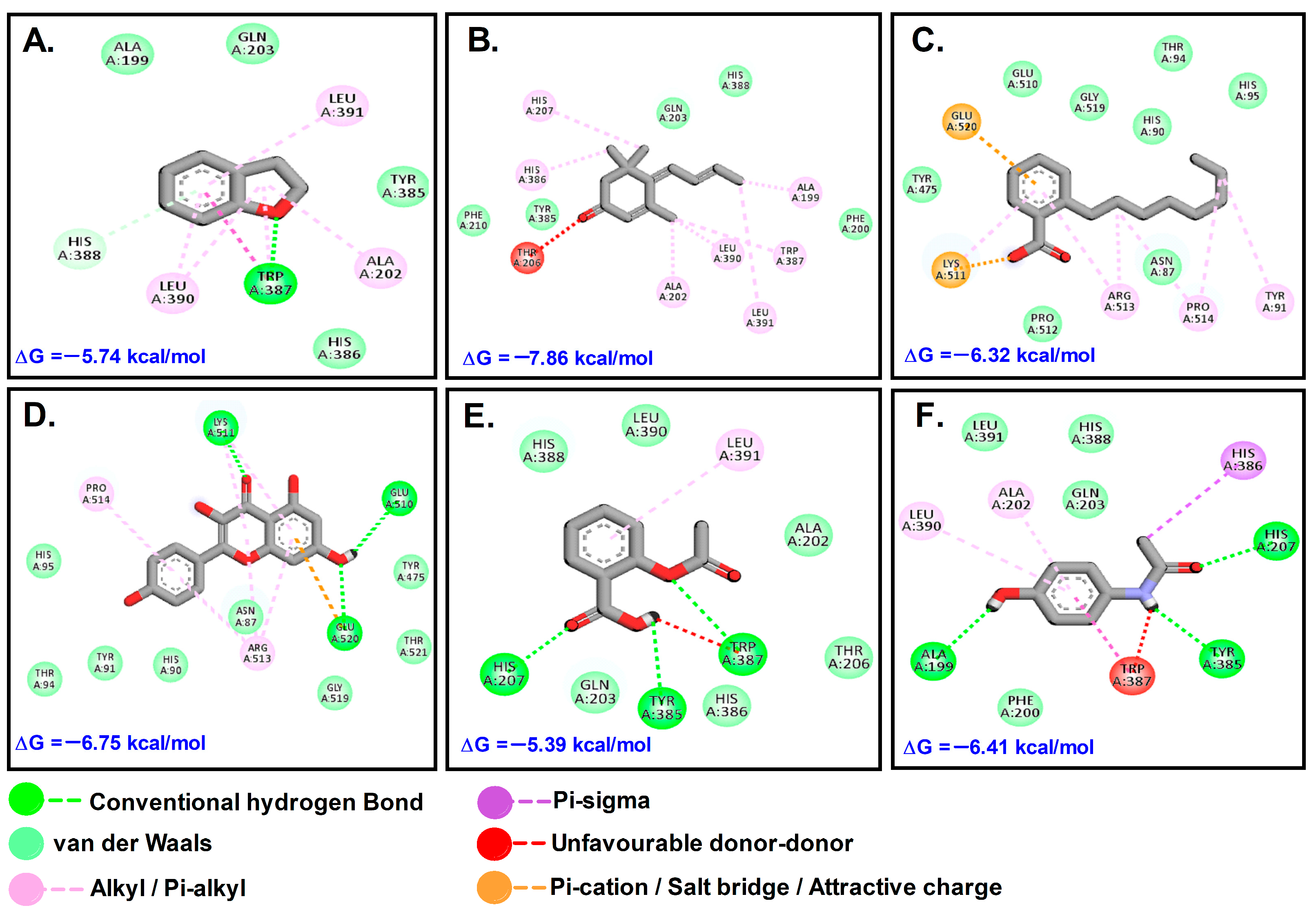
2.7. Docking Results for Selected Compounds of P. dicoccos with Selected Breast Cancer Receptors
2.7.1. Akt/Protein Kinase B
2.7.2. COX-2
2.7.3. HER2
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Collection
4.3. Phytoextraction
4.4. Phytochemical Analysis
4.5. Anti-Bacterial Activity
4.6. Antioxidant Activity
4.6.1. DPPH• o Free Radical-Scavenging Assay
4.6.2. ABTS•+ Assay
4.7. Anti-Inflammatory Activity of P. dicoccos Leaf Extract
4.8. Cytotoxicity of P. dicoccos Leaf Extract
4.9. GC-MS and LC-MS Profiling of the Methanol Leaf Extracts of P. dicoccos
4.10. Molecular Docking Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary Phytochemicals in Breast Cancer Research: Anticancer Effects and Potential Utility for Effective Chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef]
- Haidinger, R.; Bauerfeind, I. Long-Term Side Effects of Adjuvant Therapy in Primary Breast Cancer Patients: Results of a Web-Based Survey. Breast Care 2019, 14, 111–116. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals Targeting NF-κB Signaling: Potential Anti-Cancer Interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef]
- El Menyiy, N.; Aboulaghras, S.; Bakrim, S.; Moubachir, R.; Taha, D.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Hermansyah, A.; Ming, L.C.; et al. Genkwanin: An emerging natural compound with multifaceted pharmacological effects. Biomed Pharmacother. 2023, 165, 115159. [Google Scholar] [CrossRef]
- Mates, L.; Rusu, M.E.; Popa, D.S. Phytochemicals and Biological Activities of Walnut Septum: A Systematic Review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Chali, B.U.; Melaku, T.; Berhanu, N.; Mengistu, B.; Milkessa, G.; Mamo, G.; Alemu, S.; Mulugeta, T. Traditional Medicine Practice in the Context of COVID-19 Pandemic: Community Claim in Jimma Zone, Oromia, Ethiopia. Infect. Drug Resist. 2021, 14, 3773. [Google Scholar] [CrossRef]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of Novel Microbial Secondary Metabolites on the Pharma Industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef]
- Benali, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahlia, S.; Hammani, K.; Ghaleb, A.; et al. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Amalraj, S.; Murugan, R.; Gangapriya, P.; Krupa, J.; Divya, M.; Gurav, S.S.; Ayyanar, M. Evaluation of Phytochemicals, Enzyme Inhibitory, Antibacterial and Antioxidant Effects of Psydrax Dicoccos Gaertn. Nat. Prod. Res. 2021, 36, 5772–5777. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, M.A.T.M.; Parkes, E.E. When Breaks Get Hot: Inflammatory Signaling in BRCA1/2-Mutant Cancers. Trends Cancer 2022, 8, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Gideon, D.A.; Annadurai, P.; Parashar, A.; Murthy, R.G.; Dhandayuthapani, K.; Srinivasan, S.; Sivaramakrishnan, R.; Jasmine, R. Herbal Medicinal Compounds and Their Anti–Breast Cancer Actions: A Mechanistic Perspective. In Phytopharmaceuticals and Biotechnology of Herbal Plants; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-329402-3. [Google Scholar]
- Ma, Y.; Iyer, R.P.; de Castro Brás, L.E.; Toba, H.; Yabluchanskiy, A.; Deleon-Pennell, K.Y.; Hall, M.E.; Lange, R.A.; Lindsey, M.L. Chapter 4—Cross Talk Between Inflammation and Extracellular Matrix Following Myocardial Infarction. In Inflammation in Heart Failure; Blankesteijn, W.M., Altara, R., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 67–79. ISBN 978-0-12-800039-7. [Google Scholar]
- Kumar, R.; Rao, S. Streptozotocin-Induced Oxidative Stress in Diabetic Rats—A Defensive Effect of Psydrax Dicoccos. Asian J. Pharm. Clin. Res. 2018, 11, 378–380. [Google Scholar] [CrossRef]
- Umaiyambigai, D.; Saravanakumar, K.; Adaikala Raj, G. Phytochemical Profile and Antifungal Activity of Leaves Methanol Extract from the Psydrax Dicoccos (Gaertn) Teys. & Binn. Rubiaceae Family. Int. J. Pharmacol. Phytochem. Ethnomed. 2017, 7, 53–61. [Google Scholar] [CrossRef]
- Farjam, M.H. Comparative Study of the Antimicrobial Activity of Essential Oil and Two Different Extract from Salvia Urmiensis Bunge. Asian Pac. J. Trop. Biomed. 2012, 2, S1680–S1682. [Google Scholar] [CrossRef]
- Qawoogha, S.S.; Shahiwala, A. Identification of Potential Anticancer Phytochemicals against Colorectal Cancer by Structure-Based Docking Studies. J. Recept. Signal Transduct. Res. 2020, 40, 67–76. [Google Scholar] [CrossRef]
- Taher, R.F.; Al-Karmalawy, A.A.; Maksoud, A.I.A.E.; Khalil, H.; Hassan, A.; El-Khrisy, E.-D.A.; El-Kashak, W. Two New Flavonoids and Anticancer Activity of Hymenosporum Flavum: In Vitro and Molecular Docking Studies. J. Herbmed Pharmacol. 2021, 10, 443–458. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Moukette Moukette, B.; Constant Anatole, P.; Nya Biapa, C.P.; Njimou, J.R.; Ngogang, J.Y. Free Radicals Quenching Potential, Protective Properties against Oxidative Mediated Ion Toxicity and HPLC Phenolic Profile of a Cameroonian Spice: Piper Guineensis. Toxicol. Rep. 2015, 2, 792–805. [Google Scholar] [CrossRef]
- Hammond, M.L.; Kopka, I.E.; Zambias, R.A.; Caldwell, C.G.; Boger, J.; Baker, F.; Bach, T.; Luell, S.; MacIntyre, D.E. 2,3-Dihydro-5-Benzofuranols as Antioxidant-Based Inhibitors of Leukotriene Biosynthesis. J. Med. Chem. 1989, 32, 1006–1020. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The Anti-Inflammatory Flavones Quercetin and Kaempferol Cause Inhibition of Inducible Nitric Oxide Synthase, Cyclooxygenase-2 and Reactive C-Protein, and down-Regulation of the Nuclear Factor kappaB Pathway in Chang Liver Cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, Z.; Liu, J.; Hou, Y. The Akt–mTOR Network at the Interface of Hematopoietic Stem Cell Homeostasis. Exp. Hematol. 2021, 103, 15–23. [Google Scholar] [CrossRef]
- Dan, H.C.; Ebbs, A.; Pasparakis, M.; Van Dyke, T.; Basseres, D.S.; Baldwin, A.S. Akt-Dependent Activation of mTORC1 Complex Involves Phosphorylation of mTOR (Mammalian Target of Rapamycin) by IκB Kinase α (IKKα). J. Biol. Chem. 2014, 289, 25227–25240. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.R.; Pelly, V.S.; Moeini, A.; Chiang, S.-C.; Flanagan, E.; Bromley, C.P.; Clark, C.; Earnshaw, C.H.; Koufaki, M.A.; Bonavita, E.; et al. Chemotherapy-Induced COX-2 Upregulation by Cancer Cells Defines Their Inflammatory Properties and Limits the Efficacy of Chemoimmunotherapy Combinations. Nat. Commun. 2022, 13, 2063. [Google Scholar] [CrossRef]
- Ristimäki, A.; Sivula, A.; Lundin, J.; Lundin, M.; Salminen, T.; Haglund, C.; Joensuu, H.; Isola, J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002, 62, 632–635. [Google Scholar]
- Prathyusha, T.; Khaliq, S.A.; Nikhat, S.; Prashanth, S.; Hemalatha, R. Evaluation of Anti-Ulcer Activity of Methanolic Extract of Psydrax Dicoccos in Experimental Rats. Int. J. Res. Pharmacol. Pharmacother. 2022, 11, 103–109. [Google Scholar]
- Vedeanu, N.; Voica, C.; Magdas, D.A.; Kiss, B.; Stefan, M.G.; Simedrea, R.; Georgiu, C.; Berce, C.; Vostinaru, O.; Boros, R.; et al. Subacute Co-Exposure to Low Doses of Ruthenium(III) Changes the Distribution, Excretion and Biological Effects of Silver Ions in Rats. Environ. Chem. 2020, 17, 163–172. [Google Scholar] [CrossRef]
- Autor, E.; Cornejo, A.; Bimbela, F.; Maisterra, M.; Gandía, L.M.; Martínez-Merino, V. Extraction of Phenolic Compounds from Populus Salicaceae Bark. Biomolecules 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; García-Plazaola, J.I.; Hernández, A.; Fernández-Marín, B. On the Recalcitrant Use of Arnon’s Method for Chlorophyll Determination. New Phytol. 2018, 217, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, K. Microplastics Contamination in Soil Affects Growth and Root Nodulation of Fenugreek (Trigonella Foenum-graecum L.) and 16 s rRNA Sequencing of Rhizosphere Soil. J. Hazard. Mater. Adv. 2022, 7, 100146. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Analysis of the Chemical Composition of Natural Carbohydrates—An Overview of Methods. Food Chem. 2022, 394, 133466. [Google Scholar] [CrossRef]
- Morales-Palomo, S.; Liras, M.; González-Fernández, C.; Tomás-Pejó, E. Key Role of Fluorescence Quantum Yield in Nile Red Staining Method for Determining Intracellular Lipids in Yeast Strains. Biotechnol. Biofuels Bioprod. 2022, 15, 37. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid Determination of Total Lipids in Mosquitoes. J. Am. Mosq. Control Assoc. 1985, 1, 302–304. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative Estimation of Protein in Sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef]
- Troll, W.; Cannan, R.K. A Modified Photometric Ninhydrin Method for the Analysis of Amino and Imino Acids. J. Biol. Chem. 1953, 200, 803–811. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Molole, G.J.; Gure, A.; Abdissa, N. Determination of Total Phenolic Content and Antioxidant Activity of Commiphora mollis (Oliv.) Engl. Resin. BMC Chem. 2022, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Erhonyota, C.; Edo, G.I.; Onoharigho, F.O. Comparison of Poison Plate and Agar Well Diffusion Method Determining the Antifungal Activity of Protein Fractions. Acta Ecol. Sin. 2022, 43, 684–689. [Google Scholar] [CrossRef]
- Meshaal, A.K.; Hetta, H.F.; Yahia, R.; Abualnaja, K.M.; Mansour, A.T.; Al-Kadmy, I.M.S.; Alghamdi, S.; Dablool, A.S.; Emran, T.B.; Sedky, H.; et al. In Vitro Antimicrobial Activity of Medicinal Plant Extracts against Some Bacterial Pathogens Isolated from Raw and Processed Meat. Life 2021, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Annadurai, P.; Annadurai, V.; Yongkun, M.; Pugazhendhi, A.; Dhandayuthapani, K. Phytochemical Composition, Antioxidant and Antimicrobial Activities of Plecospermum spinosum Trecul. Process. Biochem. 2021, 100, 107–116. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Annadurai, P.; Gideon, D.A.; Nirusimhan, V.; Sivaramakrishnan, R.; Dhandayuthapani, K.; Pugazhendhi, A. Deciphering the Pharmacological Potentials of Aganosma cymosa (Roxb.) G. Don Using in Vitro and Computational Methods. Process. Biochem. 2022, 114, 119–133. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kanthi Gudimella, K.; Gedda, G.; Kumar, P.S.; Babu, B.K.; Yamajala, B.; Rao, B.V.; Singh, P.P.; Kumar, D.; Sharma, A. Novel Synthesis of Fluorescent Carbon Dots from Bio-Based Carica Papaya Leaves: Optical and Structural Properties with Antioxidant and Anti-Inflammatory Activities. Environ. Res. 2022, 204, 111854. [Google Scholar] [CrossRef] [PubMed]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Emran, T.B.; Siddiqui, S.A. Membrane Stabilization as a Mechanism of the Anti-Inflammatory Activity of Ethanolic Root Extract of Choi (Piper chaba). Clin. Phytosci. 2020, 6, 59. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Hong, J. Application of the MTT-Based Colorimetric Method for Evaluating Bacterial Growth Using Different Solvent Systems. LWT 2022, 153, 112565. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Ralte, L.; Khiangte, L.; Thangjam, N.M.; Kumar, A.; Singh, Y.T. GC–MS and Molecular Docking Analyses of Phytochemicals from the Underutilized Plant, Parkia Timoriana Revealed Candidate Anti-Cancerous and Anti-Inflammatory Agents. Sci. Rep. 2022, 12, 3395. [Google Scholar] [CrossRef]


| Targets | Types of Analysis | Ligands | ΔG (kcal/mol) | Ligand Efficiency | Ki (μM) |
|---|---|---|---|---|---|
| 3CQW chain A (Akt1) | GC-MS | MST | −6.62 | −0.47 | 14.03 |
| OBZ | −6.29 | −0.37 | 24.53 | ||
| DHB | −5.65 | −0.63 | 72.8 | ||
| LC-MS | KMF | −7.2 | −0.34 | 5.29 | |
| Control drugs | AFT | −9.36 | −0.28 | 0.138 | |
| LTB | −10.02 | −0.25 | 0.045 | ||
| 5F1A Chain A (COX2) | GC-MS | MST | −7.86 | −0.56 | 1.75 |
| OBZ | −6.32 | −0.37 | 23.46 | ||
| DHB | −5.74 | −0.64 | 62.3 | ||
| LC-MS | KMF | −6.75 | −0.32 | 11.23 | |
| Control drugs | ASP | −5.39 | −0.41 | 112.58 | |
| PCM | −6.41 | −0.55 | 37.59 | ||
| 3PP0 Chain A (HER2) | GC-MS | OBZ | −7.49 | −0.44 | 3.24 |
| MST | −7.39 | −0.53 | 3.8 | ||
| DHB | −5.05 | −0.56 | 198.31 | ||
| LC-MS | KMF | −6.9 | −0.33 | 8.78 | |
| Control drugs | PTB | −9.5 | −0.23 | 0.109 | |
| NTB | −9.51 | −0.24 | 0.107 |
| Types of Interaction | 3CQW (Akt-1) | |||||
|---|---|---|---|---|---|---|
| OBZ | MST | DHB | KMF | LTB | AFT | |
| Conventional hydrogen bond/Carbon–hydrogen bond | - | Ala230 | Leu213 | Glu228, Ala230, Glu198, Leu156 | Phe161, Glu234, Asp439, Asp292 | Gly159, Asp292, Lys156, Glu284, Gly157, Asp439 |
| Van der waals | Glu228, His207, Leu210, Arg206, Leu213, Leu202, Thr211, Ser205 | Tyr229, Glu228, Thr211, Thr291, Asp292, Glu278, Glu234, Asn279, Phe438 | His207, Tyr474, Leu210, Thr211, Leu202, Gln203 | Leu202, Gly157, Thr211, Lys179, Tyr229, Tyr229 | Glu191, His194, Gly294, Asn279, Asn274, Gly162, Lys163, Val164, Lys158, Gly157, Gly159, Leu156, Thr160, Glu278, Lys276, Phe237 | Lys163, Thr291, Leu156, Phe237, Tyr437, Gly233, Phe442, Phe236, Glu278 |
| Salt bridge/Attractive charge/Pi-lone pair/Pi-anion/Pi-sulfur Halogen bond | Lys419, Lys289 | - | Ser205 | Asp292, Met227 | Phe438, Phe442 | Met281, Gly162 |
| Pi-stacked/Pi-Tshaped/Alkyl/Pi-alkyl | Ala212, Pro208 | Met227, Ala177, Val164, Leu156, Met281 | Ala212, Arg206 | Leu295 | Val164, Phe438, Lys179 | |
| Unfavorable Donor-Donor | - | - | - | Phe293 | - | - |
| Types of Interaction | 5F1A (COX-2) | |||||
|---|---|---|---|---|---|---|
| OBZ | MST | DHB | KMF | ASP | PCM | |
| Conventional hydrogen bond/Carbon–hydrogen bond/Pi-donor hydrogen bond | - | - | Trp387, His388 | Lys511, Glu510, Glu520 | His207, Tyr385, Trp387 | Ala199, Tyr385, His207 |
| Van der waals | Tyr475, Glu510, Gly519, His90, Thr94, His95, Pro512, Asn87 | Phe207, Tyr385, Gln203, His388, Phe200 | Ala199, Gln203, Tyr385, His386 | - | Gln203, His388, Leu390, Ala202, Thr206, Thr386 | Phe200, Leu391, His388, Glu203 |
| Salt bridge/Attractive charge/Pi-lone pair/Pi-anion/Halogen bond | Lys511, Glu520 | - | - | - | - | - |
| Pi-stacked/Pi-Tshaped/Alkyl/Pi-alkyl/Unfavorable acceptor-Acceptor | Arg518, Pro514, Tyr91 | His207, His286, Ala202, Leu390, Ala199, Trp387, Leu391, Thr391 | Leu391, Leu390, Ala202 | Arg513 | Leu391 | His386, Leu390, Ala202 |
| Types of Interaction | 3PP0 (HER-2) | |||||
|---|---|---|---|---|---|---|
| OBZ | MST | DHB | KMF | NTB | PTB | |
| Conventional hydrogen bond/Carbon–hydrogen bond/Pi-donor hydrogen bond | Tyr877 | Lys753 | - | Thr798, Thr862, Glu770 | Arg756, Asn850, Arg849 | Arg849, Asp843, Asp863 |
| Van der waals | Val884, Lys883, Glu766, Gly865, Ala763, Glu770 | Thr793, Val797, Ile752, Ala751, Thr862, Asp863, Phe864, Glu770 | Arg784, Ala771, Glu770, Thr798, Ser783, Thr862, Asp863 | Ap863, Leu852, Ser783, Arg784 | Thr798, Asp863, Gly729, Val884, Gly727 | Ala730, Phe731, Lys383, Val884, Leu866, Leu807, Gly727, Gly804, Leu852, Thr862, Thr798, Leu796, Ser728, Asn850 |
| Salt bridge/Attractive charge/Pi-lone pair/Pi-anion/Halogen bond | Lys762, Arg868, Arg844, Gly882 | - | - | - | Ser728, Pro885, Lys883, Asp845 | - |
| Pi-Sigma/Pi-stacked/Pi-Tshaped/Alkyl/Pi-alkyl/Unfavorable acceptor-Acceptor | Phe731, Ile767, Leu866, Ala879 | Leu796, Met774, Ala771, Leu785 | Phe864, Met774, Leu785 | Met774, Phe864, Leu785, Leu796, Ala751, Val734, Ly753 | Thr862, Ala730, Phe731, Lys753, Leu852, Val734, Lys805 | Pro885, Leu726, Val734, Ala751, Lys758, Gly729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeramuthu, K.; Ahuja, V.; Annadurai, P.; Gideon, D.A.; Sundarrajan, B.; Rusu, M.E.; Annadurai, V.; Dhandayuthapani, K. Chemical Profiling and Biological Activity of Psydrax dicoccos Gaertn. Molecules 2023, 28, 7101. https://doi.org/10.3390/molecules28207101
Veeramuthu K, Ahuja V, Annadurai P, Gideon DA, Sundarrajan B, Rusu ME, Annadurai V, Dhandayuthapani K. Chemical Profiling and Biological Activity of Psydrax dicoccos Gaertn. Molecules. 2023; 28(20):7101. https://doi.org/10.3390/molecules28207101
Chicago/Turabian StyleVeeramuthu, Kamaraj, Vishal Ahuja, Pushparaj Annadurai, Daniel A. Gideon, Balamurugan Sundarrajan, Marius Emil Rusu, Vinothkanna Annadurai, and Kandavel Dhandayuthapani. 2023. "Chemical Profiling and Biological Activity of Psydrax dicoccos Gaertn" Molecules 28, no. 20: 7101. https://doi.org/10.3390/molecules28207101
APA StyleVeeramuthu, K., Ahuja, V., Annadurai, P., Gideon, D. A., Sundarrajan, B., Rusu, M. E., Annadurai, V., & Dhandayuthapani, K. (2023). Chemical Profiling and Biological Activity of Psydrax dicoccos Gaertn. Molecules, 28(20), 7101. https://doi.org/10.3390/molecules28207101








