Abstract
The development of multiple-drug-resistant pathogens has prompted medical research toward the development of new and effective antimicrobial therapies. Much research into novel antibiotics has focused on bacterial and fungal compounds, and on chemical modification of existing compounds to increase their efficacy or reactivate their antimicrobial properties. In contrast, cyanobacteria have been relatively overlooked for antibiotic discovery, and much more work is required. This may be because some cyanobacterial species produce environmental toxins, leading to concerns about the safety of cyanobacterial compounds in therapy. Despite this, several cyanobacterial-derived compounds have been identified with noteworthy inhibitory activity against bacterial, fungal and protozoal growth, as well as viral replication. Additionally, many of these compounds have relatively low toxicity and are therefore relevant targets for drug development. Of particular note, several linear and heterocyclic peptides and depsipeptides with potent activity and good safety indexes have been identified and are undergoing development as antimicrobial chemotherapies. However, substantial further studies are required to identify and screen the myriad other cyanobacterial-derived compounds to evaluate their therapeutic potential. This study reviews the known phytochemistry of cyanobacteria, and where relevant, the effects of those compounds against bacterial, fungal, protozoal and viral pathogens, with the aim of highlighting gaps in the literature and focusing future studies in this field.
Keywords:
cyanotoxins; cyclic lipopeptides; depsiptide; microcystin; lectins; antibacterial; antimicrobial 1. Introduction
Cyanobacteria (commonly known as blue-green algae) are photosynthetic prokaryotes that are amongst the oldest oxidative photosynthetic organisms. They have existed for approximately 3.5 billion years, and over that time they have contributed to the Earth’s atmospheric oxygen production [1]. They live in a diverse range of environments, including freshwater, marine environments, soil and bare rock surfaces. They tolerate wide pH, temperature, light and salinity ranges [2]. Cyanobacteria also exist in a range of morphologies, including unicellular (suspended/benthic and aggregate forms) and filamentous forms [3]. Whilst individual cyanobacteria are microscopic, many species are readily visible in nature as they form extensive colonies that form surface crusts or blooms in their environment.
Many cyanobacteria readily switch metabolic pathways between oxygenic and anoxygenic photosynthesis (using sulphide as the electron donor) depending on the environmental conditions [3]. Under normal atmospheric oxygen conditions, all cyanobacteria preferentially use oxygenic photosynthesis for their energy metabolism. However, some species may switch to anoxygenic photosynthesis in the dark or in low-oxygen environments. This metabolic adaptability allows cyanobacteria to survive and flourish in diverse and relatively harsh conditions. The ecological and metabolic variability of cyanobacteria results in their ability to produce a myriad of bioactive compounds, many of which are yet to be rigorously examined for their biotechnology applications [4]. Furthermore, their relatively rapid growth rate compared to higher organisms such as plants and their ability to grow on otherwise non-productive land or in brackish and saline water, industrial wastewater or freshwater make them attractive targets for the production of useful secondary metabolites.
Several reviews have summarised the potential of cyanobacteria as a resource for biofuel and biofertiliser production, and for bioremediation purposes [2,4] and the reader is referred to those reviews for a comprehensive examination of the potential of cyanobacteria for those purposes. Similarly, the nutritional value of cyanobacteria has also been summarised elsewhere [2]. Whilst the therapeutic properties of cyanobacteria and cyanobacterial compounds have also been previously reviewed, most studies have narrow focuses, with the pharmaceutical potential of filamentous marine cyanobacteria being particularly well reported [5]. Furthermore, many reviews into the therapeutic potential of cyanobacteria place substantially greater emphasis on the antioxidant and anticancer properties of cyanobacterial compounds than on other bioactivities [2,4,5,6]. This is perhaps not surprising due to the diversity of cytotoxic compounds identified in cyanobacteria [7]. Whilst environmental production of these toxins is often hazardous, they also have potential in treating cancers. However, the toxicity of some cyanobacterial compounds may also mitigate their usefulness for other therapeutic purposes. For this reason, this review begins by examining some noteworthy toxic cyanobacterial compounds, as they may also have antimicrobial activities. Whilst several reviews have also examined the antimicrobial properties of cyanobacterial compounds [2,4,5,6,8], these activities are often reviewed less comprehensively, or the reviews preferentially focus on the antibacterial and/or antifungal properties of these compounds, with less extensive reporting of their antiprotozoal and antiviral activities. Our study updates the earlier reviews and summarises and extends the antimicrobial properties of cyanobacterial compounds against all classes of infective agents (bacteria, fungi, protozoa, viruses).
2. Toxicology
Multiple cyanobacteria spp. secrete toxins into their environment, which are believed to be used as protective mechanisms against competing bacteria, fungi, zooplankton and eukaryotic microalgae. These toxins are particularly harmful to zooplankton that feed on cyanobacteria, directly killing the plankton as well as inhibiting their reproduction [9]. These toxins also cause substantial ecological damage and are threats to wildlife, livestock and human health [10]. Indeed, a recent study reviewed 468 separate reports of cyanobacterial toxin poisoning in humans and animals (including 337 reported incidences since 2000) [11]. The vast majority of these poisonings were due to Microcystis spp. (~60% of cases reported in that study), Anabaena spp. (~36%), Aphanizomenon spp. (~9%), Planktothrix spp. (~9%) and Oscillatoria spp. (~7%). Notably, the incidence of toxic cyanobacterial blooms has increased rapidly in recent years and has been linked to both human factors (e.g., increased input of nutrients from agricultural fertilisers into water courses) as well as to global climate change events (increased temperatures, increased atmospheric carbon dioxide levels, increased UV intensity) [10]. The increasing threat posed by toxic cyanobacteria blooms requires increased environmental vigilance and improved water quality monitoring. However, it is important to note that not all cyanobacteria blooms produce significant levels of toxins and there are wide discrepancies in opinions, with estimates of toxic blooms ranging from 25 to 75% of total cyanobacterial blooms (as reviewed in [10]).
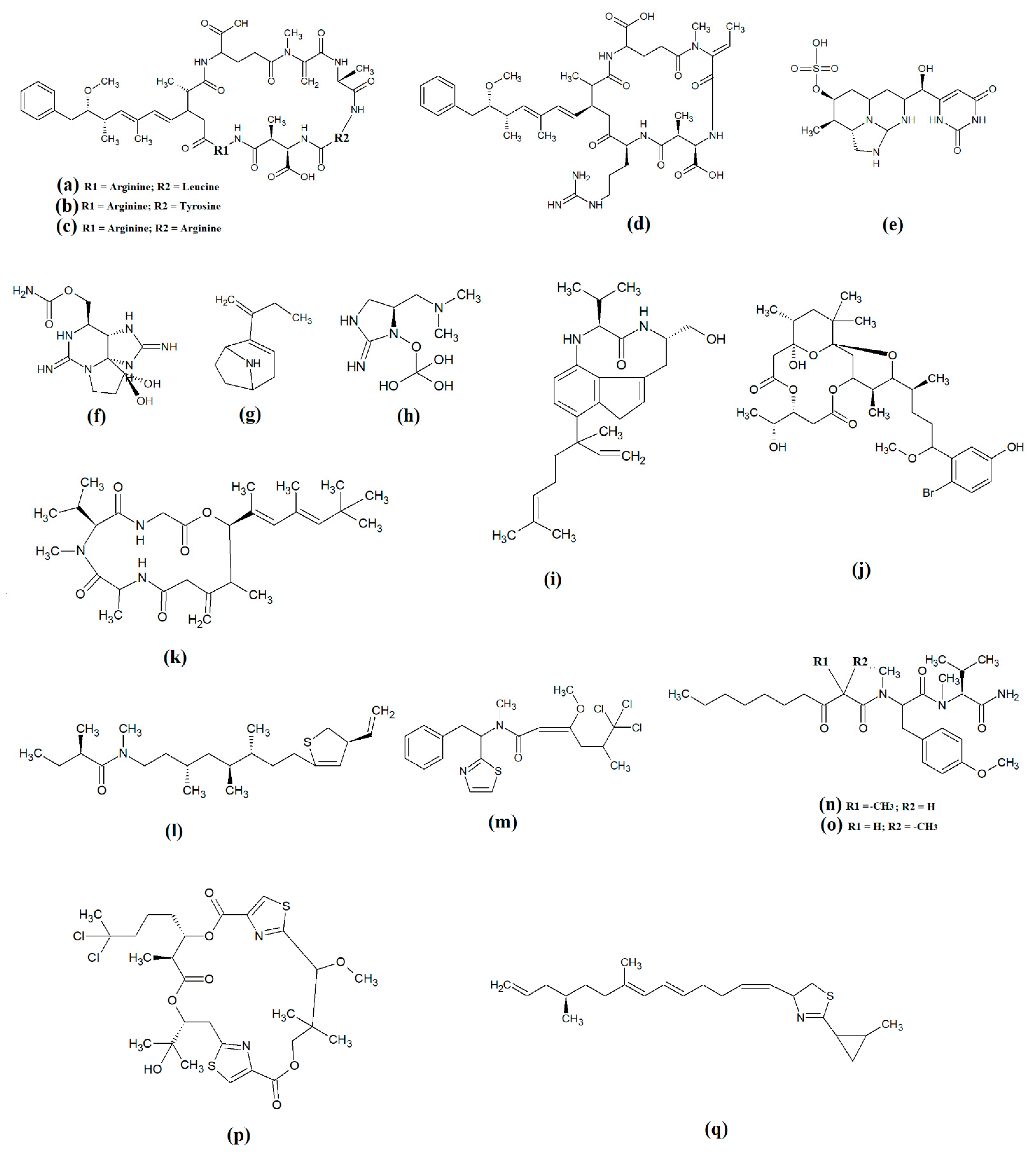
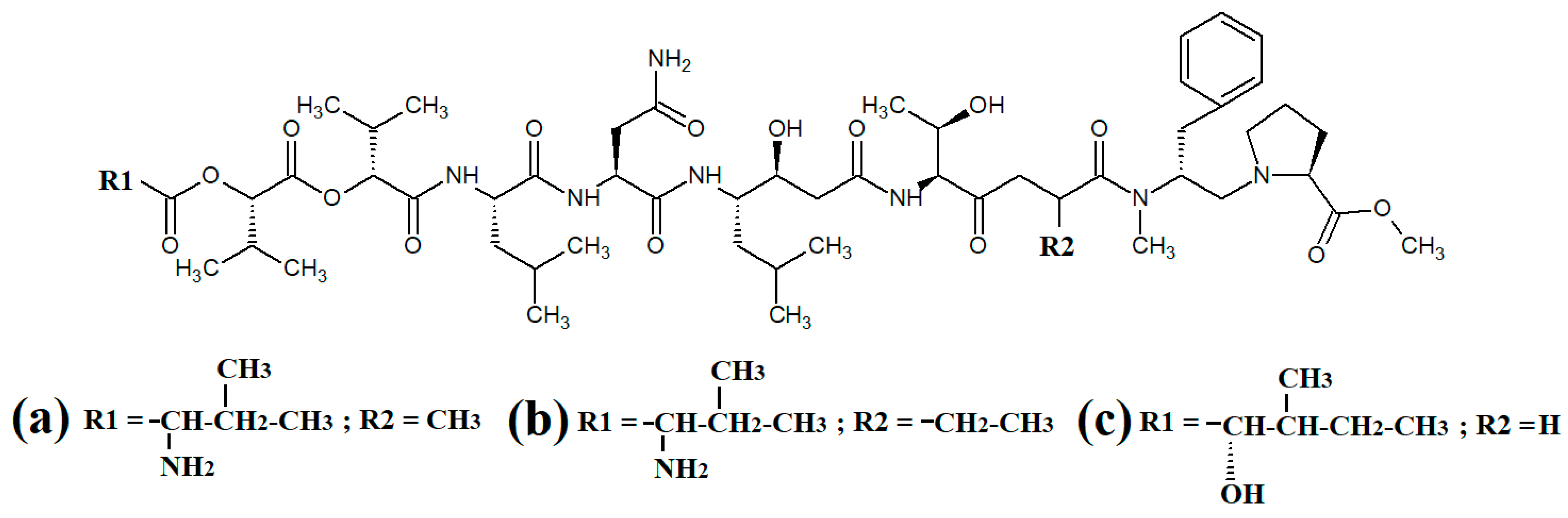
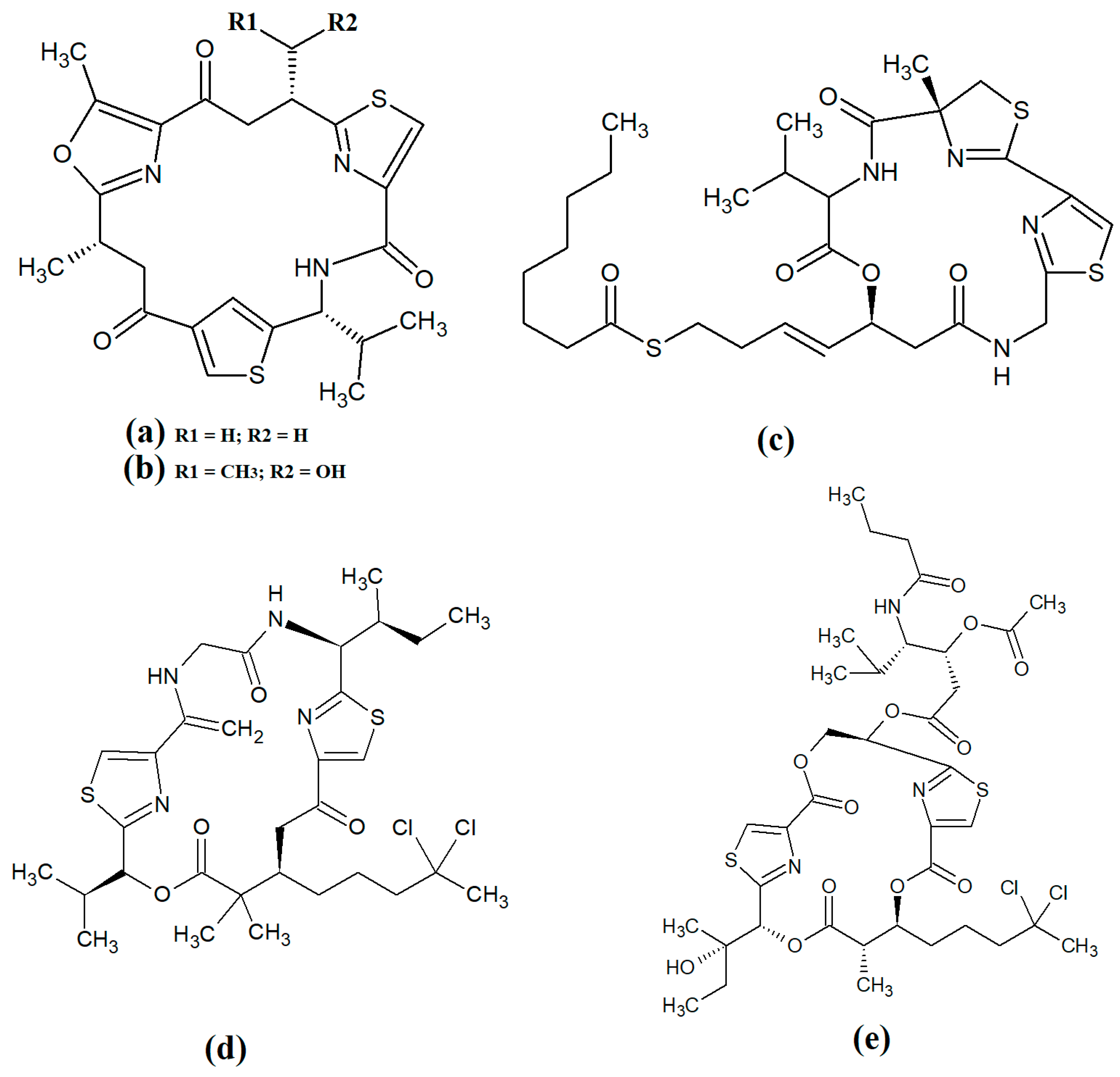
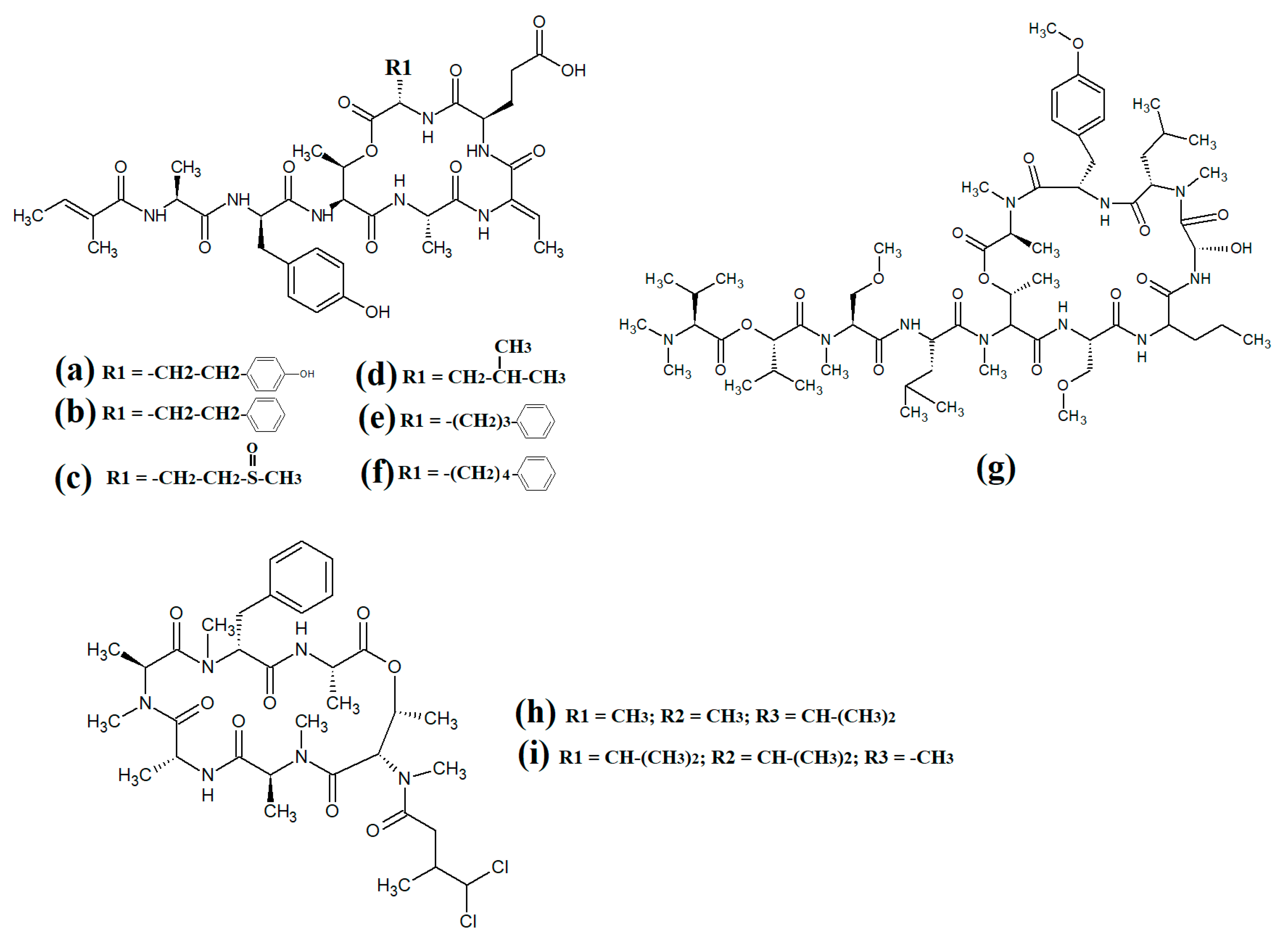
The most common cyanobacterial toxins are the cyclic peptide microcystin compounds, particularly microcystins LR (Figure 1a), YR (Figure 1b) and RR (Figure 1c). These are produced mainly by freshwater Microcystis spp., particularly Microcystis aeruginosa [10,12]. However, several other freshwater cyanobacterial species, including some from the Planktothrix, Anabaena, Oscillatoria, Spirulina, Synechococcus and Trichodesmium genera, also produce microcystins. Approximately 250 microcystins have been reported [13]. All share similar heptapeptide structures, differing by the specific amino acid moieties in their peptide loops. Microcystins are hepatotoxic and function by covalently binding to the protein phosphatases PP1 and PP2A [7]. Nodularins (Figure 1d), which are produced by Nodularia spp. (particularly Nodularia spumigena), share similar cyclic peptide structures with the microcystins and also target protein phosphatase enzymes.
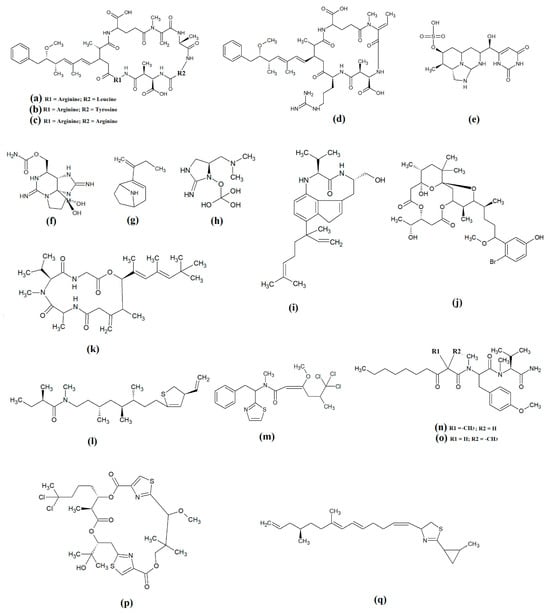
Figure 1.
Noteworthy cyanobacterial toxins: (a) microcystin-LR; (b) microcystin-YR; (c) microcystin-RR; (d) nodularin; (e) cylinderspermopsin; (f) saxitoxin; (g) anatoxin-a; (h) guanotoxin; (i) lyngbyatoxin; (j) aplysiatoxin; (k) antillatoxin; (l) kalkitoxin; (m) barbamide; (n) majuscalamide A; (o) majuscalamide B; (p) hectochlorin; (q) curacin A.
Cylinderospermopsins (Figure 1e) are relatively common cyanotoxins that are mainly produced by freshwater cyanobacteria of the Cylinderospermopsis, Anabaena, Aphanizomen, Dolichospermum, Lyngbya and Umezakia genera [7]. Whilst they are also hepatotoxins, they have very different structures and toxic mechanisms to the cyclic peptide cyanotoxins. Structurally, the cylinderospermopsins consist of a tricyclic guanidine group, linked to a uracil ring moiety. The structure is zwitterionic, making it highly water-soluble. Cylinderospermopsins function by inhibiting protein synthesis [14] and modifying DNA and RNA structure in hepatocytes [15,16]. In addition to their acute toxicity, cylinderospermopsins may also be carcinogenic and have been reported to initiate tumour formation in mice [17], although similar effects are yet to be verified in humans.
Saxitoxins (Figure 1f) are highly potent neurotoxic alkaloids that are best known as shellfish toxins due to their paralytic effects in people that have consumed toxic cyanobacteria of the Anabaena, Aphanizomenon, Cuspidothrix, Cylinderospermopsis, Doliospermum, Fischerella and Geitlerinema genera [7]. Approximately 60 saxitoxins have been reported, all of which consist of tricyclic 3,4-perhydropurine systems containing two guanidinium moieties [18]. All saxitoxins are highly toxic and exert their effects by selectively and reversibly blocking voltage-gated sodium channels in neuronal cell synapses, thereby blocking neurotransmission [7].
Anatoxin-a (Figure 1g) is also known as very fast death factor (VFDF) due to its rapid neurotoxic effects. It is produced by multiple species of the Anabaena, Aphanizomenon, Arthrospira, Cuspidothrix, Cylinderospermum, Dolichospermum, Oscillatoria and Phormidium genera [7]. Structurally, anatoxin-a contains 2-acetyl-9-aza-bicyclo(4.2.1)non-2-ene, which binds to nicotinic acetylcholine receptors and functions as a receptor agonist [19]. Guanotoxin (previously known as anatoxin-a(s); Figure 1g) has only been reported to be produced by Anabaena spp. Its structure has been determined to be (5S)-2-amino-1-((hydroxylmethoxyphosphinyl) oxy)-N,N-dimethyl-4,5-dihydri-1H-imidazole-5-methanamine (Figure 1h) [7]. Like anatoxin-a, guanotoxin also inhibits neurotransmission, although this is achieved via inhibition of the enzyme acetylcholinesterase [7,20].
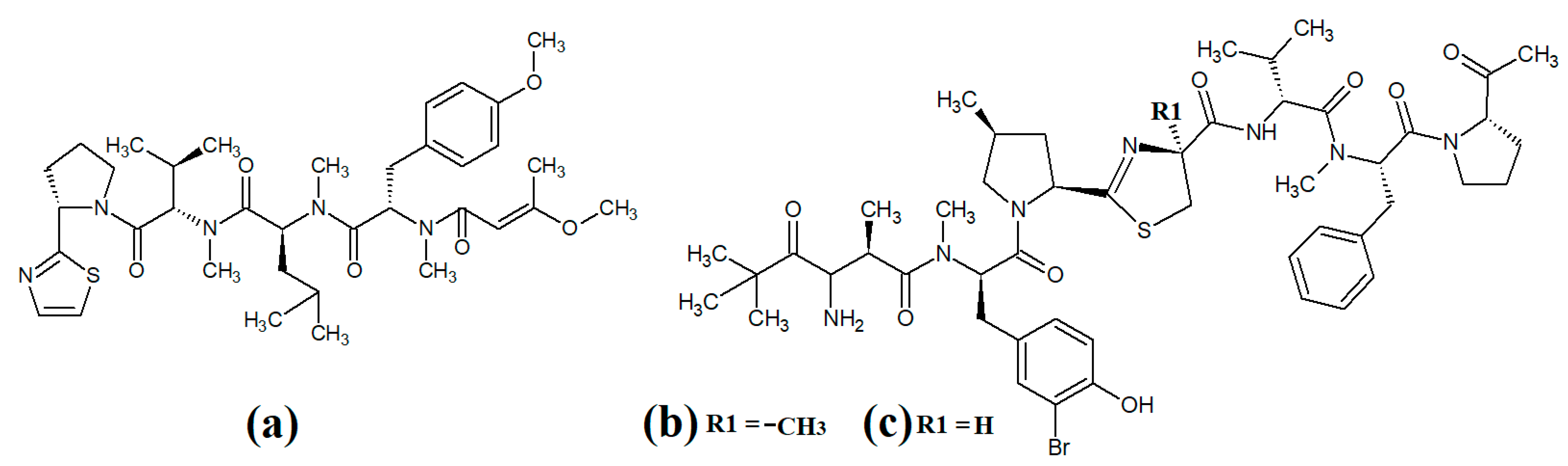
Lyngbyatoxins (Figure 1i) are dermatoxic alkaloids that are produced by several Lyngbya, Oscillatoria and Schizothrix species, particularly Lynbya majuscula. To date, seven lyngbyatoxins have been reported [7]. Structurally, all are based on indolactam ring structures, with linayl side chains attached at C-7 [21]. Lyngbyatoxin exposure activates protein kinase enzymes, inducing inflammation and skin blisters [22]. Aplysiatoxins (Figure 1j) have substantially different structures, consisting of 3,4-dihydroxyvaleric acid and 4,6,6,10,12-pentamethyl-3,7,9,11,15-tetraoxy-15-phenylpentadecanoic acid bislactones. Despite these structural differences, aplysiatoxins have similar dermatoxic effects to the lyngbyatoxins [7].
Seven lipoprotein cyanotoxins have also been identified. Antillatoxins (Figure 1k) are predominantly produced by the marine cyanobacteria Lyngbya majuscula [23]. They consist of a tripeptide moiety that is linked via both ester and amide linkages with the lipid portion of the molecule [24]. Antillatoxins induce neurotoxic effects via activation of voltage-gated sodium channels [23]. Kalkitoxin (Figure 1l) has a structure consisting of 2,4-disubstituted thiazoline linked to a lipophilic chain [25]. It exerts neurotoxic effects in humans by blocking voltage-gated sodium channels. It also strongly suppresses cell proliferation by blocking cell division and it inhibits inflammation. Lyngbya majuscula also produces barbamide (Figure 1m), which has strong molluscicidal activity, although no risks to humans have yet been reported [7]. Additionally, Lyngbya majuscula produces two majuscalamide cyanotoxins (majuscalamide A and B; Figure 1n and Figure 1o, respectively), which are epimers of N-((2R)-2-methyl-3-oxodecanoyl)-d-N,O-dimethyltyrosyl-l-N-methylvalinamide [7]. These compounds have therapeutic potential as they have antifungal activity, as well as selective cytotoxicity towards PANC-1 pancreatic and U251N glioblastoma cell lines [26]. Lyngbya majuscula also produces hectochlorin (Figure 1p), which has antifungal activity towards Candida albicans [27]. That study also reported that hectochlorin modulates actin polymerization and therefore has potent cytotoxic activity against multiple cancer cell lines. Additionally, Lyngbya majuscula also produces curacin A (Figure 1q), which exerts potent cytotoxic effects by binding to the colchicine binding site on tubulin, thereby blocking microtubule formation and cell division.
3. Cyanobacterial Chemistry
In addition to the potent cyanotoxins discussed above, cyanobacteria also produce a wealth of interesting compounds with therapeutic potential. Perhaps because of the well-established cytotoxicity of some cyanobacterial compounds, the greatest emphasis on cyanobacterial drug research to date has focused on compounds with anticancer activity. However, with the diversity of cyanobacterial compounds already identified, other therapeutic properties should not be neglected. In particular, cyanobacteria contain a wealth of peptides and lipopeptides (both linear and cyclic), as well as lectins, terpenoids and polyphenolic compounds, some of which are summarised below. Whilst the majority of the available chemical literature focusses on marine cyanobacteria, we review the known components from marine, freshwater and soil cyanobacteria herein.
3.1. Linear Peptides and Lipopeptides
Cyanobacteria are a rich source of multiple classes of linear peptides and lipopeptides. Whilst species growing in all environments produce these compounds, the peptide and lipopeptide composition of marine cyanobacteria have been particularly well reported [28]. Therefore, this review has a greater emphasis on linear peptides and lipopeptides identified in marine cyanobacteria. The marine cyanobacterium Lyngbya majuscula has been particularly well studied and has yielded a wealth of bioactive structurally diverse secondary metabolites. Whilst our review emphasises the identification of marine cyanobacterial compounds, similar compounds are also produced by freshwater and terrestrial cyanobacterial species, and these are also discussed where relevant in the following sections.
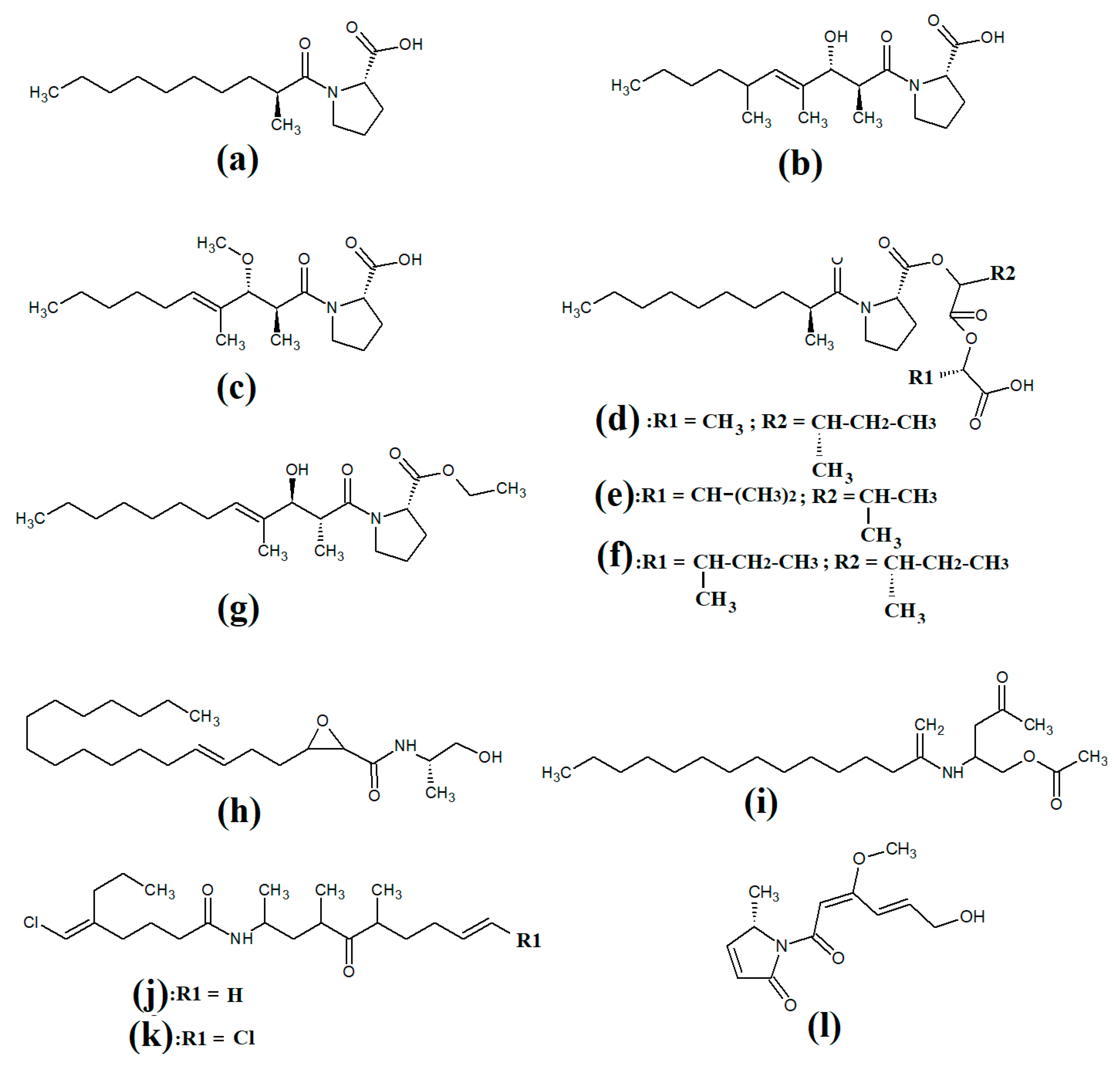
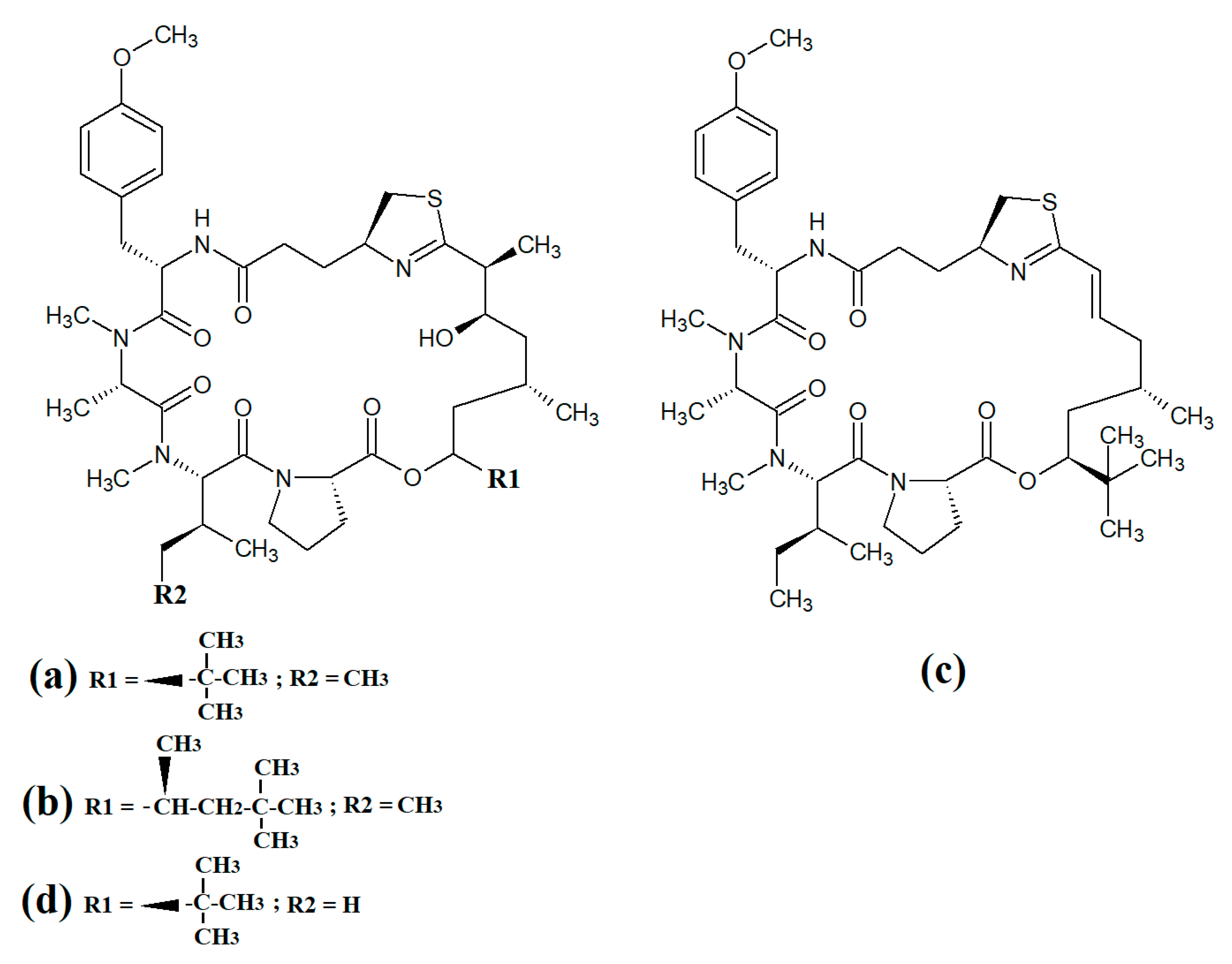
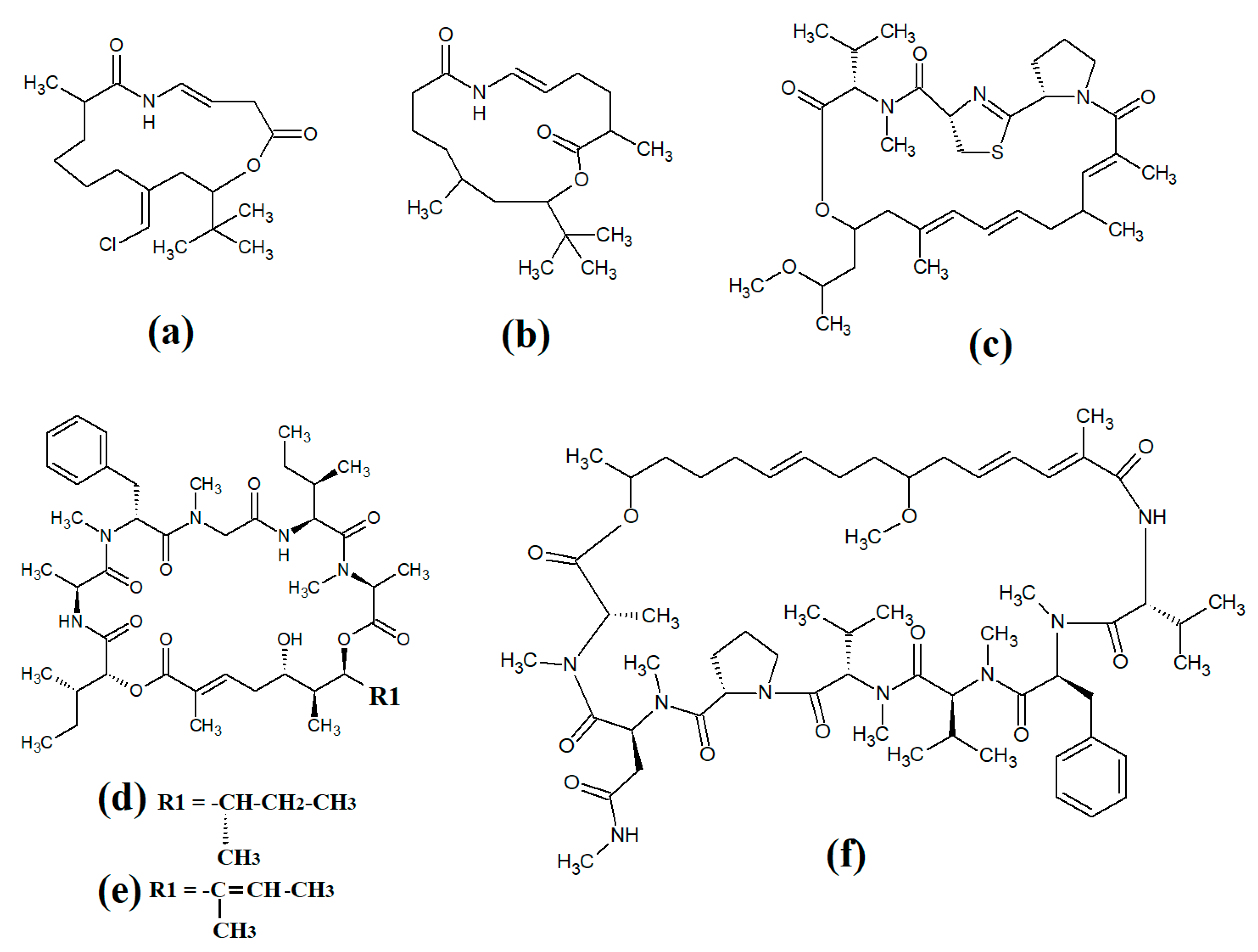
Tumonoic acid and its derivatives are some of the most abundant linear peptides in many cyanobacteria. Three tumonoic acids (A–C) were initially identified in the marine cyanobacterial species Lyngbya majuscula and Schizothrix calcicola in 1999 [29]. Since that time, tumonic acid D (Figure 2a), tumonic acid E (Figure 2b), tumonic acid F (Figure 2c), tumonic acid G (Figure 2d), tumonic acid H (Figure 2e) and tumonic acid I (Figure 2f) were identified in Blennothrix cantharidomum [30]. Because the tumonoic acids share structural characteristics with homoserine lactones (which function as bacterial signalling molecules), the authors of that study tested their ability to inhibit quorum sensing in Vibrio harveyi. Inhibitory activity was reported for all of the tumonoic acids, although tumonoic acid F was particularly potent, with an IC50 of 62 μM. That study also reported that tumonoic acid I had noteworthy antimalarial activity (IC50 = 2 μM). Subsequently, the tumonoic acid derivative ethyl tumonoate A (Figure 2g) was identified in Oscillatoria margaritefera and shown to have anti-inflammatory activity against RAW264.7 murine macrophages in a nitric oxide inhibition assay (IC50 = 9.8 μM) [31]. Besarhanamide A (Figure 2h) and besarhanamide B (Figure 2i) were also isolated from Lyngbya majuscula extracts and their structures were reported [32]. The therapeutic potential of the besarhanamides remain to be rigorously explored, although the authors of that study reported that they had moderate toxicity in an Artemia nauplii lethality assay.
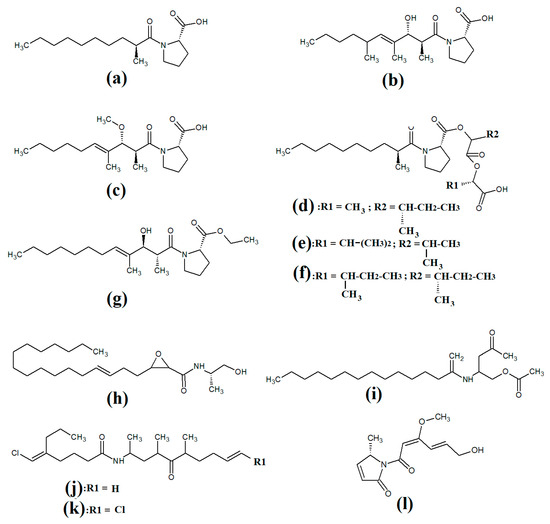
Figure 2.
Cyanobacterial tumonoic acid-, besarhananmide-, grenadamine- and palmyrroline-class linear lipopeptides: (a) tumonic acid D; (b) tumonic acid E; (c) tumonic acid F; (d) tumonic acid G; (e) tumonic acid H, (f) tumonic acid I; (g) ethyl tumonoate A; (h) besarhanamide A, (i) besarhanamide B; (j) grenadamine B; (k) grenadamine C; (l) palmyrrolinone.
In another study, grenadamide B (Figure 2j) and grenadamide C were isolated from lipophilic Lyngbya majuscula extracts [33]. Both compounds have planar fatty acid amide structures. Grenadamide C (Figure 2k) was determined to have a substituted vinyl chloride group. The same study also reported that both of these compounds have weak insecticidal properties against armyworms, with mortality rates 38–50% at 1 mg/mL. Other biological activities remain relatively unexplored for these compounds and further studies are required.
Lipopeptides of the malyngamide class are also common across multiple cyanobacteria of the genus Lyngbya. Indeed, more than 30 malyngamide class lipopeptides have been reported in multiple cyanobacterial species, with the majority of these identified in Lyngbya majuscula [34,35]. Whilst these compounds vary considerably, all contain a 7S-methoxytetradec-4(E)-enoic acid (commonly known as lyngbic fatty acid; Figure 3a) chain, or a lyngbic acid derivative. Several of the malyngamides have been reported to have noteworthy bioactivities. In particular, the malyngamide stereoisomers 8-epi-malyngamide C (Figure 3b) and 8-O-acetyl-8-epi-malyngamide C (Figure 3c) are strongly cytotoxic against NCI-H460, Neuro-2a and HT29 cells, with IC50 values ranging from 3.1 to 23.9 μM [34,35]. Additionally, 8-epi-malyngamide C also inhibits bacterial quorum sensing in transformed Escherichia coli [35]. Isomalyngamide K (Figure 3d) was isolated from Lyngbya majuscula extracts in another study, although no bioactivities were reported for this compound [36] and further studies are required to evaluate its therapeutic potential.
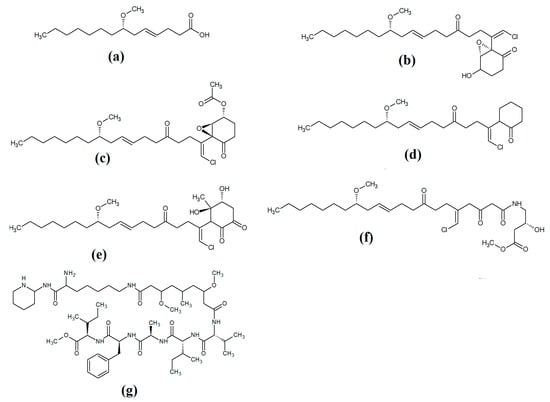
Figure 3.
Cyanobacterial malyngamide-class linear lipopeptides: (a) lyngbic acid; (b) 8-epi-malyngamide C; (c) 8-O-acetyl-8-epi-malyngamide C; (d) isomalyngamide K; (e) malyngamide 2; (f) malyngamide 3; (g) mitosoamide A.
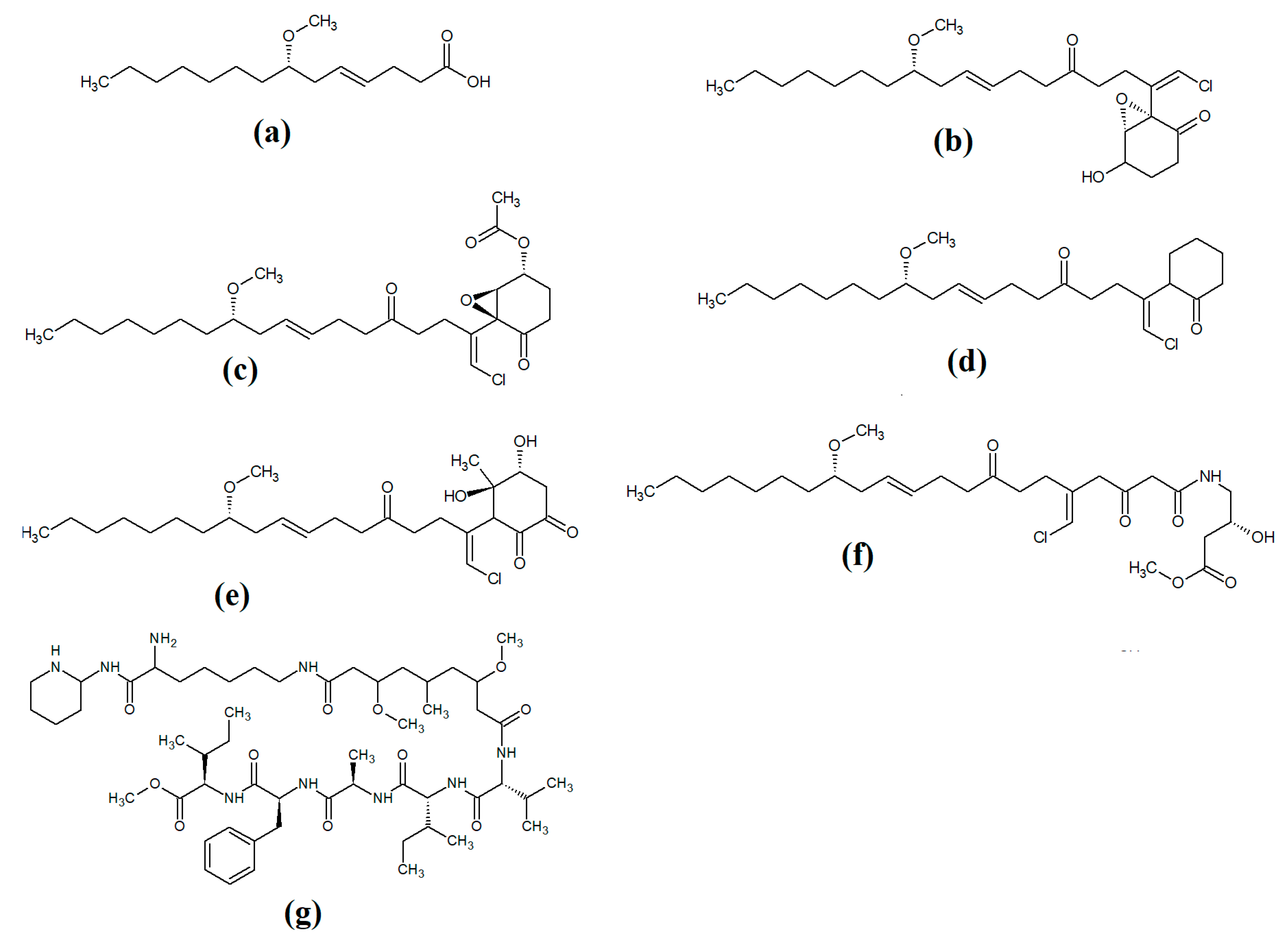
Another study isolated malyngamide 2 (Figure 3e) from Lyngbya sordida and determined that it contains a trihydroxy cyclohexanone ring structure [37]. The authors reported noteworthy nitric oxide inhibitory activity against LPS-stimulated RAW264.7 murine macrophages (IC50 = 8 μM). They also reported that malyngamide 2 had moderate cytotoxic activity against H-460 human lung cancer cells (IC50 = 8 μM). However, the antimicrobial properties of this compound are yet to be rigorously evaluated. Another study reported the isolation and identification of malyngamide 3 (Figure 3f) from Lyngbya majuscula and reported that it has weak cytotoxicity against MCF-7 breast cancer cells (IC50 = 29 μM) and HT-29 colon cancer cells (IC50 = 48 μM) [38]. Additionally, mitsoamide A (Figure 3g) was isolated from a marine cyanobacterium of the Geitlerinema genus and was reported to have several unusual structural features [39]. The structure contains a homolysine, 3,7-dimethoxy-5-methyl-nonanedioic acid and a piperidine moiety, linked by an alanine, isoleucine, N-methyl-isoleucine, phenylalanine and valine peptide. The authors of that study reported that mitsoamide A was cytotoxic to human NCI-H460 lung cells (IC50 = 0.46 μM).
The lipodepsipeptide gallinamide A (Figure 4a) was isolated from Schizithrix spp., although the specific species was not identified [40]. Interestingly, gallinamide A had moderate antiprotozoal activity against Plasmodium falciparum (IC50 = 8.4 μM) and Leishmania donovani (IC50 = 9.3 μM). The same study also reported moderate cytotoxicity against human NCI-H460 lung cancer cells and neuro-2a mouse neuroblastoma cells. A different study isolated and identified the structural isomer symplostatin 4 (Figure 4b) from Symploca spp., although the specific species was not identified [41]. The authors of that study reported that symplostatin 4 had noteworthy cytotoxicity against HT-29 human colon cancer cells (IC50 = 53 μM) and HeLa cervical cancer cells (IC50 = 12 μM) due to its antimitotic activity. Symplostatin 4 disrupted intracellular microtubule formation at 50 μM, and completely depolymerised microtubules at 100 μM. Additionally, the authors also reported that symplostatin 4 and largazole (which was also produced by the Symploca spp. cells) synergised the cytotoxicity of the combination against HT-29 cells, although fractional IC50 values of the combination components were not reported. Largazole functions via a different cytotoxic mechanism, through inhibition of histone deacetylases [42], possibly accounting for these effects.
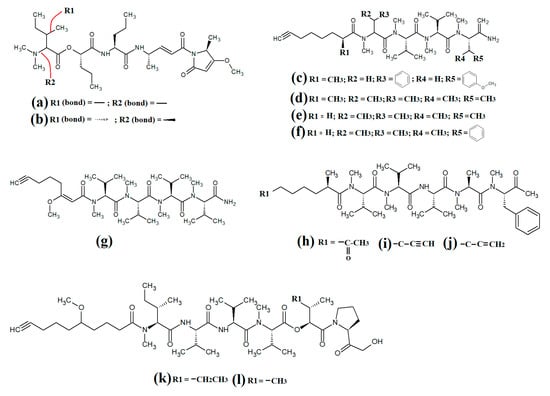
Figure 4.
Cyanobacterial gallinamide-, symplostatin- and dragonamide-class linear lipopeptides: (a) gallinamide A; (b) symplostatin 4; (c) dragomabin; (d) dragomabin B; (e) dragomabin D; (f) dragomabin E; (g) dragomabin C; (h) almiramide A; (i) almiramide B; (j) almiramide C; (k) viridamide A; (l) viridamide B.
Dragomabin (Figure 4c) and the dragonamides B (Figure 4d), D (Figure 4e), E (Figure 4f) and C (Figure 4g), as well as the almiramides A (Figure 4h), B (Figure 4i) and C (Figure 4j) and viridamides A (Figure 4k) and B (Figure 4l), are a group of structurally related linear lipopeptides. All contain an eight-carbon polyketide moiety linked to amino acids via amide bonds, with all of these compounds except the viridamides terminating in a primary amide functional group [7]. Dragomabin and dragonamides A and B were isolated from Lyngbya majuscula and screened against a chloroquine-resistant Plasmodium falciparum strain [43]. The authors reported IC50 values between 4.3 and 7.7 μM for all compounds except dragonamide B, which was completely inactive against the chloroquine-resistant Plasmodium falciparum strain, indicating that the aromatic moiety is required for antimalarial activity. Additionally, the same study also determined the cytotoxicity of these compounds against Vero cells, with IC50 values ≤ 182 μM for all of the tested compounds. Dragonamides C and D were isolated from Lyngbya majuscula and Lyngbya polychroa in another study and reported to have noteworthy inhibitory activity against Leishmania donovani (IC50 = 5.1 μM) [44] and U2OS osteocarcoma cells (IC50 = 56–59 μM).
Almiramides A–C were isolated from Lyngbya majuscula, identified and screened for antiprotozoal activity against Leishmania donovani [44]. The inhibitory activity of almiramides B and C were particularly noteworthy (IC50 values of 2.4 and 1.9 μM, respectively). Another study screened the almiramides against a chloroquine-resistant Plasmodium falciparum strain and reported good antimalarial activity for dramonamide A (IC50 = 7.7 μM) [45]. Another study isolated viridamide A (Figure 4k) and viridamide B (Figure 4l) from the marine cyanobacteria Oscillatoria nigro-viridis and determined that the structures consisted of N-methylated amino acids, hydroxyl acids and a 5-methoxydec-9-ynoic acid moiety [46]. The authors of that study reported noteworthy antiparasitic activity for viridamide A against Leishmania mexicana, Plasmodium falciparum and Trypanosoma cruzi (IC50 values 1.1–5.8 μM). Contrastingly, the effects of all of these compounds against bacteria, fungi, viruses and other protozoal pathogens are yet to be rigorously examined.
Grassystatins are linear decadapsipeptides that are potent inhibitors of cathepsin E enzyme [5]. Grassystatins A (Figure 5a), B (Figure 5b) and C (Figure 5c) were isolated and identified from the marine cyanobacterium Lyngbya conferoides [47]. That study reported that these compounds are potent inhibitors of both cathepsin D (IC50 = 16.9 nM) and E (IC50 = 0.62 nM). As cathepsins D and E are involved in antigen processing via the HHC class II pathway and bioactive protein degradation [48], it is likely that grassystatins may block pathogen activation mechanisms, and thereby modulate the course of multiple infections. Notably, grassystatins have profound effects on the course of viral diseases. By inhibiting cathepsin enzymes, grassystatins block viral attachment glycoprotein activation and therefore inhibit the entry of virus into the target cells, as well as directly inhibiting viral replication [49].
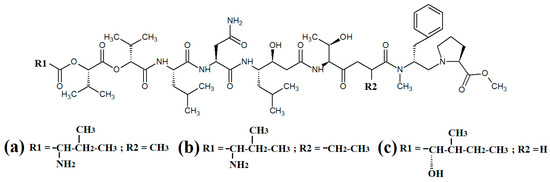
Figure 5.
Cyanobacterial grassystatin linear lipopeptides: (a) grassystatin A; (b) grassystatin B; (c) grassystatin C.
3.2. Linear Lipopeptides and Peptides Containing Heterocyclic Moieties
Lyngbyapeptin D (Figure 5a) and three structural analogues (27-deoxylyngbyabellin A, lyngbyabellin J and laingolide B) have been identified in Lyngbya boullonii extracts [50]. That study reported that all of these compounds were moderately cytotoxic towards HT29 colorectal adenocarcinoma and HeLa cervical cancer cell lines, and therefore may have potential as cancer therapeutics. Notably, the effects of lyngbyapeptin D and its analogues have yet to be tested against bacterial, fungal, protozoal and viral pathogens and substantially more work is required.
The cytotoxic linear peptide bisebromoamide (Figure 6b) was isolated from an unidentified Lyngbya spp. in 2009 [51]. A subsequent study also identified the structural analog norbisebromamide (Figure 6c) [52]. Bisebromoamide is highly cytotoxic against an extensive panel of human cancer cells with an average GI50 value of 40 nM [53]. That study reported that bisebromoamide exerts its anticancer effects via modulation of extracellular signal-regulated protein kinase (ERK) pathways. Another study reported that bisebromoamide also destabilises actin filaments [54]. However, the effects of bisebromoamide and norbisebromoamide are yet to be elucidated.
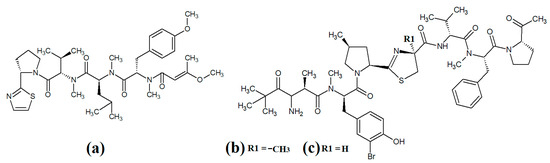
Figure 6.
Cyanobacterial lyngbypeptin- and bisebromoamide-class heterocycle containing linear lipopeptides: (a) lyngbyapeptin D; (b) bisebromoamide; and (c) norbisebromoamide.
3.3. Cyclic Depsipeptides and Peptides
Cyclic depsipeptides are a diverse group of compounds containing ring structures composed of amino and hydroxyl acid moieties linked by amide and ester bonds. Differences in the ring structure and the nature of the side chains provides substantial diversity in the structure and function of this class of compounds [55].
3.4. Cyclic Depsipeptides Containing Heterocyclic Moieties
The epimeric cyclic depsipeptides porpoisamide A (Figure 7a) and porpoisamide B (Figure 7b) were isolated from an unidentified marine Lyngbya spp. cyanobacterium and the structure was elucidated [56]. Both compounds contained alanine, N-methyl-phenylalanine, 2-hydroxy-3-methylpentanoic acid and 3-amino-2-methyl-octanoic acid moieties and differed only in the stereochemistry of the amino acid at C2 of the ring structure. That study reported that both porpoisamides have weak cytotoxic activity against human HCT-116 colorectal (IC50 = 21 μM) and U2OS osteosarcoma cell lines (IC50 = 28 μM).
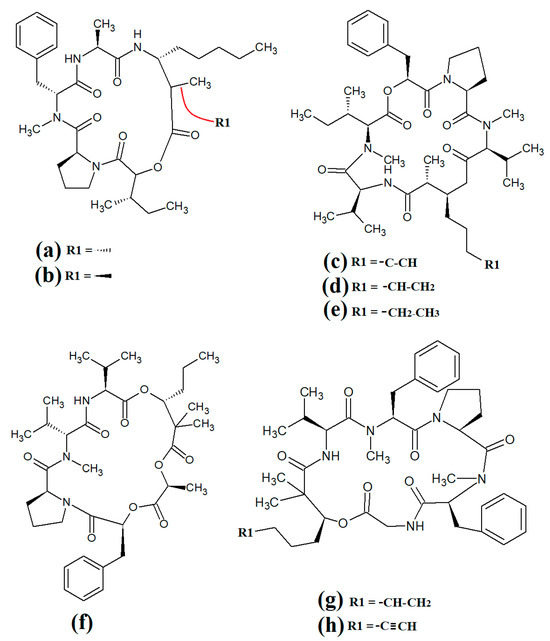
Figure 7.
Cyclic cyanobacterial lipopeptides: (a) porpoisamide A; (b) porpoisamide B; (c) hantupeptin A; (d) hantupeptin B; (e) hantupeptin C; (f) palmyramide A; (g) cocosamide A; (h) cocosamide B.
Hantupeptin A (Figure 7c) and its structural analogues hantupeptin B (Figure 7d) and hantupeptin C (Figure 7e) have been isolated from Lyngbya majuscula extracts [57]. These compounds each contain a cyclic structure consisting of four amino acids and two hydroxy acid residues, one of which is a PKS-derived 3-hydroxy-2-methyloctynoic acid. The hantupeptins differ only in the nature of a chain extension that occurs at C2 of this residue. Subsequent studies by the same group reported significant cytotoxic activity against MOLT-4 leukaemia and MCF-7 breast cancer cell lines, with IC50 values between 32 and 3000 nM [58]. Hantupeptin A was particularly potent against MOLT-4 cells (32 nM). However, the potential of these compounds in the treatment and inhibition of pathogenic diseases remains to be examined.
The 19-membered cyclodepsipeptide palmyramide A (Figure 7f) has been reported in Lyngbya majuscula extracts [59]. Its structure was reported to consist of five amino acid moieties, including three valine residues, as well as one residue each of N-methyl-valine and proline. It also contains the three hydroxy acids 2,2-dimethyl-3-hydroxyhexanoic acid, lactic acid and 3-phenyllactic acid. The authors of that study tested palmyramide A in sodium channel blocking assays and reported noteworthy inhibition of veratridine and ouabain-induced sodium channel overload, resulting in considerable cytotoxicity in neuro-2a murine neuroblast cells (IC50~17 μM) and in H-460 human lung cancer cells (IC50~40 μM).
The cyclodepsipeptides cocosamide A (Figure 7g) and cocosamide B (Figure 7h) have also been isolated from Lyngbya majuscula extracts in another study [38]. Both compounds contain two N-methyl-phenylalanine residues, proline, glycine, valine, and either 2,2-dimethyl-3-hydroxy-7-octynoic acid or 2,2-dimethyl-3-hydroxy-7-octenoic acid. Both compounds were moderately cytotoxic against HT-29 and MCF-7 breast cancer cells, with IC50 values ranging from 11 to 39 μM. The effects of these compounds against bacterial, fungal, protozoal and viral pathogens remain to be investigated.
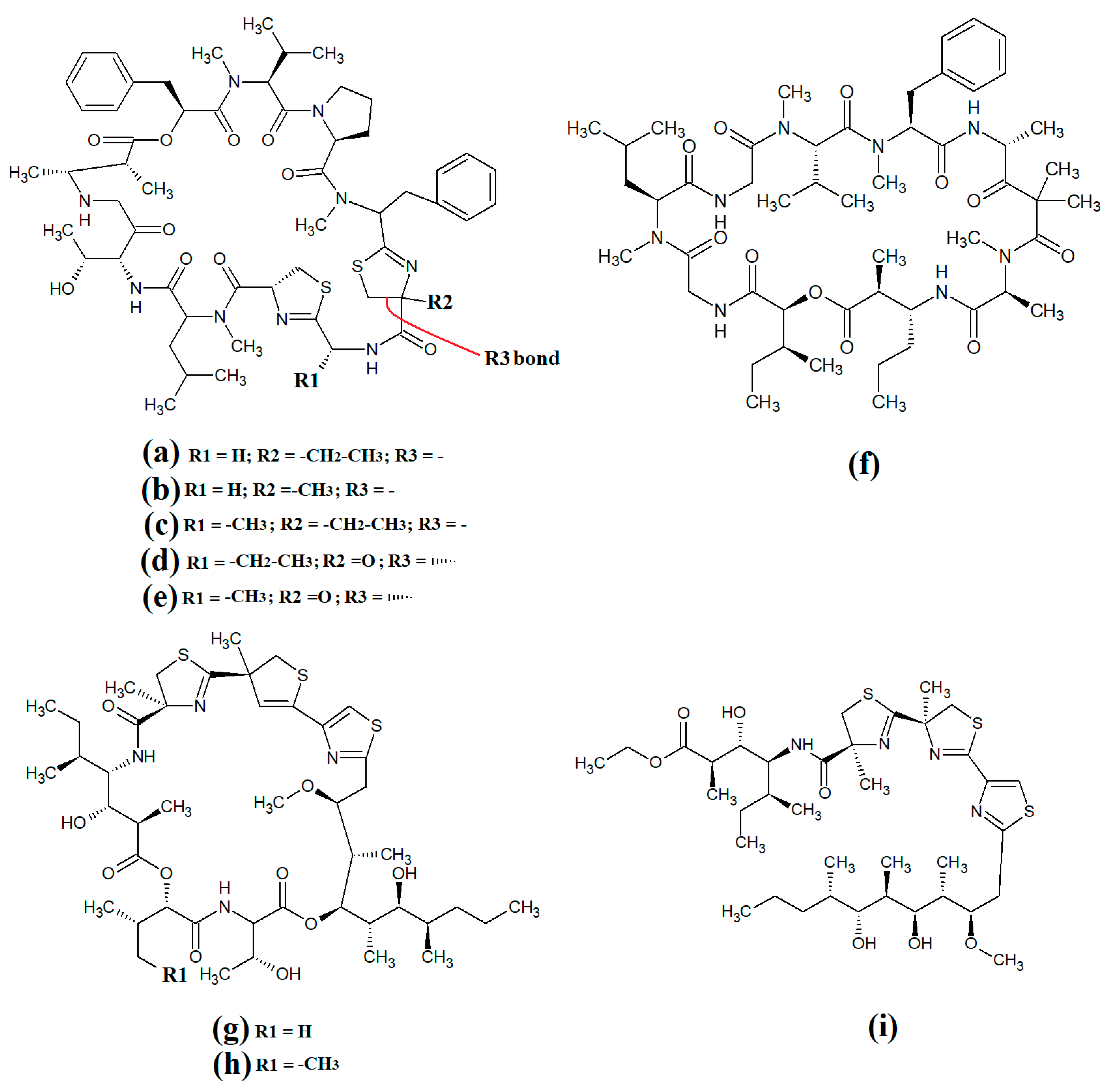
Carriebowmide (Figure 8a) was isolated from Lyngbya majuscula extracts and the structure was determined to consist of a 21-membered cyclic depsipeptide structure, which consists of alanine, N-methyl-leucine, phenylalanine, methionine, N-methyl-phrnylanine and a 2-hydroxy-3-methylbutyric acid moiety [60]. It also contains the two rare amino acids 3-amino-2-methyl-hexanoic acid and methionine sulfoxide. Another study reported that carriebowmide is moderately cytotoxic towards human HEK-293 embryonic kidney cells, with an IC50 of approximately 50 μM [33]. However, we are unaware of any studies to date that have screened carriebowmide against any pathogens and substantially more work is needed to evaluate the therapeutic potential of this compound.
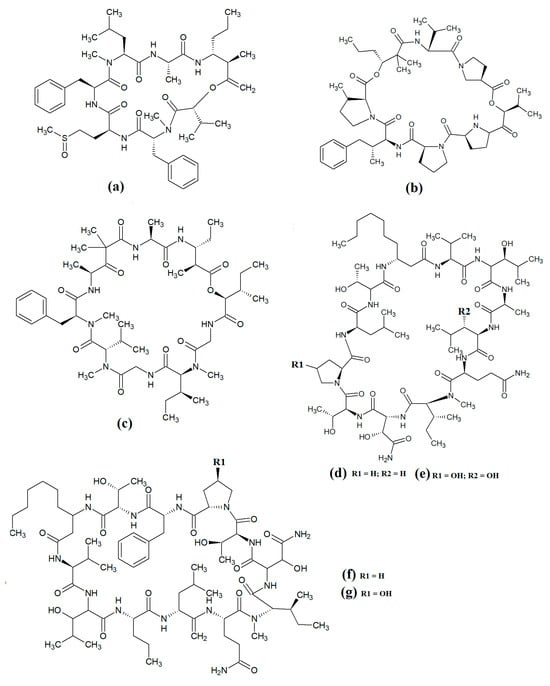
Figure 8.
Cyclic cyanobacterial lipopeptides: (a) carriebowmide; (b) pitiprolamide; (c) desmethoxymajusculamide C; (d) laxaphycin B2; (e) laxaphycin B3; (f) lynbyacyclamide A; (g) lynbyacyclamide B.
Pitiprolamide (Figure 8b) was also identified in Lyngbya majuscula extracts [61]. Its structure was reported to be similar to that of dolastatin, although it contains four proline residues, as well as a valine, dolaphenvaline and 2-hydroxy-isovaleric acid moieties. The authors of that study reported that pitiprolamide was mildly cytotoxic towards human MVC7 breast cancer and HCT116 colorectal cancer cell lines (IC50 = 33 μM for both). Of further interest, pitiprolamide also showed antibacterial activity against Mycobacterium tuberculosis in a disc diffusion assay at 50 μg. However, the MIC was not determined, making it impossible to compare with the potency of other compounds tested in other studies. Additionally, the disc diffusion assay was perhaps not the most appropriate assay system to test antibacterial activity of this compound. Pitiprolamide has a molecular mass of 905.54 Da and its size would hinder its diffusion in agar gels, thereby providing falsely low results. Furthermore, dolastatin (which is structurally similar) has low water solubility, also hampering its diffusion in agar. Therefore, liquid-based assay systems may have been more appropriate for testing the antibacterial potency of this compound. Despite that, the reported mycobacterial activity is promising, and future studies should focus on testing pitiprolamide against other pathogens using more appropriate assays.
Another cyclic depsipeptide was isolated from the marine cyanobacterium Lyngbya majuscula and was identified as desmethoxymajusculamide C (Figure 8c) [62]. Notably, desmethoxymajusculamide C has highly potent and selective cytotoxicity, with an IC50 value of 20 nM against human HCT-116 colorectal cells, although it is substantially less potent against other cell lines. The authors of that study determined the HCT-116 cytotoxicity to be mediated through inhibition of microfilament production.
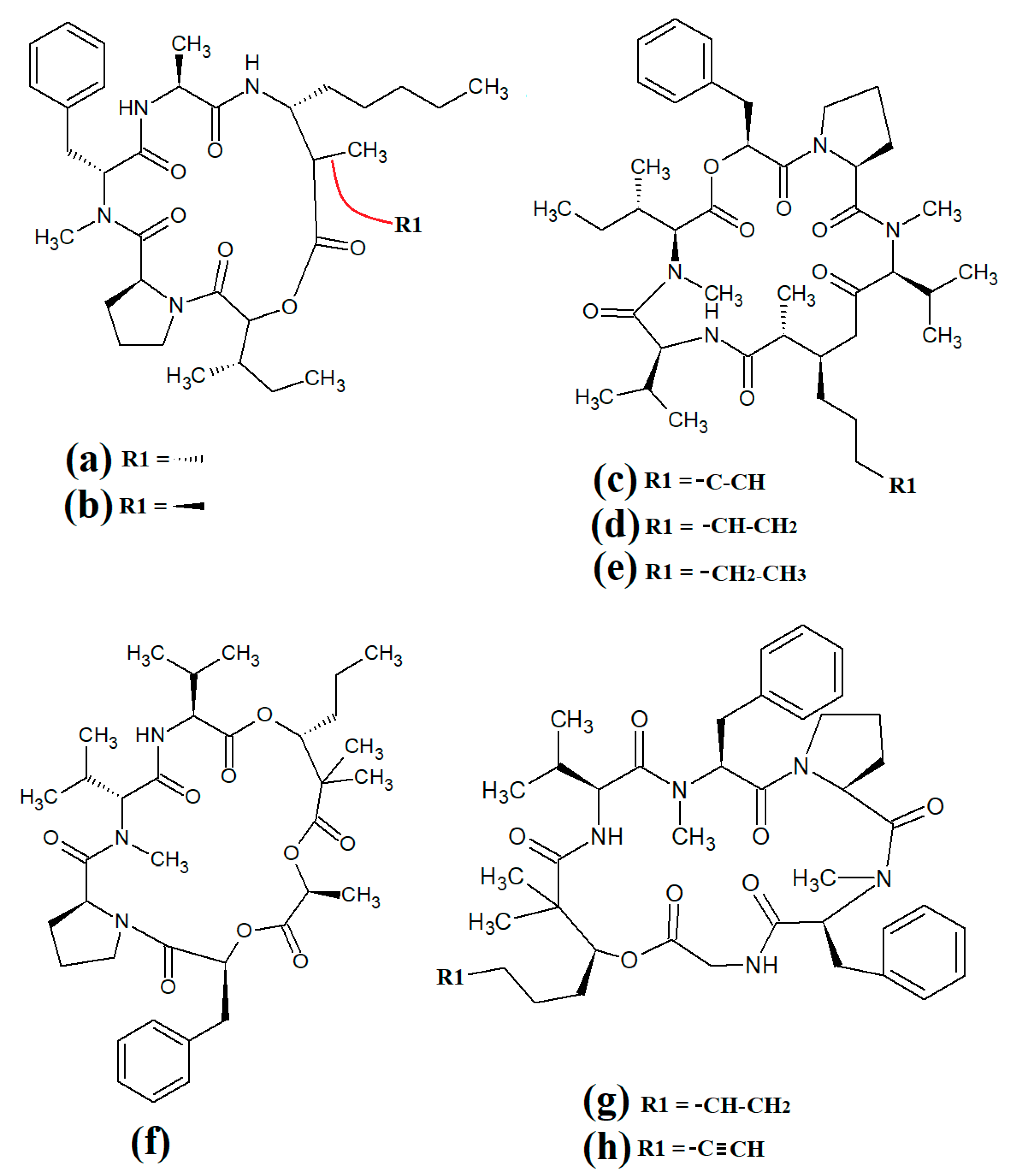
Laxaphycin B2 (Figure 8d) and laxaphycin B3 (Figure 8e) were detected in Annabaena torulosa extracts, albeit in low abundance [63]. Both compounds were reported to have noteworthy anti-proliferative activity towards a panel of drug-resistant and drug-sensitive solid lymphoblastic cancer cells, with IC50 values generally between 1 and 20 μM. Interestingly, these compounds substantially potentiated each other’s anti-proliferative activity when tested in combination, with at least a two-fold increase in potency against all of the cell lines tested. Lynbyacyclamide A (Figure 8f) and lynbyacyclamide B (Figure 8g) are structurally related to the laxaphycins, which were originally isolated from Lyngbya majuscula extracts [64]. Structurally, they contain 37-member rings consisting of 11 α-amino acids and 1 β-amino acid (β-amino-decanoic acid). Similar to laxaphycin B2 and laxaphycin B3, the lynbyacyclamides are strongly cytotoxic, both with IC50 values of 0.7 μM against mouse B16 melanoma cancer cells. Interestingly, whilst the cytotoxic properties of the laxaphycin and lynbyacyclamides are well established, we were unable to find reports testing their activities against any pathogens and substantial further work is required.
Several recent studies have focused on cyanobacterial compounds for antiprotozoal activity, particularly against malaria-, leishmaniasis- and schistomiasis-causing pathogen species. Several promising compounds have been identified with noteworthy antiprotozoal activities. In particular, ventutramide A (Figure 9a) and ventutramide B (Figure 9b) that were isolated from Oscillatoria spp. are reported to have good antimalarial activity. Ventutramide A was a particularly good inhibitor of Plasmodium falciparium growth, with an IC50 of 8.2 μM [65]. Both compounds also inhibited Trypanasoma cruzi and Leishmania donovani, albeit with substantially higher IC50 values. The ventutramides are structurally unusual, containing two thiazole and one methyl-oxazole ring moieties, which may contribute to the antiprotozoal activity of these compounds. The effects of these compounds on non-protozoal pathogens have been less extensively examined and substantially more work is required to determine the therapeutic potential of these molecules.
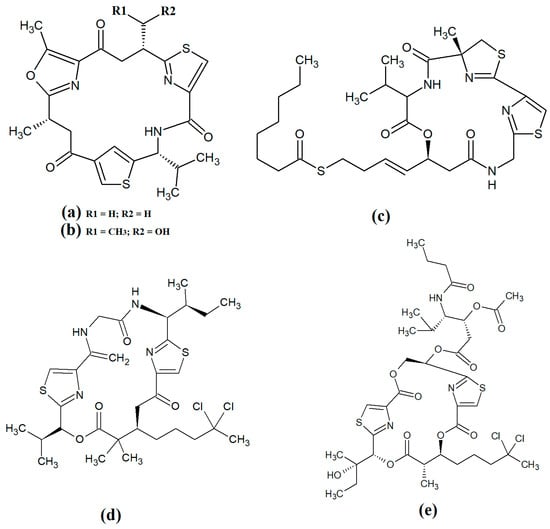
Figure 9.
Cyclic cyanobacterial depsipeptides/peptides: (a) ventutramide A; (b) ventutramide B; (c) largazole; (d) 27-deoxylyngbyaybellin A; (e) lyngbyabellin J.
Largazole (Figure 9c) was initially isolated from marine cyanobacteria of the genus Symploca in 2008 [42]. This compound has attracted substantial interest since that time as it is a potent class I histone deacetylase (HDAC) inhibitor. As HDACs regulate HIV latency [66], largazole has potential as an anti-retroviral therapy for use in HIV-AIDS. Additionally, the HDAC inhibitory activity of largazole also makes it a promising target for anticancer drug development. Notably, largazole (as well as its analogues) has anti-proliferative activity towards the NCI 60 panel of human cancer cell lines [67]. Further studies demonstrated that largazole also has cytostatic effects in a human HCT116 xenograft mouse model via stimulation of histone hyperacetylation, as well cytotoxic effects by inducing apoptosis [67]. Notably, these compounds are yet to be tested for anti-infective properties against pathogens of interest to human health.
Lyngbyabellins are a group of cyclic lipopeptides that are characterised by the presence of thiazole/thaizoline moieties and dichlorination of a polyketide moiety. The structural lyngbyabellin analogues 27-deoxylyngbyaybellin A (Figure 9d) and lyngbyabellin J (Figure 9e) were isolated and characterised from Lyngbya bouillonii extracts and tested for cytotoxic activity against human HT29 colorectal and HeLa cervical carcinoma cell lines [50]. Whilst both compounds displayed good cytotoxic activity, 27-deoxylyngbyaybellin A was particularly potent, with IC50 values of 12 and 7.3 nM against the HT29 and HeLa cells, respectively. Lyngbyabellin J also had noteworthy activity, albeit with significantly higher IC50 values (54 and 41 nM, respectively).
Multiple members of the apratoxin class of compounds have also been identified in cyanobacteria. These compounds have cyclic structures that contain a thiazoline unit, as well as an extensive polyketide moiety. Apratoxin A (Figure 10a) was reported over twenty years ago in the marine cyanobacterium Lyngbya majuscula [68]. Since that time, apratoxin D (Figure 10b), apratoxin E (Figure 10c) and apratoxin G (Figure 10d) have also been isolated from Lyngbya majuscula, as well as from Lyngbya sordida [69]. Apratoxin D was reported to have potent cytotoxic activity against human H-460 lung cancer cells (IC50 = 2.6 nM) [69]. Apratoxin E also has potent cytotoxicity against HT29, HeLa and U2OS cell lines (IC50s of 21, 72 and 59 nM, respectively) [70]. Similarly, apratoxin G was cytotoxic to human H-460 cells (IC50 = 14 nM) [71]. However, these compounds are yet to be rigorously tested against pathogens.
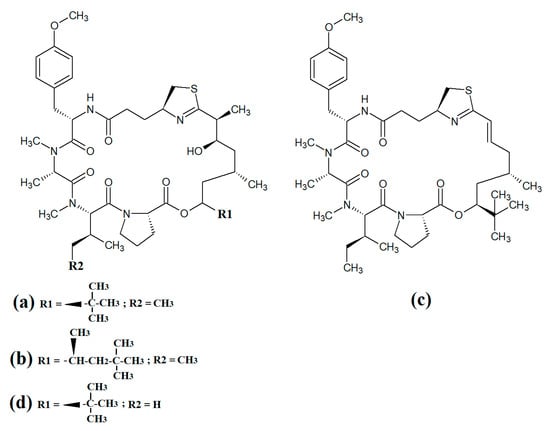
Figure 10.
Cyclic cyanobacterial apratoxins: (a) apratoxin A; (b) apratoxin D; (c) apratoxin E; (d) apratoxin G.
Grassypeptolide A (Figure 11a) and grassypeptolide B (Figure 11b) were isolated and characterised from Lyngbya confervoides [72,73]. The authors of those studies also screened these compounds and reported noteworthy cytotoxic activity for grassypeptolide A against human U2OS osteosarcoma, HeLa cervical cancer, HT29 colorectal and IMR-32 neuroblastoma cells, with IC50 values of 2.2, 1, 1.5 and 4.2 μM, respectively [72]. Subsequent studies demonstrated that grassypeptolides A and C inhibit cell cycle progression at G1 phase in low concentrations, and also at the G2/M phase when tested at higher concentrations [73]. More recently, grassypeptolide D (Figure 11c), grassypeptolide F (Figure 11d) and grassypeptolide G (Figure 11e) were isolated from the marine cyanobacteria Leptolyngbya spp. and Lyngbya majuscula and tested for cytotoxicity [74,75]. Grassypeptolides D and E were particularly cytotoxic against HeLa (IC50 = 335 and 192 nM, respectively) and mouse neuro-2a-blastoma cells (IC50 = 599 and 407 nM, respectively) [74]. In contrast, grassypeptolides F and G were only moderate inhibitors of transcription factor AP-1 (IC50 = 5.2 and 6 μM, respectively) [75].
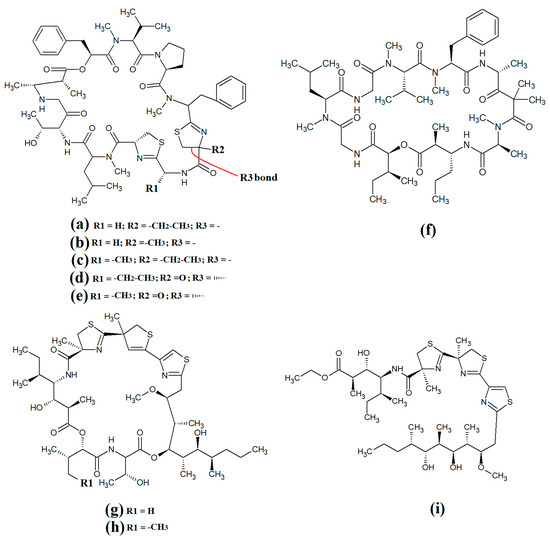
Figure 11.
Cyanobacterial grassypeptolide- and hoiamide-class depsipeptides/peptides: (a) grassypeptolide A; (b) grassypeptolide B; (c) grassypeptolide D; (d) grassypeptolide F; (e) grassypeptolide G; (f) ibu-epidemethoxylyngbyastatin; (g) hoiamide A; (h) hoiamide B; (i) hoiamide C.
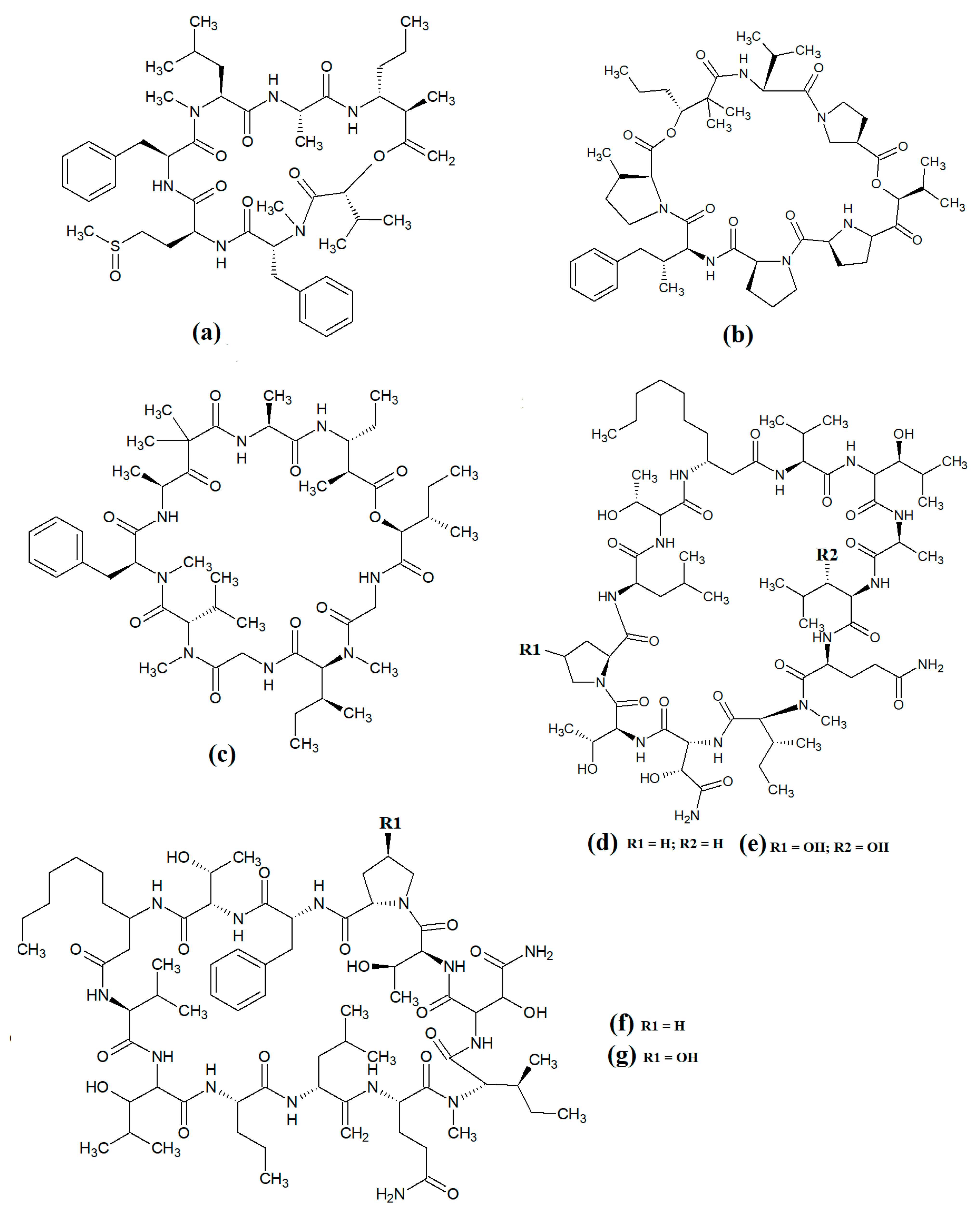
The hoiamides are a class of cyclic depsipeptides that have been isolated from several filamentous marine cyanobacteria, including Lyngbya majuscula and Phormidium gracile [76]. Hoiamide A (Figure 11g) contains an extended isoleucine moiety, two methylated thiazoles, a thiazole moiety and a highly methylated and oxygenated polyketide group [76]. The same study also reported that hoiamide A is a potent inhibitor of [3H]-batrachotoxin binding to voltage-gated sodium channels (VGSCs), and therefore increases sodium influx in neural cells (IC50 = 93 nM; EC50 = 2.3 μM). A subsequent study isolated and identified hoiamide B (Figure 11h) and hoiamide C (Figure 11i) from other marine cyanobacteria [77]. Notably, that study reported that hoiamide B also promotes sodium influx into neocortical neural cells (EC50 = 3.9 μM)
3.5. Lariat-Type Cyclic Depsipeptides
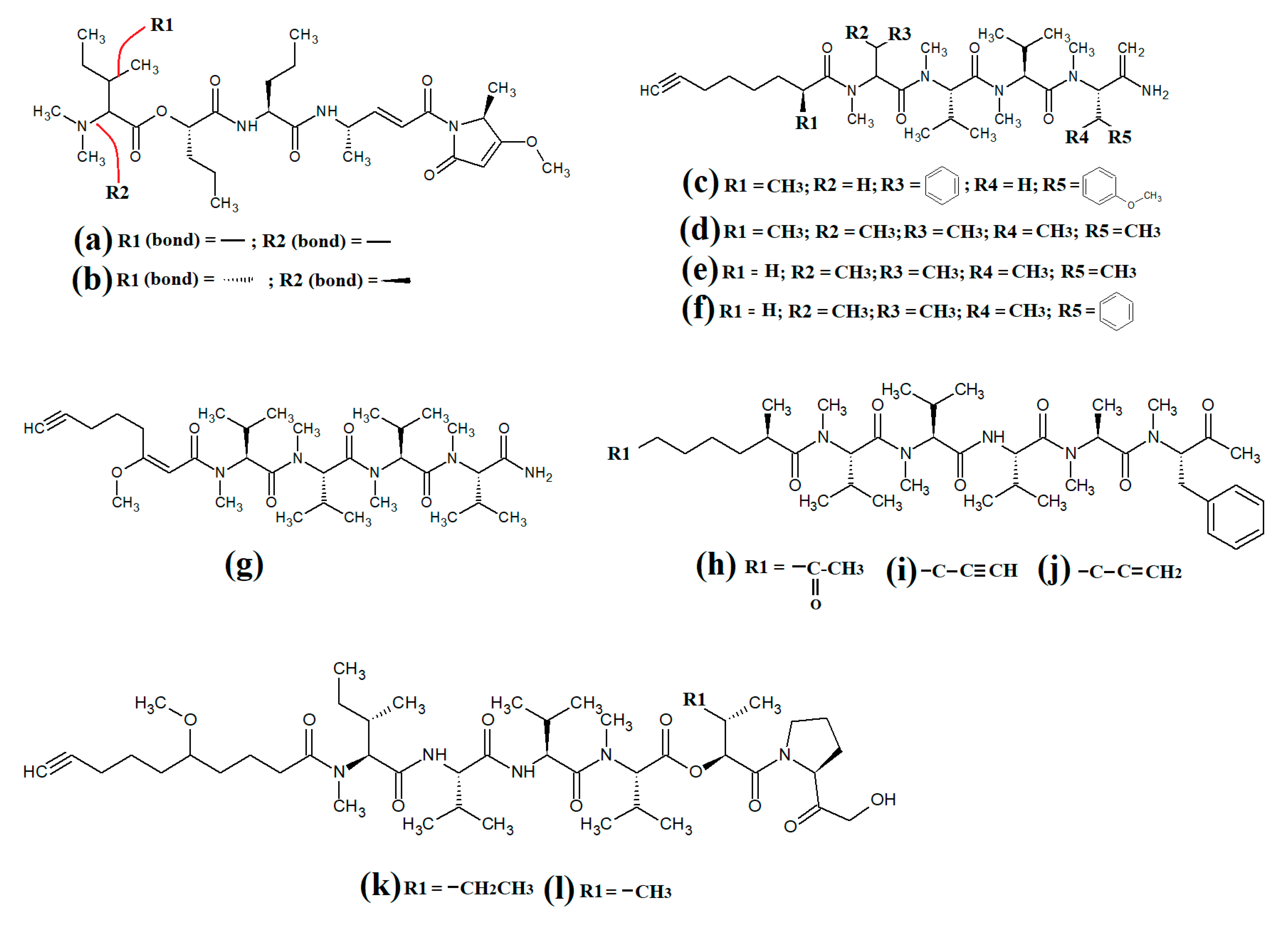
Lariat-type cyclic depsipeptides are a class of compounds with a wide range of ring sizes and tail lengths. What they all have in common is that they contain non-peptide polyketide regions within their structures [78]. Many of these compounds have relatively low polarity and therefore have good membrane permeability, allowing them to affect intracellular targets. Additionally, many compounds of this class have good serine protease inhibitory activity. The lyngbyastatins are one such class of molecules with noteworthy biological properties. Cyanobacterial-derived lynbyastatins include lynbyastatin 7 (Figure 12a), bouillomide A (Figure 12b), bouillomide B (Figure 12c), kempopeptin A (Figure 12d), kempopeptin B (Figure 12e), molassamide (Figure 12f), symplocamide A (Figure 12g), pompanopeptin A (Figure 12h) and pompanopeptin B (Figure 12i). As serine proteases are involved in multiple important processes in human health, including immunological responses and blood clotting, compounds that modulate serine protease activities may have profound effects on inflammation, cancer, cardiac health, etc.
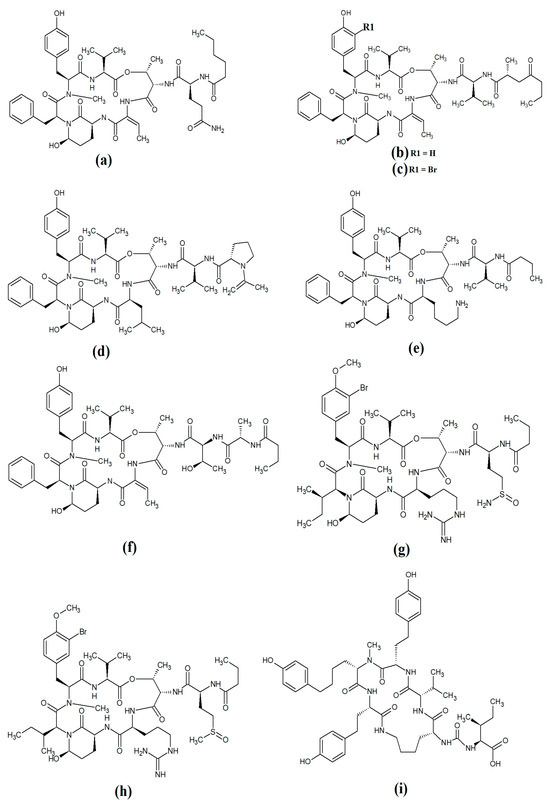
Figure 12.
Cyanobacterial lynbyastatins and kempopeptins: (a) lynbyastatin 7; (b) bouillomide A; (c) bouillomide B; (d) kempopeptin A; (e) kempopeptin B; (f) molassamide; (g) symplocamide A; (h) pompanopeptin A; (i) pompanopeptin B.
Lynbyastatin 7 was originally isolated and characterised from Lyngbya confervoides [79]. The authors of that study also reported strong elastase inhibitory activity for lynbyastatin 7 (IC50 = 8.3 nM), as well as similar activity for the structural analogues lynbyastatins 5 and 6. In another study, bouillomide A and bouillomide B were isolated from Lyngbya bouillonii and their structures were elucidated [80]. These compounds were reported to be selective inhibitors of serine proteases, with substantial inhibitory effects against elastase (IC50 = 1.2 μM for both compounds) and chymotrypsin (IC50 values of 2.6 and 0.32 μM for bouillomides A and B, respectively). Notably, their inhibitory activity was substantially lower against the other serine proteases tested.
Kemopopetins A and B were isolated from an unidentified marine cyanobacterium of the genus Lyngbya in 2008 [81]. These compounds were noteworthy inhibitors of elastase and chymotrypsin activity, although kemopopetin A had the most potent inhibitory activity (IC50 values of 0.32 and 2.6 μM against elastase and chymotrypsin, respectively). In a different study, molassamide was isolated from the cyanobacterium Dichothrix utahensis, making it the first natural product identified in any species from the genus Dichothrix [82]. Molassamide was also a good inhibitor of elastase and chymotrypsin activity (IC50 values of 32 and 234 nM, respectively).
In another study, symplocamide A was identified in an unidentified marine bacterium of the Symploca genus [83]. The structure was unusual amongst this class of compounds as it contained citrilline and N,O-dimethyl-bromo-tyrosine residues within the cyclic backbone structure. The authors of that study reported that symplocamide A was a good inhibitor of chymotrypsin and trypsin protease activity (IC50 = 0.38 and 80.2 μM, respectively). Additionally, this compound had potent cytotoxicity towards NCI-H460 human lung cancer cells (IC50 = 40 nM) and murine neuro-2a neuroblastoma cells (IC50 = 29 nM). Symplocamide A was also a noteworthy inhibitor of Plasmodium falciparum growth (IC50 = 950 nM).
Pompanopeptins A (Figure 12h) and B (Figure 12i) were isolated and characterised from Lyngbya confervoides [84]. Despite their similar names, these compounds are not structurally related, due to the presence of a N-methyl-2-amino-6-(4′-hydroxy-phenyl) hexanoic acid moiety in the cyclic backbone of pompanopeptin B. Pompanopeptin A (but not pompanopeptin B) was determined to be a selective inhibitor of trypsin, with an IC50 of 2.4 μM. Notably, with the exception of the antiprotozoal activities of some compounds discussed above, the anti-pathogenic properties of all lyngbyastatins have been largely neglected and substantially more work is required to understand their therapeutic potential.
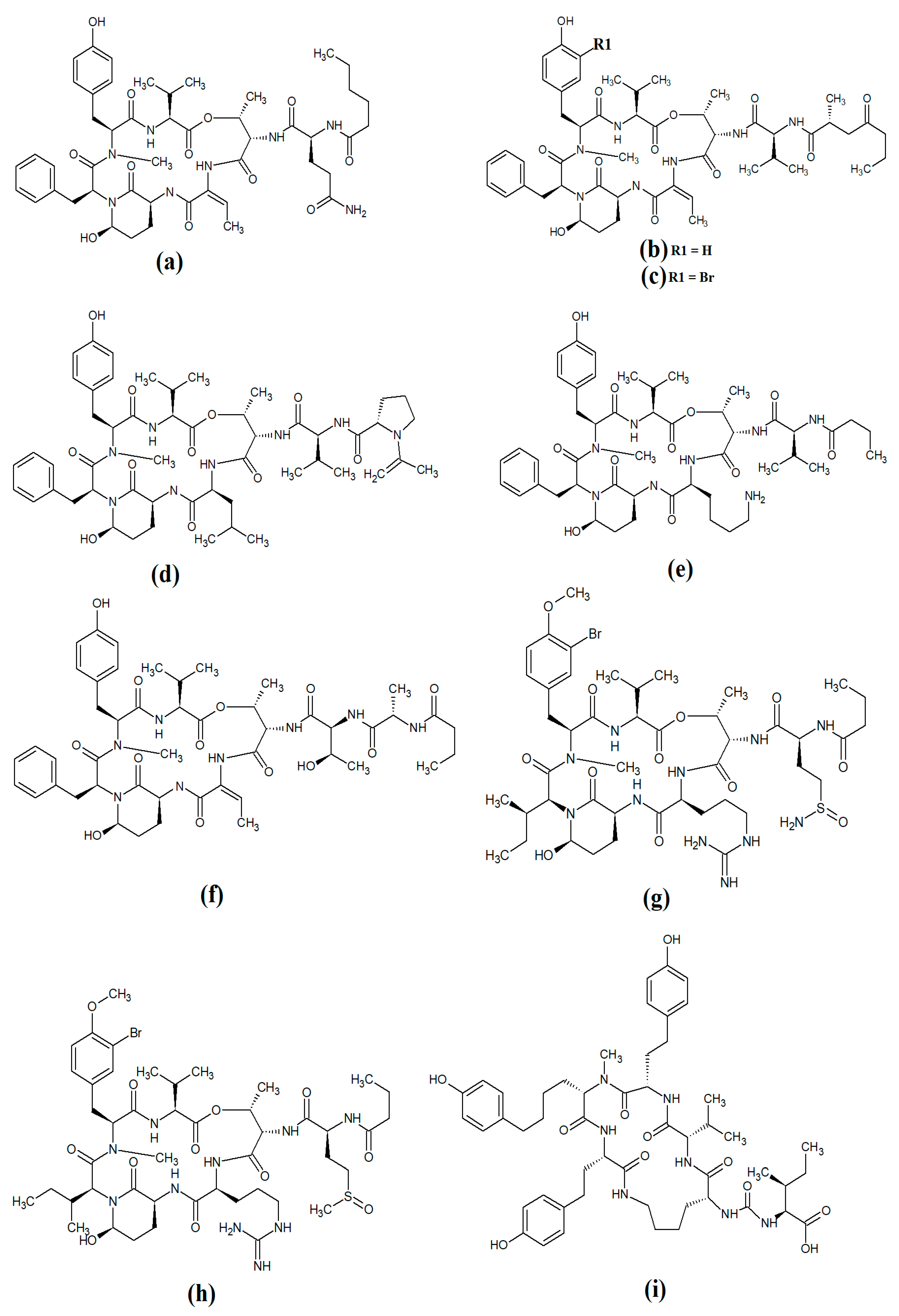
Further lariat-type cyclic depsipeptides have been isolated from Lyngbya confervoides. In particular, the structural analogues tiglicamide A (Figure 13a), tiglicamide B (Figure 13b), tiglicamide C (Figure 13c), largamide A (Figure 13d), largamide B (Figure 13e) and largamide C (Figure 13f) were identified and their structures were determined to differ by a single amino acid residue in the cyclic backbone [85]. The authors reported that these compounds were moderate inhibitors of elastase activity, with IC50 values ranging from 0.53 to 7.3 μM.
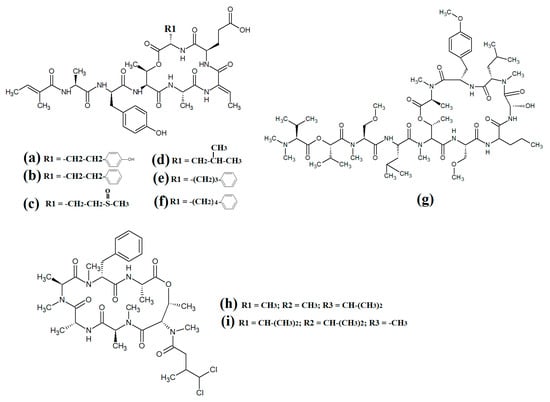
Figure 13.
Lariat-type cyanobacterial compounds: (a) tiglicamide A; (b) tiglicamide B; (c) tiglicamide C; (d) largamide A; (e) largamide B; (f) largamide C; (g) coibamide A; (h) itralamide A; (i) itralamide B.
Another study reported the isolation and characterisation of coibamide A (Figure 13g) [86]. The authors reported that the compound was highly N-methylated, with eight of the eleven amino acids in the macrocyclic group containing N-methyl moieties. Interestingly, coibamide A displayed potent cytotoxicity against human NCI-H460 lung cancer cells and murine neuro-2a cells (LC50 values < 23 nM against both cell lines). Additionally, coibamide A was screened against the 60 human cancer cell lines of the NCI panel, and the authors reported substantial toxicity against MDA-MB-231 (IC50 = 2.8 nM), LOX IMVI (IC50 = 7.4 nM), HL-60(TB) (IC50 = 7.4 nM) and SNB-75 cells (IC50 = 7.6 nM) [86]. However, we were unable to find studies that screened coibamide A against any pathogens of importance to human health.
Itralamide A (Figure 13h) and itralamide B (Figure 13i) were isolated from Lyngbya majuscula and their structures were reported [33]. Interestingly, both compounds contained a branched chlorinated group (4,4-dichloro-3-methylbutanoic acid) linked to N-methyl-threonine. The itralamides are both toxic, although itralamide B was particularly toxic towards human HEK-293 embryonic kidney cells (IC50 = 6 μM).
3.6. Cyclic Depsipeptides with Extensive Polyketide Chain
Laingolide B (Figure 14a) was isolated from the marine cyanobacteria Lyngbya bouillonii and structurally characterised [50]. Unfortunately, this compound is highly labile and biological activity testing was not undertaken in that study. Palmyrolide (Figure 14b) contains a similar cyclic structure, with extensive polyketone groups, and was isolated from mixed cyanobacterial cultures rich in Oscillatoria spp. [87]. Interestingly, palmyrolide A was a moderate inhibitor of calcium influx in cerebrocortical neurons (IC50 = 3.7 μM) via blocking of sodium channels (IC50 = 5.2 μM). In another study, alotamide A (Figure 14c) was isolated from Lyngbya bouillonii and it was determined to consist of a macrocyclic structure containing seven acetate units, as well as N-methylvaline, a thiazoline moiety and a proline, linked by a polyketide group [88]. Interestingly, alotamide A was reported to activate calcium influx in cerebrocortical neurons (IC50 = 4.2 μM).
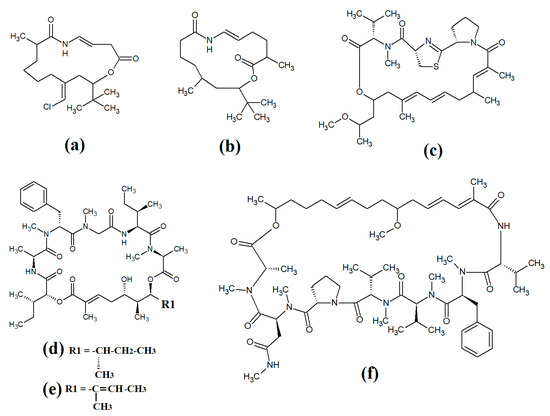
Figure 14.
Cyclic cyanobacterial depsipeptides with extensive polyketone chains: (a) laingolide B; (b) palmyrolide A (c) alotamide A; (d) lagunamide A; (e) lagunamide B; (f) malevamide E.
A related class of compounds (lagunamides) were also isolated from Lyngbya majuscula extracts and their structures were elucidated [89]. The authors of that study reported that both lagunamide A (Figure 14d) and langunamide B (Figure 14e) were strongly cytotoxic towards murine P338 leukaemia cells (IC50 values of 6.4 and 20.5 nM, respectively). These compounds also had significant antimalarial potential, with IC50 values of 0.2 and 0.9 μM against Plasmodium falciparium. Additionally, lagunamide A and lagunamide B had substantial anti-swarming activity against Pseudomonas aeruginosa, although IC50 values were not reported in that study, making comparisons difficult. Despite this wide range of promising bioactivities, most pathogens of human importance are yet to be tested using these compounds.
Malevamide E (Figure 14f) is a structural analogue of dolastatin that was isolated from the marine cyanobacterium Symploca laete-viridis [90]. This compound is a noteworthy inhibitor of calcium entry into immortalised human HEK embryonic kidney cells treated with thapsigargin. However, the mechanism of inhibition was not reported. We were unable to find studies that tested this compound against any pathogen of medicinal importance in humans. Substantially more work is required.
4. Antimicrobial Properties
4.1. Antibacterial Activity
Compounds isolated from cyanobacteria possess inhibitory activities towards multiple bacterial species, as summarised in Table 1. This is interesting given the wide structural dissimilarity between many of the molecules that have been tested. Additionally, many of the experiments were performed on agar, where purified preparations of the molecules produce a zone of inhibition, or in microdilution broth assays where minimum inhibitory concentrations (MICs) can be quantified. Together, these provide evidence of the effectiveness of molecules derived from cyanobacteria in inhibiting the growth of various pathogens.

Table 1.
Cyanobacterial natural products.
The abietane diterpenes produced MIC values of 14–22 µg/mL against S. aureus, S. epidermidis, S. typhi, V. cholerae, B. cereus, B. subtilis, E. coli and K. pneumoniae [91]. Aeruginazole A inhibits B. subtilis, S. aureus and S. epidermidis with IC50 values of 2.2 µM, although it did not inhibit E. coli [92,93]. Ambigols A–C inhibited B. megaterium in agar diffusion assays at a concentration of 100 nM [94,95]. However, in all these studies, the toxicities of the compounds were not determined. Ambiguine isonitriles are potent inhibitors of B. anthracis, M. tuberculosis and S. aureus, where MIC values as low as 78 ng/mL have been observed with a moderate toxicity of 40 µM observed against Vero cells for ambiguine G nitrile [96,97,98]. Anaephenes A, B and C inhibit S. aureus, as well as its resistant counterpart, MRSA, in addition to M. luteus and B. cereus, with low MIC values and moderate toxicity towards HCT116 cells being reported [99,100]. Anyhdrohapaloxindole A possesses moderate-to-strong activities against M. tuberculosis, M. smegmatis, S. aureus, E. coli and A. baumannii but is toxic towards Vero cells [101], while antillatoxin B is a potent inhibitor of B. cereus growth but is weaker against S. typhimurium and L. monocytogenes [102], although toxicity measurements were not conducted in this study.
Bastadin has antibacterial properties, although the affected microbial species were not identified in the relevant study, nor was the activity or toxicity of bastadin quantified [104]. Bromoanaindolone has mild inhibitory activity towards B. cereus, with an MIC value of 530 µM [107], while Mycobacteria spp. are effectively inhibited by brunsicamines A, B and C [108]. In both these studies, toxicity determinations were not conducted. Both carbamidocyclophane F and G strongly inhibit Mycobactetium tuberculosis, with lower activities towards Acinitobacter baumannii, P. aeruginosa, S. aureus, S. pneumoniae and E. faecalis [111]. Additionally, they are toxic against two different cancer cell lines. Carriebowlinol is an inhibitor of Vibrio and Fusarium spp., although its toxicity is yet to be assessed [114]. Bacillus cereus, E. coli and S. epidermidis are inhibited by comnostins A–E [115] and are highly cytotoxic towards Caco-2 and KB cells, while low MIC values (4–32 µg/mL) have been reported for cybastacines A and B against E. faecalis, E. faecium, M. abscessus, N. carnea, N, cyriacigeorgica, S. pyogenes, S. aureus and S. epidermidis, although the toxicities of these compounds were not determined [122]. The cylindrofridins A–C and cylindrocyclophanes A–C are also potent inhibitors of S. aereus, with MIC values of 1 μg/mL, although the MIC values measured were substantially higher against MRSA, S. pneumoniae, E. faecium and other bacteria [123]. The compounds were toxic towards the HaCaT keratinocyte cell line. Corioloc acid and dimorphecolic acid are active towards B. subtilis, M. flavus, S. aureus and S. epidermidis on agar, although large masses of these compounds (50–100 µg per disc) were used in the assays [124]. Unfortunately, that study did not determine an MIC, making it impossible to evaluate the potency of those compounds, or to benchmark their activity against other antibacterial molecules, and the toxicities of these compounds were not reported. Another study by Choi et al. [116] reported that the crossbyanols (B–D) produce low MIC values against both the susceptible S. aureus bacteria as well as the resistant MRSA pathogen, indicating their potential as antibiotic chemotherapies. However, crossbyanol B was toxic in brine shrimp lethality assays, with all compounds showing weak or no activity in lung cancer cell line assays. These different bioactivity profiles provide information on the safety and potential therapeutic usefulness of the four compounds.
Mycobacterium tuberculosis growth can be strongly suppressed with eucapsitrione (MIC values ranging from 3.1 to 6.4 µM), although this compound was determined to be considerably weaker against M. smegmatis, S. aureus and E. coli, and has limited cytotoxicity towards Vero cells [127]. Fischerellins A and B generate MIC values of 2–100 µM against S. aureus, M. tuberculosis, M. smegmatis and E. coli, whilst fischambiguine B is equally as effective against S. aureus, B. anthracis, M. tuberculosis and M. smegmatis [97]. Both molecules show a low level of cytocoxity towards Vero cells. Several different hapalindole compounds are moderately toxic while possessing potent activities towards a wide range of bacteria on agar, including S. aureus B. subtilis, E. coli, E. faecium, S. epidermidis S. pyogenes, S. pneumoniae, H. influenza, K. pneumoniae, P. morganii, Salmonella spp. and S. sonnei, producing zones of inhibition (ZOIs) as large as 36 mm and MIC values ranging from 0.5 to 16 µg/mL [129,130]. Kawaguchipeptins A and B mildly inhibit S. aureus growth, although their toxicities are unreported [137], while lagunamides A, B and C inhibit the swarming properties of P. aeruginosa and show strong cytotoxicity towards multiple cancer cell lines [89,138]. Laxaphycins A, B and B3 are moderately active against B. cereus, S. typhimurium and L. monocytogenes but have not been assessed for their toxicities [102]. Agar disc diffusion assays to evaluate the antibacterial activity of linoleic acid reported varying degrees of activity across the bacterial species B. subtilis, Micrococcus flavus, S. aureus and S. epidermidis when 100 µg of each compound was infused in the discs [124]. However, linoleic acid is quite nonpolar and therefore agar diffusion assays are not the best choice of assay to evaluate its activity. ϒ-Linolenic acid is generally a more effective antibacterial compound against S. aureus, E. coli, K. aerogenes, P. aeruginosa and S. typhi based on their relatively low MIC values of 2–16 μg/mL [142]. The toxicity of these molecules is as yet unknown. Shaala et al. [144] demonstrated that lyngbic acid and the malyngamides (4, A and B) inhibit M. tuberculosis, whilst other studies report that the lyngbyazothrins strongly inhibit B. subtilis and E. coli growth on agar [146,147,148]. Inhibition of B. cereus, S. aureus and S. pyogenes by malyngoloide has been reported but was not quantified, making it difficult to compare their activity to that of other natural products and clinical antibiotics [149]. The toxicity of the lyngbyazothrins is not known, while they have been determined for the other compounds using several different mammalian cell lines.
Majusculamide compounds have moderate antibacterial activity against S. typhimurium and L. monocytogenes, although they are more potent inhibitors of B. cereus [102]. These compounds are yet to be tested for their cytotoxicities. Microcystin-LR is a strong inhibitor of Mycobacterium spp. where low MIC values (60 nM–1.93 µM) have been documented against M. chelonae, M. kansaii, M. terrae and M. tuberculosis [154]. These findings are particularly promising given the seriousness of mycobacterial infections and their limited treatment options, and also given that they were found to be nontoxic against a hepatoma cell line [154]. Additionally, these compounds should be tested against drug-resistant mycobacterial strains in order to determine their effectiveness against these particularly dangerous organisms. Low levels of activity were observed for muscoride against B. subtilis in agar diffusion assays, although its toxicity was not reported [157]. The two compounds 20-nor-3α-acetoxy-abieta-5,7,9,11,13-pentaene and 20-nor-3α-acetoxy-12 hydroxy-abieta-5,7,9,11,13-pentaene were not assessed for their toxicity levels; however, they do appear to have broad-spectrum properties since they can inhibit S. aureus, S. epidermidis, S. typhi, V. cholerae, B. subtilis, B. cereus, E. coli and K. pneumoniae, with their strongest activity reported against Staphylococcus spp. [91]. Norharmane (9H-pyrido(3,4-b)indole and 4,4’-dihydroxybiphenol also showed good antibacterial activity (MIC values of 16–160 μg/mL) against E. coli, P. aeruginosa, S. aureus, B. subtilis and B. cereus [158]. Activity was also reported for noscomin against B. cereus, S. epidermidis and E. coli [159]. These compounds were not examined for their cytotoxicities. Propionibacterium acnes growth was also inhibited in agar diffusion when 50 μg of nostocionone, nostocionone D1, nostocionone D2 or nostocionone D3 was infused into discs [161], although their toxicities were not reported in this work.
Potent inhibition of S. aureus and B. subtilis was observed for the compound nostocyclyne A, with MIC values of 30–36 nM [163] indicating their potential for therapeutic use, although their toxicities still need to be measured. Low MICs (2–16 μg/mL) were also reported for nostotrebin 7 and nostolactone 7 against several bacteria, including E. faecium, B. subtilis, S. aureus, M. tuberculosis, E. aerogenes, S. typhi, P. aeruginosa and E. coli [129,164], while toxicity studies for these molecules are lacking. Pahayokolides A and B are acutely toxic in zebrafish assays However, they also inhibited the growth of B. subtilis, M. megaterium, P. aeruginosa, M. luteus, E. coli and S. epidermidis, with substantially stronger inhibition noted for Bacillus spp., with 5 μg/mL of these compounds individually producing ZOIs as large as 32 mm [167,168]. The potencies of these compounds were not determined, and the use of agar diffusion methods may not be a suitable measure of activity due to the large sizes of these molecules and thus their lowered capacity to move through the agar medium. C-Phycocyanin also inhibits B. subtilis, although it is less effective against Pseudomonas and Xanthamonus spp. [169]. This study also showed that this compound is nontoxic in a silkworm assay. Parsiguine has inhibitory properties towards S. epidermidis, while its toxicity is undetermined [170]. Phycocyanin, which was isolated from three different cyanobacterial species, showed good antibacterial activity on agar towards S. aureus, E. coli and Pseudomonas and Klebsiella spp. when using 4–100 µg of the compound and, in microdilution broth assays, yielded MIC values of 50–125 µg/mL against these bacteria [171,173]. Future studies on this compound should include an examination of its cytotoxicities. The pitipeptolides A and B are strong inhibitors of M. tuberculosis in agar diffusion assays, although MIC values were not determined in that study and only weak cytotoxicity was observed against Vero cells [174]. Similarly, pitiprolamide inhibited B. cereus growth in disc diffusion assays with a low cytotoxicity [175]. Falch and colleagues [94] reported antibacterial activity against M. luteus, B. subtilis and E. coli by protoamides, although the potency was not determined in the study and toxicity studies were not performed. Additionally, schizotrin A was shown to produce large ZOIs on agar at concentrations of 7 nM against B. subtilis in another early study [177] but again, toxicity assays were not conducted.
The compound scytoscalarol was shown to be weakly cytotoxic in a Vero cell assay and it exerted inhibitory activity towards B. anthracis, S. aureus, E. coli and M. tuberculosis, with MIC values varying between 2 and 110 µM [184]. Three tiahuramide molecules (A, B and C) are inhibitors of S. baltica, A. salmonicida, V. anguillarum, M. luteus and E. coli and have anticancer properties against neuroblastoma cells [192]. Falch et al. [193] state that they were able to demonstrate inhibition of M. luteus, B. subtilis and E. coli by tjipanazole D, but did not quantify the inhibition nor did they assess the toxicity of the compound.
4.2. Antifungal Activity
Considerable evidence has accumulated on the inhibitor effects of compounds isolated from cyanobacteria with antifungal properties. Candida albicans has been particularly well studied and is susceptible to growth inhibition by several cyanobacterial molecules. Scytonema sp. produce scytoscalarol, a sesterterpenene containing a guanidino group, which was isolated by bioactivity-guided fractionation. This compound was shown via microdilution broth assays to inhibit C. albicans, with an MIC of 4 µM [184]. Thecyanobacteria Fischerella ambigua also contains indole alkaloids, including ambiguine isonitriles, which have similar potency towards C. albicans [98]. Additionally, halopindane-related alkaloids isolated from Fischerella ambigua have noteworthy antifungal activity against C. albicans. Fischambiguine A and ambiguine P were particularly good inhibitors of C. albicans growth, with MIC values in the range 15–30 µM [97]. Both Westiellopsis sp. and Fischerella muscicola contain anhydrohapaloxindole A, which strongly inhibits the growth of C. albicans, with an MIC of 1.9 µM [101]. Calothrix fusca contains the cyclic decapeptide calophycin, which inhibits C. albicans on agar at 1.2 µg/disc and in broth at an MIC value of 1.25 µg/mL [109]. An environmental Nostoc spp. strain was found to contain carbamidocyclophane compounds that are active against C. albicans, with some producing MIC values ranging from 1 to 10 µM [111]. Other compounds showing activity towards C. albicans include lyngbyabellin B from the marine cyanobacterium Lyngbya majuscula ([145], tanikolide from Scytonema spp. [190,191] and the cyclic peptides tolybyssidins A and B, which were isolated from Tolypothrix byssoides [194]. Norharmane (9H-pyrido(3,4-b)indole) is a cyanobacterial β-carboline exometabolite from Nodularia harveyana and Nostoc insulare that possesses noteworthy inhibitory activities in microdilution broth assays towards C. albicans, with the biphenyl compound 4,4’-dihydroxybiphenol being a slightly more potent growth inhibitor [158]. However, an anthraquinone derivative, eucapsitrione, isolated from Eucapsis spp., was inactive against C. albicans when tested at a concentration of 55 µM [127].
Many compounds purified from cyanobacteria inhibit the growth of other fungal strains and/or show broad-spectrum antifungal properties. For example, Anabaena cylindrica contains the balticidins A–D which exert potent and specific antifungal activity against Candida maltosa on agar, with ZOIs of 3–12 mm at 10 µg [103]. However, that study did not determine MIC values, making comparisons with other studies difficult. Fischerella ambigua isolated from soil samples in Iran was found to contain a novel compound named parsiguine, which inhibited Candida krusei with an MIC value of 20 µg/mL [170]. Methanolic extracts prepared from the terrestrial blue-green alga, Nostoc commune, contains a lipopeptide nostofungicidine that has potent antifungal activity against Aspergillus candidus in microdilution broth assays with an MIC value of 1.6 µg/mL [160]. The allelochemical compounds fischerellins A and B identified in Ficscherella muscicola elicit full inhibition of the agronomically important fungal strains Uromyces appendiculatus and Erysiphe graminis at 250 ppm and 1000 ppm, respectively [128]. The authors of that study only tested these two relatively high concentrations of these compounds to allow comparisons with other compounds and other studies. The cyanobacterium Hassallia sp. contains hassallidins A and B, which possess MIC activities ranging from 4 to 16 µM against a number of different fungal strains, including C. albicans, C. krusei, C. glabrata, C. tropicalis, C. parapsilosis, C. neoformans and A. fumigatus [131,132]. Scytophytins (isolated from Scytonema spp.) and tolytoxins (isolated from Tolypothrix spp.) are further examples of cyanobacterial compounds with potent broad-spectrum antifungal activity, with ZOIs of up to 30 mm being observed on agar against the fungal strains S. pastorianus, N. crassa, C. albicans, P. ultimum, R. solani and S. homoeocarpa [178,179,180,181,182,183]. Whilst these ZOIs indicate potent activity, the compounds were tested at a single relatively high dose and MICs were not reported. As previously mentioned, calophycin purified from Calothrix fusca inhibits C. albicans; however, it is also has broad-spectrum capability in that it also inhibits P. notatum, Aspergillus oryzae, Saccharomyces cerevisae and Trichophyton mentagrophytes on agar and in broth at potencies that are comparable to the positive control antifungal drug amphotericin B [109]. The carmaphycins A and B, isolated from Symploca blue-green algae species, produced IC50 values of 2.5 and 2.6 nM against the S. cerevisiae 20S proteasome and was shown to be cytotoxic to colon and lung cancer cell lines with IC50 values of 6–43 µM [195].
Some compounds isolated from cyanobacteria act synergistically in inhibiting fungal growth. Ethanolic extracts of the blue-green algae Anabaena laxa contain laxaphycins, which have antifungal inhibitory activities on agar against C. albicans, A. oryzae and S. cerevisiae [139,140,141]. The activities of laxaphycins A–E vary between the compounds; however, synergistic antifungal activity was observed when the compounds were combined in assays. Similarly, lobocyclamides A and B (from Lyngbya confervoides) synergistically inhibited the growth of C. albicans and C. glabrata in disc diffusion assays, while compounds A–C all showed moderate activity against these fungal strains when used alone [143].
Whilst the studies summarised herein demonstrate the potential of cyanobacterial-derived compounds in treating fungal infections, they also highlight the need for further studies to further evaluate the therapeutic potential of these compounds. In particular, several of the previous studies tested one (or limited) concentrations of the compounds and have reported potent activity on the basis of those evaluations. These studies need to be repeated with the activity screened across a range of concentrations and MICs reported. This would allow the activity of these compounds to be benchmarked against other antifungal compounds. Additionally, several of the studies that tested antifungal activity did not test toxicity in parallel, making it difficult to evaluate the safety of the compounds.
4.3. Antiprotozoal Activity
Many compounds that have been isolated from cyanobacteria that inhibit bacterial growth are also effective at inhibiting some protozoal pathogens. Of these, the most studied protozoal organism is P. falciparum, the etiological agent of malaria tropica, which causes more than 600,000 deaths annually. Numerous drug-resistant strains have also emerged. However, the growth of P. falciparum and other protozoa can be inhibited by molecules derived from cyanobacteria. Shao et al. [105] reported IC50 values of 80–270 nM for bastimolides A and B against P. falciparum, alongside modest mammalian cell line toxicities of 2–3 µM, indicating their potential as anti-malarial chemotherapies. Similar findings were observed for calothrixin A and B [110], and for lagunamides A, B and C [89]. Additionally, carmabin is a highly effective inhibitor (MIC = 4.3 µM) of chloroquine-resistant P. falciparum [43]. Interestingly, dragonamide A and B do not inhibit P. falciparum or another parasite, Trypanosoma cruzi, but are effective inhibitors of Leishmania donovani, indicating that they may affect targets or processes that are specific to those protozoa [43,44]. Dragomabin, herbamide B and malyngolide dimer from Lyngbya majuscula also exhibit good antiparasitic activities of 4–20 µM against chloroquine-resistant P. falciparum [43,150].
The dudawalamide compounds (A–E) are good inhibitors of P. falciparum, T. cruzi and L. donovani growth, with MIC values ranging from 2.6 to 10 µM against all three parasites [126]. Trypanosoma brucei brucei is potently inhibited by both hoshinolactam and ikoamide, both of which produce MIC values in the nanomolar range [133,134], and can inhibit both the blood stage (P. falciparum) and liver stage (P. berghei) of Plasmodium spp., with lyngbyabellin A being the most potent inhibitor of both phases [136]. Phycocyanin, which was isolated from Nostoc muscorum, inhibited P. falciparum growth almost completely at 74 µg/mL [172]. The venturamides (A and B) inhibit P. falciparum growth [65], whilst the viridamides (A and B) inhibit T. cruzi and L. mexicana growth [46].
Marine cyanobacteria extracts containing compounds showing similarity to dioxanes, endoperoxides and spirocarbocyclic and spirooxindole substructures and have been also shown to inhibit the growth of Leishmania infantum, Giardia duodenalis and Trichomonas vaginalis parasites as adjudged by growth curve determinations [196], although IC50 values were not determined in that study. However, there are very few cyanobacterial compounds that have been examined for their antiparasitic activity against these protozoans. An exception is carmaphycin-17 (an analog derived from carmaphycin B from Symploca cyanobacteria), which has been shown to selectively inhibit the T. vaginalis 20S proteasome [197]. The proliferation of another parasite, Encephalitozoon cuniculi, has been reported to be inhibited by up to 50% by sulphated polysaccharides that were isolated from the cyanobacteria Arthrospira platensis [198,199].
4.4. Antiviral Activity
Substantially fewer studies have examined the antiviral activities of cyanobacterial compounds against viral pathogens than against the other classes of pathogens. Several reports have highlighted the potential of cyanobacterial compounds to treat viral diseases, although the range of viruses screened remains relatively narrow. Interestingly, antiviral activity has been most extensively studied against human immunodeficiency virus (HIV-1). Noteworthy anti-HIV activity was reported for the sulphated polysaccharide calcium spirulan, which was isolated from the marine cyanobacterium Spirulina platensis [112]. Indeed, an IC50 value of 9.3 μg/mL was determined in that study by determining viral release assays. This activity compared favourably to that of the dextran sulphate positive control (9.6 μg/mL) in that study. The same study also examined the effects of calcium spirulan on CD4 viability in HIV-infected cells, and the effects on HIV-induced syncytium formation, with noteworthy effects reported for all assays. Unfortunately, the toxicity of calcium spirulan was not evaluated in the same study, making it impossible to determine a therapeutic index (TI). However, a different study reported LC50 values of 2900–7900 μg/mL against several eukaryotic cell lines [113], indicating a therapeutic index of 300–800. At these high TIs, it is likely that calcium spirulan would be safe to use at therapeutic doses, although these studies should be repeated to test the antiviral activities and toxicity in parallel, within the same study. Notably, the Rechter et al. study [113] also screened calcium spirulan against human cytomegalovirus (HCMV), poliovirus and herpes simplex virus (HSV) and reported noteworthy activity (IC50 values 0.92–23 μg/mL), indicating that this compound is effective against a broad spectrum of viral pathogens. Further studies are required to evaluate the effects of calcium spirulan against other viruses.
Interestingly, several other sulphur-containing cyanobacterial compounds also have good anti-HIV activity. In one study, an uncharacterised sulpholipid fraction isolated from Lyngbya lagerhimii and Phormidium tenue had noteworthy anti-HIV activity between 1 and 100 μg/mL [188]. However, IC50 values were not reported in that study, making it difficult to compare the activity with other studies. Furthermore, that study did not screen these lipids for toxicity. Future studies are therefore required to evaluate its safety for therapeutic use. A different study reported similar antiviral activity for a similar sulfoglycolipid against HIV, with IC50 values as low as 24 nM [189]. The authors also reported that study determined that this compound inhibited the activity of DNA polymerase and postulated that the anti-HIV activity may be due to inhibition of HIV reverse transcriptase, although this has yet to be confirmed.
Microvirin (isolated from Microcystis aeruginosa) was also a potent inhibitor of HIV replication, with IC50 values in the range 2–12 nM against both HIV-1 and HIV-2 [155,156]. Notably, microviron was reported to be nontoxic against MT-4 and MVN T cells in those studies at concentrations up to 7 μM. These results equate to Tis of approximately 1000, indicating the safety of this compound for therapeutic use in the treatment of HIV. Another study reported good anti-HIV activity for scytovirin (isolated from Scytonema varium) against several HIV strains, with IC50 values 0.3–22 nM [186]. Interestingly, other studies have also reported scytovirin to have good inhibitory activity against several other serious human viral pathogens, including Ebola virus, Marburg virus and hepatitis C virus, with IC50 values between approximately 3 and 96 nM [117,185,187]. Furthermore, the toxicity was also evaluated in those studies, with LC50 values > 400 nM, indicating its safety for therapeutic use. Similarly, cyanovirin (isolated from Nostoc ellipsosporum) was also evaluated against HIV and reported to inhibit HIV cell entry via binding to HIV gp120 protein [118]. Other studies have also reported cyanovirin to inhibit the replication of Ebola virus, Marburg virus, hepatitis C, influenza and parainfluenza, with IC50 values as low as 50 nM [117,119,120,121]. None of these studies screened this compound for toxicity. Further studies are therefore required to determine the safety of cyanovirin for therapeutic use.
The antiviral effects of several cyanobacterial compounds have also been screened against herpes simplex virus (HSV). Three bauerines (A–C) isolated from Dichotrix baueriana were reported to have noteworthy anti-HSV-2 activity (IC50 = 3 μg/mL) [106]. That study also evaluated toxicity in LoVo cells and reported an LC50 of 5 μg/mL. This equates to a TI of substantially less than 2, which indicates that this compound may not be safe for treatment of HSV-2. Similar results were reported for a novel lectin isolated from Ocillatoria acuminate and Ocillatoria agarghii, with IC50 values in the range 90–130 μg/mL [165,166]. However, the LC50 values of 107 and 254 μg/mL against Huh-7 and MCF-7 cells, respectively, indicate that this compound is toxic and highlight the need for further studies to evaluate its safety for therapeutic use. In contrast, substantially better therapeutic potential was reported for nostoflan (isolated from Nostoc flagelliforme) against both HSV-1 and HSV-2 (IC50 values 0.4–100 μg/mL) [162]. Additionally, the authors of that study reported nostoflan to be nontoxic, with LC50 values 5–10 mg/mL. Thus, nostoflan is likely to be a substantially better antiviral option for treating HSV infections, although in vivo studies are required to further evaluate is potential.
Whilst the antiviral studies reviewed herein highlight the potential of some cyanobacterial compounds as antiviral therapeutics, they also highlight the substantial amount of work required. The compounds tested for antiviral activity represent only a small percentage of the cyanobacterial compounds identified to date. Furthermore, these compounds have only been tested against a limited panel of viral pathogens, and with a few notable exceptions, the antiviral mechanism(s) remain to be determined. Additionally, all of these reports have screened the compounds using in vitro assays and therefore do not account for the bioavailability of the compounds. Where noteworthy antiviral activities and Tis have been determined, the efficacy should also be verified in in vivo systems.
5. Conclusions
The development of multiple-drug-resistant pathogens has highlighted the need to develop new antimicrobial therapies. Much of the antibiotic development pipeline of new antibiotics over the last century has relied on studies evaluating bacteria (particularly soil bacteria) and fungi for compounds with antibacterial activity. In contrast, cyanobacterial compounds have been relatively neglected as antimicrobial therapies and substantially more work is warranted. Multiple novel compounds with antibacterial, antifungal, antiprotozoal and antiviral activity have already been reported. However, substantially more work is required to thoroughly evaluate the potency of these compounds, and to evaluate their safety for therapeutic use. Additionally, many more cyanobacterial species and countless strains are yet to be evaluated for similar activities, and where relevant, the noteworthy compounds identified. This review summarises the compounds that have been evaluated and highlights gaps in the literature, with the aim of stimulating interest in this field and focusing future studies.
Author Contributions
Conceptualization, I.E.C.; formal analysis, I.E.C. and M.J.C.; data curation, I.E.C. and M.J.C.; writing—original draft preparation, I.E.C. and M.J.C.; writing—review and editing, I.E.C. and M.J.C.; project administration, I.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to Griffith University for providing administrative and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schopf, W.; Packer, B. Early Archean (3.3-billion to 3.5 billion year old) microfossils from Warrawoona group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. Introduction to the cyanobacteria. In Ecology of Cyanobacteria II 2012; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar]
- Abed, R.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T. Pharmaceutical agents from filamentous marine cyanobacteria. Drug Discov. 2013, 18, 863–871. [Google Scholar] [CrossRef]
- Saeed, M.U.; Hussain, N.; Shahbaz, A.; Hameed, T.; Iqbal, H.M.; Bilal, M. Bioprospecting microalgae and cyanobacteria for biopharmaceutical applications. J. Basic. Microbiol. 2022, 62, 1110–1124. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The diversity of cyanobacterial toxins on structural characterization, distribution and identification: A systematic review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef]
- Mundt, S.; Kreitlow, S.; Nowotny, A.; Effmert, U. Biochemical and pharmacological investigations of selected cyanobacteria. Int. J. Hyg. Environ. Health 2001, 203, 327–334. [Google Scholar] [CrossRef]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef]
- Bláha, L.; Babica, P.; Marsálek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of microcystins and nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; Volume 20, pp. 526–537. [Google Scholar]
- Ruebhart, D.R.; Wickramasinghe, W.; Cock, I.E. Protective efficacy of the antioxidants vitamin E and Trolox against Microcystis aeruginosa and microcystin-LR in Artemia franciscana nauplii. J. Toxicol. Environ. Health Part A 2009, 72, 1567–1575. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Shaw, G.R.; Seawright, A.A.; Moore, M.R.; Lam, P.K. Cylindrospermopsin, a cyanobacterial alkaloid: Evaluation of its toxicologic activity. Ther. Drug Monit. 2000, 22, 89–92. [Google Scholar] [CrossRef]
- Shen, X.; Lam, P.K.; Shaw, G.R.; Wickramasinghe, W. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Toxicon 2002, 40, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Humpage, A.R. Preliminary evidence for in vivo tumour initiation by oral administration of extracts of the blue-green alga Cylindrospermopsis raciborskii containing the toxin cylindrospermopsin. Environ. Toxicol. 2001, 16, 192–195. [Google Scholar] [CrossRef]
- Wiese, M.; D’agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.J.; Main, B.J.; Samardzic, K. Cyanobacterial neurotoxins: Their occurrence and mechanisms of toxicity. Neurotox. Res. 2018, 33, 168–177. [Google Scholar] [CrossRef]
- Matsunaga, S.; Moore, R.E.; Niemczura, W.P.; Carmichael, W.W. Anatoxin-a (s), a potent anticholinesterase from Anabaena flosaquae. J. Am. Chem. Soc. 1989, 111, 8021–8023. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Marner, F.J.; Moore, R.E. Seaweed dermatitis: Structure of lyngbyatoxin A. Science 1979, 204, 193–195. [Google Scholar] [CrossRef]
- Osborne, N.J.; Webb, P.M.; Shaw, G.R. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ. Int. 2001, 27, 381–392. [Google Scholar] [CrossRef]
- Nogle, L.M.; Okino, T.; Gerwick, W.H. Antillatoxin B, a neurotoxic lipopeptide from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 983–985. [Google Scholar] [CrossRef]
- Orjala, J.; Nagle, D.G.; Hsu, V.; Gerwick, W.H. Antillatoxin: An exceptionally ichthyotoxic cyclic lipopeptide from the tropical cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 1995, 117, 8281–8282. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; de Marsac, N.T. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef]
- Caro-Diaz, E.J.; Valeriote, F.A.; Gerwick, W.H. Highly convergent total synthesis and assignment of absolute configuration of majusculamide D, a potent and selective cytotoxic metabolite from Moorea sp. Organ. Lett. 2019, 21, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.V.; Sorrels, C.M.; Gerwick, W.H. Cloning and biochemical characterization of the hectochlorin biosynthetic gene cluster from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhao, P.; Quan, C.; Zhao, Z.; Gao, W.; Li, J.; Zu, X.; Fu, D.; Feng, S.; Bai, X.; et al. Cyanobacteria-derived peptide antibiotics discovered since 2000. Peptides 2018, 107, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, G.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Nagle, D.G.; Biggs, J.; Park, P.U.; Paul, V.J. Tumonoic acids, novel metabolites from a cyanobacterial assemblage of Lyngbya majuscula and Schizothrix calcicola. J. Nat. Prod. 1999, 62, 464–467. [Google Scholar] [CrossRef]
- Clark, B.R.; Engene, N.; Teasdale, M.E.; Rowley, D.C.; Matainaho, T.; Valeriote, F.A.; Gerwick, W.H. Natural products chemistry and taxonomy of the marine cyanobacterium Blennothrix cantharidosmum. J. Nat. Prod. 2008, 71, 1530–1537. [Google Scholar] [CrossRef]
- Engene, N.; Choi, H.; Esquenazi, E.; Byrum, T.; Villa, F.A.; Cao, Z.; Murray, T.F.; Dorrestein, P.C.; Gerwick, L.; Gerwick, W.H. Phylogeny-guided isolation of ethyl tumonoate A from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 1737–1743. [Google Scholar] [CrossRef]
- Tan, L.T.; Chang, Y.Y.; Ashootosh, T. Besarhanamides A and B from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2008, 69, 2067–2069. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Vansach, T.; Yoshida, W.Y.; Sakamoto, B.; Pörzgen, P.; Horgen, F.D. Halogenated fatty acid amides and cyclic depsipeptides from an eastern Caribbean collection of the cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2009, 72, 1573–1578. [Google Scholar] [CrossRef]
- Gross, H.; McPhail, K.L.; Goeger, D.E.; Valeriote, F.A.; Gerwick, W.H. Two cytotoxic stereoisomers of malyngamide C, 8-epi-malyngamide C and 8-O-acetyl-8-epi-malyngamide C, from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 1729–1735. [Google Scholar] [CrossRef]
- Kwan, J.C.; Teplitski, M.; Gunasekera, S.P.; Paul, V.J.; Luesch, H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscule. J. Nat. Prod. 2010, 73, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Reinscheid, U.M.; Gerwick, W.H.; Gross, H. The structure elucidation of isomalyngamide K from the marine cyanobacterium Lyngbya majuscula by experimental and DFT computational methods. J. Molec. Struct. 2011, 989, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Malloy, K.L.; Villa, F.A.; Engene, N.; Matainaho, T.; Gerwick, L.; Gerwick, W.H. Malyngamide 2, an oxidized lipopeptide with nitric oxide inhibiting activity from a Papua New Guinea marine cyanobacterium. J. Nat. Prod. 2011, 74, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Owle, C.S.; Montaser, R.; Luesch, H.; Paul, V.J. Malyngamide 3 and cocosamides A and B from the marine cyanobacterium Lyngbya majuscula from Cocos Lagoon, Guam. J. Nat. Prod. 2011, 74, 871–876. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; Goeger, D.; Gerwick, W.H. Mitsoamide: A cytotoxic linear lipopeptide from the Madagascar marine cyanobacterium Geitlerinema sp. Pure Appl. Chem. 2007, 79, 593–602. [Google Scholar] [CrossRef]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Ureña, L.D.; Kyle, D.E.; Gerwick, W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009, 72, 14–17. [Google Scholar] [CrossRef]
- Taori, K.; Liu, Y.; Paul, V.J.; Luesch, H. Combinatorial strategies by marine cyanobacteria: Symplostatin 4, an antimitotic natural dolastatin 10/15 hybrid that synergizes with the coproduced HDAC inhibitor largazole. ChemBioChem 2009, 10, 1634–1639. [Google Scholar] [CrossRef]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef]
- McPhail, K.L.; Correa, J.; Linington, R.G.; González, J.; Ortega-Barría, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef]
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.D.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Della Togna, G.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A− C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef]
- Simmons, T.L.; Engene, N.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, L.; Gerwick, W.H. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 2008, 71, 1544–1550. [Google Scholar] [CrossRef]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins A− C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.; Kalbacher, H. Cathepsin E: A mini review. Biochem. Biophys. Res. Commun. 2008, 367, 517–522. [Google Scholar] [CrossRef]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The key role of lysosomal protease cathepsins in viral infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef]
- Matthew, S.; Salvador, L.A.; Schupp, P.J.; Paul, V.J.; Luesch, H. Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam. J. Nat. Prod. 2010, 73, 1544–1552. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Fukazawa, H.; Suenaga, K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngba sp.: Isolation, stereostructure, and biological activity. Org. Lett. 2009, 11, 5062–5065. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Teruya, T.; Fukazawa, H.; Suenaga, K. Revised structure and structure–activity relationship of bisebromoamide and structure of norbisebromoamide from the marine cyanobacterium Lyngbya sp. Tetrahedron 2011, 67, 990–994. [Google Scholar] [CrossRef]
- Li, W.; Yu, S.; Jin, M.; Xia, H.; Ma, D. Total synthesis and cytotoxicity of bisebromoamide and its analogues. Tetrahedron Lett. 2011, 52, 2124–2127. [Google Scholar] [CrossRef]
- Sumiya, E.; Shimogawa, H.; Sasaki, H.; Tsutsumi, M.; Yoshita, K.i.; Ojika, M.; Suenaga, K.; Uesugi, M. Cell-Morphology Profiling of a natural product library identifies bisebromoamide and miuraenamide A as actin filament stabilizers. ACS Chem. Biol. 2011, 6, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Taevernier, L.; Wynendaele, E.; Gevaert, B.; De Spiegeleer, B. Chemical classification of cyclic depsipeptides. Curr. Protein Pept. Sci. 2017, 18, 425–452. [Google Scholar] [CrossRef] [PubMed]
- Meickle, T.; Gunasekera, S.P.; Liu, Y.; Luesch, H.; Paul, V.J. Porpoisamides A and B, two novel epimeric cyclic depsipeptides from a Florida Keys collection of Lyngbya sp. Bioorg. Med. Chem. 2011, 19, 6576–6580. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.; Tan, L.T. Hantupeptin A, a cytotoxic cyclic depsipeptide from a Singapore collection of Lyngbya majuscula. J. Nat. Prod. 2009, 72, 29–32. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.; Tan, L.T. Hantupeptins B and C, cytotoxic cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 307–311. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a cyclic depsipeptide from a Palmyra Atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Ritson-Williams, R.; Paul, V.J. Carriebowmide, a new cyclodepsipeptide from the marine cyanobacterium Lyngbya polychroa. J. Nat. Prod. 2008, 71, 2060–2063. [Google Scholar] [CrossRef]
- Montaser, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Pitiprolamide, a proline-rich dolastatin 16 analogue from the marine cyanobacterium Lyngbya majuscula from Guam. J. Nat. Prod. 2011, 74, 109–112. [Google Scholar] [CrossRef]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef]
- Bonnard, I.; Rolland, M.; Salmon, J.M.; Debiton, E.; Barthomeuf, C.; Banaigs, B. Total structure and inhibition of tumor cell proliferation of laxaphycins. J. Med. Chem. 2007, 50, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
- Maru, N.; Ohno, O.; Uemura, D. Lyngbyacyclamides A and B, novel cytotoxic peptides from marine cyanobacteria Lyngbya sp. Tetrahedron Lett. 2010, 51, 6384–6387. [Google Scholar] [CrossRef]
- Linington, R.G.; González, J.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H. Venturamides A and B: Antimalarial constituents of the Panamanian marine cyanobacterium Oscillatoria sp. J. Nat. Prod. 2007, 70, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Kirchherr, J.L.; Sung, J.A.; Clutton, G.; Sholtis, K.; Xu, Y.; Allard, B.; Stuelke, E.; Kashuba, A.D.; Kuruc, J.D.; et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J. Clin. Investig. 2017, 127, 3126–3135. [Google Scholar] [CrossRef]
- Liu, Y.; Salvador, L.A.; Byeon, S.; Ying, Y.; Kwan, J.C.; Law, B.K.; Hong, J.; Luesch, H. Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J. Pharmacol. Exper. Ther. 2010, 335, 351–561. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine Cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a potent cytotoxic cyclodepsipeptide from Papua New Guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a cytotoxic peptolide from a Guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116. [Google Scholar] [CrossRef]
- Tidgewell, K.; Engene, N.; Byrum, T.; Media, J.; Doi, T.; Valeriote, F.A.; Gerwick, W.H. Evolved diversification of a modular natural product pathway: Apratoxins F and G, two cytotoxic cyclic depsipeptides from a Palmyra collection of Lyngbya bouillonii. ChemBioChem 2010, 11, 1458–1466. [Google Scholar] [CrossRef]
- Kwan, J.C.; Rocca, J.R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Total structure determination of grassypeptolide, a new marine cyanobacterial cytotoxin. Org. Lett. 2008, 10, 789–792. [Google Scholar] [CrossRef]
- Kwan, J.C.; Ratnayake, R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Grassypeptolides A− C, cytotoxic bis-thiazoline containing marine cyclodepsipeptides. J. Org. Chem. 2010, 75, 8012–8023. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Thimmaiah, M.; Shaala, L.A.; Hau, A.M.; Malmo, J.M.; Ishmael, J.E.; Youssef, D.T.; McPhail, K.L. Cyclic depsipeptides, grassypeptolides D and E and Ibu-epidemethoxylyngbyastatin 3, from a Red Sea Leptolyngbya cyanobacterium. J. Nat. Prod. 2011, 74, 1677–1685. [Google Scholar] [CrossRef]
- Popplewell, W.L.; Ratnayake, R.; Wilson, J.A.; Beutler, J.A.; Colburn, N.H.; Henrich, C.J.; McMahon, J.B.; McKee, T.C. Grassypeptolides F and G, cyanobacterial peptides from Lyngbya majuscula. J. Nat. Prod. 2011, 74, 1686–1691. [Google Scholar] [CrossRef]
- Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Hoiamide a, a sodium channel activator of unusual architecture from a consortium of two Papua New Guinea cyanobacteria. Chem. Biol. 2009, 16, 893–906. [Google Scholar] [CrossRef]
- Choi, H.; Pereira, A.R.; Cao, Z.; Shuman, C.F.; Engene, N.; Byrum, T.; Matainaho, T.; Murray, T.F.; Mangoni, A.; Gerwick, W.H. The hoiamides, structurally intriguing neurotoxic lipopeptides from Papua New Guinea marine cyanobacteria. J. Nat. Prod. 2010, 73, 1411–1421. [Google Scholar] [CrossRef]
- Kelly, C.N.; Townsend, C.E.; Jain, A.N.; Naylor, M.R.; Pye, C.R.; Schwochert, J.; Lokey, R.S. Geometrically diverse lariat peptide scaffolds reveal an untapped chemical space of high membrane permeability. J. Am. Chem. Soc. 2020, 143, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.K.; Parrish, S.M.; Yoshida, W.; Schupp, P.J.; Schils, T.; Williams, P.G. Depsipeptides from a Guamanian marine cyanobacterium, Lyngbya bouillonii, with selective inhibition of serine proteases. Tetrahedron Lett. 2010, 51, 6718–6721. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Kempopeptins A and B, serine protease inhibitors with different selectivity profiles from a marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 2008, 71, 1625–1629. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Miller, M.W.; Kwan, J.C.; Luesch, H.; Paul, V.J. Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J. Nat. Prod. 2010, 73, 459–462. [Google Scholar] [CrossRef]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef]
- Matthew, S.; Ross, C.; Paul, V.J.; Luesch, H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron 2008, 64, 4081–4089. [Google Scholar] [CrossRef]
- Matthew, S.; Paul, V.J.; Luesch, H. Tiglicamides A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry 2009, 70, 2058–2063. [Google Scholar] [CrossRef]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; McPhail, K.L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Amer. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Cao, Z.; Engene, N.; Soria-Mercado, I.E.; Murray, T.F.; Gerwick, W.H. Palmyrolide A, an unusually stabilized neuroactive macrolide from Palmyra Atoll cyanobacteria. Org. Lett. 2010, 12, 4490–4493. [Google Scholar] [CrossRef] [PubMed]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef]
- Adams, B.; Pörzgen, P.; Pittman, E.; Yoshida, W.Y.; Westenburg, H.E.; Horgen, F.D. Isolation and structure determination of malevamide E, a dolastatin 14 analogue, from the marine cyanobacterium Symploca laete-viridis. J. Nat. Prod. 2008, 71, 750–754. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Flores, A.M.; Solis, R.V.; Jimenez, J.C. Two new antibacterial norbietane diterpenoids from cyanobacterium Micrococcus lacustris. J. Nat. Med. 2008, 62, 328–331. [Google Scholar] [CrossRef]
- Raveh, A.; Carmeli, S. Aeruginazole A, a novel thiazole-containing cyclopeptide from the cyanobacterium Microcystis sp. Org. Lett. 2010, 12, 3536–3539. [Google Scholar] [CrossRef]
- Bruno, P.; Pena, S.; Just-Baringo, X.; Albericio, F.; Alvarez, M. Total synthesis of aeruginazole A. Org. Lett. 2011, 13, 4648–4651. [Google Scholar] [CrossRef]
- Falch, B.S.; Konig, G.M.; Wright, A.D.; Sticher, O.; Rüegger, H.; Bernardinelli, G. Ambigol A and B: New biologically active polychlorinated aromatic compounds from the terrestrial blue-green alga Fischerella ambigua. J. Org. Chem. 1993, 58, 6570–6575. [Google Scholar] [CrossRef]
- Wright, A.D.; Papendorf, O.; König, G.M. Ambigol C and 2, 4-dichlorobenzoic acid, natural products produced by the terrestrial cyanobacterium Fischerella ambigua. J. Nat. Prod. 2005, 68, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Raveh, A.; Carmeli, S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp collected in Israel. J. Nat. Prod. 2007, 70, 196–201. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Santarsiero, B.D.; Franzblau, S.G.; Orjala, J. Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Krunic, A.; Chlipala, G.; Orjala, J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009, 72, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Brumley, D.; Spencer, K.A.; Gunasekera, S.P.; Sauvage, T.; Biggs, J.; Paul, V.J.; Luesch, H.Z. Isolation and characterization of anaephenes A-C, alkylphenols from a filamentous cyanobacterium (Hormoscilla sp., Oscillatoriales). J. Nat. Prod. 2018, 81, 2716–2721. [Google Scholar] [CrossRef]
- Kukla, D.L.; Canchola, J.; Mills, J.J. Synthesis of the cyanobacterial antibiotics anaephenes A and B. J. Nat. Prod. 2020, 83, 2036–2040. [Google Scholar] [CrossRef]
- Kim, H.; Lantvit, D.; Hwang, C.H.; Kroll, D.J.; Swanson, S.M.; Franzblau, S.G.; Orjala, J. Indole alkaloids from two cultured cyanobacteria, Westiellopsis sp. and Fischerella muscicola. Bioorg. Med. Chem. 2012, 20, 5290–5295. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Vansach, T.; Horgen, F.D.; Lacroix, M. Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem. 2016, 199, 114–118. [Google Scholar] [CrossRef]
- Bui, T.H.; Wray, V.; Nimtz, M.; Fossen, T.; Preisitsch, M.; Schröder, G.; Wende, K.; Heiden, S.E.; Mundt, S. Balticidins A–D, antifungal hassallidin-like lipopeptides from the Baltic Sea cyanobacterium Anabaena cylindrical Bio33. J. Nat. Prod. 2014, 77, 1287–1296. [Google Scholar] [CrossRef]
- Miao, S.; Anderson, R.J.; Allen, T.M. Cytotoxic metabolites from the sponge Ianthella basta collected in Papua New Guinea. J. Nat. Prod. 1990, 53, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.L.; Linington, R.G.; Balunas, M.J.; Centeno, A.; Boudreau, P.; Zhang, C.; Engene, N.; Spadafora, C.; Mutka, T.S.; Kyle, D.E.; et al. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta. J. Org. Chem. 2015, 80, 7849–7855. [Google Scholar] [CrossRef]
- Larsen, L.K.; Moore, R.E.; Patterson, G.M.L. Beta-carbolines from the blue-green alga Dichothrix baueriana. J. Nat. Prod. 1994, 57, 419–421. [Google Scholar] [CrossRef]
- Volk, R.B.; Girreser, U.; Al-Refai, M.; Laatsch, H. Bromoanaindolone, a novel antimicrobial exometabolite from the cyanobacterium Anabaena constricta. Nat. Prod. Res. 2009, 23, 607–612. [Google Scholar] [CrossRef]
- Shao, C.L.; Mou, X.F.; Cao, F.; Spadafora, C.; Glukhov, E.; Gerwick, L.; Wang, C.Y.; Gerwick, W.H. Bastimolide B, an antimalarial 24-membered marine macrolide possessing a tert-butyl group. J. Nat. Prod. 2018, 81, 211–215. [Google Scholar] [CrossRef]
- Moon, S.S.; Chen, J.L.; Moore, R.E.; Patterson, G.M.L. Calophycin, a fungicidal cyclic decapeptide from the terrestrial blue-green alga Calothrix fusca. J. Org. Chem. 1992, 57, 1097–1103. [Google Scholar] [CrossRef]
- Rickards, R.W.; Rothschild, J.M.; Willis, A.C.; de Chazal, N.M.; Kirk, J.; Kirk, K.; Saliba, K.J.; Smith, G.D. Calothrixins A and B, novel pentacyclic metabolites from Calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 1999, 55, 13513–13520. [Google Scholar] [CrossRef]
- Luo, S.; Kang, H.S.; Krunic, A.; Chlipala, G.E.; Cai, G.; Chen, W.L.; Franzblau, S.G.; Swanson, S.M.; Orjala, J. Carbamidocyclophanes F and G with anti-Mycobacterium tuberculosis activity from the cultured freshwater cyanobacterium Nostoc sp. Tetrahedron Lett. 2014, 55, 686–689. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I.A. Natural sulphated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS. Res. Hum. Retroviruses 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Rechter, S.; König, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dörnenburg, H.; Walter, C.; Marschall, M. Antiviral activity of Arthrospira-derived spirulan-like substances. Antiviral Res. 2006, 72, 197–206. [Google Scholar] [CrossRef]
- Soares, A.R.; Engene, N.; Gunasekera, S.P.; Sneed, J.M.; Paul, V.J. Carriebowlinol, an antimicrobial tetrahydroquinolinol from an assemblage of marine cyanobacteria containing a novel taxon. J. Nat. Prod. 2015, 78, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Orjala, J.; Sticher, O. A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 1999, 62, 502–503. [Google Scholar] [CrossRef]
- Choi, H.; Engene, N.; Smith, J.E.; Preskitt, L.B.; Gerwick, W.H. Crossbyanols A–D, toxic brominated polyphenyl ethers from the Hawaiian bloom-forming Cyanobacterium Leptolyngbya crossbyana. J. Nat. Prod. 2010, 73, 517–522. [Google Scholar] [CrossRef]
- Garrison, A.R.; Giomarelli, B.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.H.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antiviral Res. 2014, 112, 1–7. [Google Scholar] [CrossRef]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple antiviral activities of cyanovirin-N: Blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.G.; O’Keefe, B.R.; Bray, M.; Sanchez, A.; Gronenborn, A.M.; Boyd, M.R. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antiviral Res. 2003, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, A.H.; Pérez, V.T.; Corral, S.M.; Valencia, D.F.R.; Quintana, A.M.; Doménech, M.O.; Sánchez, Á.R. Cybastacines A and B: Antibiotic sesterterpenes from a Nostoc sp. cyanobacterium. J. Nat. Prod. 2018, 81, 410–413. [Google Scholar] [CrossRef]
- Preisitsch, M.; Heiden, S.E.; Beerbaum, M.; Niedermeyer, T.H.J.; Schneefeld, M.; Herrmann, J.; Kumpfmüller, J.; Thürmer, A.; Neidhardt, I.; Wiesner, C.; et al. Effects of halide ions on the carbamidocyclophane biosynthesis in nostoc sp. CAVN2. Mar. Drugs 2016, 14, 21. [Google Scholar] [CrossRef]
- Mundt, S.; Kreitlow, S.; Jansen, R. Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. J. Appl.Phycol. 2003, 15, 263–267. [Google Scholar] [CrossRef]
- Stewart, J.B.; Bomemann, V.; Chen, J.L.; Moore, R.E.; Caplan, F.R.; Karuso, H.; Larsen, L.K.; Patterson, G.M. Cytotoxic, fungicidal nucleosides from blue-green algae belonging to the Scytonemataceae. J. Antibiot. 1988, 41, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Almaliti, J.K.L.; Malloy, E.; Glukhov, C.; Spadafora, M.; Gutierrez, W.H.; Gerwick, A.-D. Dudawalamides, antiparasitic cyclic depsipeptides from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2017, 80, 1827e1836. [Google Scholar] [CrossRef]
- Sturdy, M.; Krunic, A.; Cho, S.; Franzblau, S.; Orjala, J. Eucapsitrione, an anti-Mycobacterium tuberculosis anthraquinone derivative from the cultured freshwater cyanobacterium Eucapsis sp. J. Nat. Prod. 2010, 73, 1441–1443. [Google Scholar] [CrossRef]
- Hagmann, L.; Jüttner, F. Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett. 1996, 37, 6539–6542. [Google Scholar] [CrossRef]
- Asthana, R.K.; Tripathi, M.K.; Deepali, A.; Srivastava, A.; Singh, A.P.; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Isolation and identification of a new antibacterial entity from the Antarctic cyanobacterium Nostoc CCC 537. J. Appl. Phycol. 2009, 21, 81–88. [Google Scholar] [CrossRef]
- Moore, R.E.; Cheuk, C.; Yang, X.Q.G.; Patterson, G.M.L.; Bonjouklian, R.; Smitka, T.A.; Mynderse, J.S.; Foster, R.S.; Jones, N.D.; Swartzendruber, J.K.; et al. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 1036–1043. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Seibold, M.; Preussel, K.; von Döhren, H. Hassallidin B–second antifungal member of the Hassallidin family. Bioorg. Med. Chem. Lett. 2006, 16, 4220–4222. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Preussel, K.; Dieckmann, R.; Pham, H.; Bartl, F.; von Döhren, H. Hassallidin A, a glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J. Nat. Prod. 2005, 68, 695–700. [Google Scholar] [CrossRef]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Iwatsuki, M.; Ishiyama, A.; Hokari, R.; Otoguro, K.; Omura, S.; Suenaga, K. Isolation and total synthesis of hoshinolactam, an antitrypanosomal lactam from a marine cyanobacterium. Org. Lett. 2017, 19, 890–893. [Google Scholar] [CrossRef]
- Iwasaki, K.; Iwasaki, A.; Sumimoto, S.; Matsubara, T.; Sato, T.; Nozaki, T.; Saito-Nakano, Y.; Suenaga, K. Ikoamide, an antimalarial lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2020, 83, 481–488. [Google Scholar] [CrossRef]
- Zainuddin, E.N.; Mentel, R.; Wray, V.; Jansen, R.; Nimtz, M.; Lalk, M.; Mundt, S. Cyclic depsipeptides, ichthyopeptins A and B, from Microcystis ichthyoblabe. J. Nat. Prod. 2007, 70, 1084–1088. [Google Scholar] [CrossRef]
- Sweeney-Jones, A.M.; Gagaring, K.; Antonova-Koch, J.; Zhou, H.; Mojib, N.; Soapi, K.; Skolnick, J.; McNamara, C.W.; Kubanek, J. Antimalarial peptide and polyketide natural products from the Fijian marine cyanobacterium Moorea producens. Mar. Drugs 2020, 18, 167. [Google Scholar] [CrossRef]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1997, 60, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.Y.K.; Tan, L.T. Lagunamide, A cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscule. Phytochemistry 2011, 72, 2369e2375. [Google Scholar] [CrossRef] [PubMed]
- Frankmölle, W.P.; Knübel, G.; Moore, R.E.; Patterson, G.M.L. Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa. II. Structures of laxaphycins A., B, C, D and E. J. Antibiot. 1992, 45, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Frankmölle, W.P.; Larsen, L.K.; Caplan, F.R.; Patterson, G.M.L.; Knübel, G.; Levine, I.A.; Moore, R.E. Antifungal cyclic peptides from the terrestrial bluegreen alga Anabaena laxa. I. Isolation and biological properties. J. Antibiot. 1991, 45, 1451–1457. [Google Scholar] [CrossRef]
- Bonnard, I.; Rolland, M.; Francisco, C.; Banaigs, B. Total structure and biological properties of laxaphycins A and B, cyclic lipopeptides from the marine cyanobacterium Lyngbya majuscula. Lett. Pept. Sci. 1997, 4, 289–292. [Google Scholar] [CrossRef]
- Asthana, R.K.; Srivastava, A.; Kayastha, A.M.; Nath, G.; Singh, S. P 2006. Antibacterial potential of c-linolenic acid from Fischerella sp. colonizing Neem tree bark. World J. Microbiol. Biotechnol. 2006, 22, 443–448. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Ernst-Russell, M.A.; de Ropp, J.S.; Molinski, T.F. Lobocyclamides A–C, lipopeptides from a cryptic cyanobacterial mat containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; McPhail, K.L.; Elbandy, M. Malyngamide 4, a new lipopeptide from the Red Sea marine cyanobacterium Moorea producens (formerly Lyngbya majuscula). Phytochemistry Lett. 2013, 6, 183e188. [Google Scholar] [CrossRef]
- Milligan, K.E.; Marquez, B.L.; Williamson, R.T.; Gerwick, W.H. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1440e1443. [Google Scholar] [CrossRef]
- Zainuddin, E.N.; Jansen, R.; Nimtz, M.; Wray, V.; Preisitsch, M.; Lalk, M.; Mundt, S. Lyngbyazothrins A–D, antimicrobial cyclic undecapeptides from the cultured Cyanobacterium lyngbya sp. J. Nat. Prod. 2009, 72, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.; Lantvit, D.D.; Swanson, S.M.; Orjala, J. Lyngbyaureidamides A and B, two anabaenopeptins from the cultured freshwater cyanobacterium Lyngbya sp. (SAG 36.91). Phytochemistry 2012, 74, 173–177. [Google Scholar] [CrossRef]
- Leão, P.N.; Pereira, A.R.; Liu, W.T.; Ng, J.; Pevzner, P.A.; Dorrestein, P.C.; König, G.M.; Vasconvelos, V.M.; Gerwick, W.H. Synergistic allelochemicals from a freshwater cyanobacterium. Proc. Natl. Acad. Sci. USA 2010, 107, 11183–11188. [Google Scholar] [CrossRef]
- Cardllina, J.H.; Moore, R.E.; Arnold, E.V.; Clardy, J. Structure and absolute configuration of malyngolide, an antibiotic from the marine blue-green alga Lyngbya majuscula gomont. J. Org. Chem. 1979, 44, 4039–4042. [Google Scholar] [CrossRef]
- Gutierrez, M.; Tidgewell, K.; Capson, T.L.; Engene, N.; Almanza, A.; Schemies, J.; Jung, M.; Gerwick, W.H. Malyngolide dimer, a bioactive symmetric cyclodepside from the Panamanian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 709e711. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Moore, R.E.; Mynderse, J.S.; Niemczura, W.P.; Todd, J.S. Structure of majusculamide C, a cyclic depsipeptide from Lyngbya majuscula. J. Org. Chem. 1984, 49, 236–241. [Google Scholar] [CrossRef]
- Moore, R.E.; Mynderse, J.S. Majusculamide C. U.S. Patent 4342751, 3 August 1982. [Google Scholar]
- Pettit, G.R.; Hogan, F.; Xu, J.P.; Tan, R.; Nogawa, T.; Cichacz, Z.; Pettit, R.K.; Du, J.; Ye, Q.H.; Cragg, G.M.; et al. Antineoplastic agents. New sources of naturally occurring cancer cell growth inhibitors from marine organisms, terrestrial plants, and microorganisms. J. Nat. Prod. 2008, 71, 438–444. [Google Scholar] [CrossRef]
- Ramos, D.F.; Matthiensen, A.; Colvara, W.; de Votto, A.P.S.; Trindade, G.S.; da Silva, P.E.A.; Yunes, J.S. Antimycobacterial and cytotoxicity activity of microcystins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 9. [Google Scholar] [CrossRef][Green Version]
- Shahzad-ul-Hussan, S.; Gustchina, E.; Ghirlando, R.; Clore, G.M.; Bewley, C.A. Solution structure of the monovalent lectin microvirin in complex with Manα(1–2)Man provides a basis for anti-HIV activity with low toxicity. J. Biol. Chem. 2011, 286, 20788–20796. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Férir, G.; Vermeire, K.; Kehr, J.-C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a novel α(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef]
- Nagatsu, A.; Kajitani, H.; Sakakibara, J. Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum. Tetrahedron Lett. 1995, 36, 4097–4100. [Google Scholar] [CrossRef]
- Volk, R.B.; Furkert, F.H. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol. Res. 2006, 161, 180–186. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef]
- Kajiyama, S.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an antifungal lipopeptide from the field-grown terrestrial blue-green alga Nostoc commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Itoh, T.; Tsuchida, A.; Muramatsu, Y.; Ninomiya, M.; Ando, M.; Tsukamasa, Y.; Koketsu, M. Antimicrobial and anti-inflammatory properties of nostocionone isolated from Nostoc commune Vauch and its derivatives against Propionibacterium acnes. Anaerobe 2014, 27, 56–63. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Lee, J.B.; Hayashi, K.; Takenaka, H.; Hayakawa, Y.; Endo, S.; Hayashi, T. Isolation of an antiviral polysaccharide, nostoflan, from a terrestrial cyanobacteium, Nostoc flagilliforme. J. Nat. Prod. 2005, 68, 1037–1041. [Google Scholar] [CrossRef]
- Ploutno, A.; Carmeli, S. Nostocyclyne A, a novel antimicrobial cyclophane from the cyanobacterium Nostoc sp. J. Nat. Prod. 2000, 63, 1524–1526. [Google Scholar] [CrossRef]
- Kossack, R.; Breinlinger, S.; Nguyen, T.; Moschny, J.; Straetener, J.; Berscheid, A.; Brötz-Oesterhelt, H.; Enke, H.; Schirmeister, T.; Niedermeyer, T.H.J. Nostotrebin 6 related cyclopentenediones and δ-lactones with broad activity spectrum isolated from the cultivation medium of the cyanobacterium Nostoc sp. CBT1153. J. Nat. Prod. 2020, 83, 392–400. [Google Scholar] [CrossRef]
- Saad, M.H.; El-Fakharany, E.M.; Salem, M.S.; Sidkey, N.M. In vitro assessment of dual (antiviral and antitumor) activity of a novel lectin produced by the newly cyanobacterium isolate, Oscillatoria acuminate MHM-632 MK014210. 1. J. Biomolec. Struct. Dyn. 2022, 40, 3560–3580. [Google Scholar] [CrossRef]
- Koharudin, L.M.; Furey, W.; Gronenborn, A.M. Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J. Biol. Chem. 2011, 286, 1588–1597. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Gawley, R.E.; Wang, M.; Rein, K. S 2004. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya isolated from the Florida Everglades. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2004, 139, 231–238. [Google Scholar] [CrossRef]
- An, T.; Kumar, T.Z.S.; Wang, M.; Liu, L.; Lay, J.O.; Liyanage, R.; Berry, J.; Gantar, M.; Marks, V.; Gawley, R.E.; et al. Structures of pahayokolides A and B, cyclic peptides from a Lyngbya sp. J. Nat. Prod. 2007, 70, 730–735. [Google Scholar] [CrossRef]
- Sabarinathan, K.; Ganesan, G. Antibacterial and toxicity evaluation of C-phycocyanin and cell extract of filamentous freshwater cyanobacterium. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 79–82. [Google Scholar]
- Ghasemi, Y.; Yazdi, M.T.; Shafiee, A.; Amini, M.; Shokravi, S.; Zarrini, G. Parsiguine, A novel antimicrobial substance from Fischerella ambigua. Pharm. Biol. 2008, 42, 318–322. [Google Scholar] [CrossRef]
- Sarada, D.V.L.; Sreenath Kumar, C.; Rengasamy, R. Purified C-phycocyanin from Spirulina platensis (Nordstedt) Geitler: A novel and potent agent against drug resistant bacteria. World J. Microbiol. Biotechnol. 2011, 27, 779–783. [Google Scholar] [CrossRef]
- Pankaj, P.P.; Seth, R.K.; Mallick, N.; Biswas, S. Isolation and purification of C-Phycocyanin from Nostoc Muscorum (Cyanophyceae and cyanobacteria) exhibits antimalarial activity in vitro. J. Advance Lab. Res. Bio. 2010, 1, 86–91. [Google Scholar]
- Shanmugam, A.; Sigamani, S.; Venkatachalam, H.; Jayaraman, J.D.; Ramamurthy, D. Antibacterial activity of extracted phycocyanin from Oscillatoria sp. J. App. Pharma. Sci. 2017, 7, 62–67. [Google Scholar]
- Luesch, H.; Pangilinan, R.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. A. and B. pitipeptolides, New cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscule. J. Nat. Prod. 2001, 64, 304e307. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C-F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Siqueira-Neto, J.L.; Souza, J.M.; Eribez, K.; LaMonte, G.M.; Smith, J.E.; Gerwick, W.H. Palstimolide A: A complex polyhydroxy macrolide with antiparasitic activity. Molecules 2020, 25, 1604. [Google Scholar] [CrossRef]
- Pergament, I.; Carmeli, S. Schizotrin A; a novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett. 1994, 35, 8473–8476. [Google Scholar] [CrossRef]
- Ishibashi, M.; Moore, R.E.; Patterson, G.M.L.; Xu, C.; Clardy, J. Scytophycins, cytotoxic and antimycotic agents from the cyanophyte Scytonema pseudohofmanni. J. Org. Chem. 1986, 51, 5300–5306. [Google Scholar] [CrossRef]
- Carmeli, S.; Moore, R.E.; Patterson, G.M.L. Tolytoxin and new scytophycins from three species of Scytonema. J. Nat. Prod. 1990, 53, 1533–1542. [Google Scholar] [CrossRef]
- Patterson, G.M.L.; Carmeli, S. Biological effects of tolytoxin (6-hydroxy-7-O-methyl-scytophycin b), a potent bioactive metabolite from cyanobacteria. Arch. Microbiol. 1992, 157, 406–410. [Google Scholar] [CrossRef]
- Patterson, G.M.L.; Smith, C.D.; Kimura, L.H.; Britton, B.A.; Carmeli, S. Action of tolytoxin on cell morphology, cytoskeletal organization, and actin polymerization. Cell Motil. Cytoskelet. 1993, 24, 39–48. [Google Scholar] [CrossRef]
- Patterson, G.M.L.; Bolis, C.M. Fungal cell-wall polysaccharides elicit an antifungal secondary metabolite (phytoalexin) in the cyanobacterium Scytonema ocellatum. J. Phycol. 1997, 33, 54–60. [Google Scholar] [CrossRef]
- Smith, C.D.; Carmeli, S.; Moore, R.E.; Patterson, G.M.L. Scytophycins, novelmicrofilament- depolymerizing agents which circumvent P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993, 53, 1343–1347. [Google Scholar]
- Mo, S.; Krunic, A.; Pegan, S.D.; Franzblau, S.G.; Orjala, J. An antimicrobial guanidine-bearing sesterterpene from the cultured cyanobacterium Scytonema sp. J. Nat. Prod. 2009, 72, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Fan, J.; Kitazato, K. The antiviral protein cyanovirin-N: The current state of its production and applications. Appl. Microbiol. Biotechnol. 2010, 86, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Bokesch, H.R.; O’Keefe, B.R.; McKee, T.C.; Pannell, L.K.; Patterson, G.M.L.; Gardella, R.S.; Sowder, R.C.; Turpin, J.; Watson, K.; Buckheit, R.W.; et al. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 2003, 42, 2578–2584. [Google Scholar] [CrossRef]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.J.; Shirakura, M.; et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Cardellina, J.H.; Fuller, R.W.; Wieslow, O.S.; Kiser, R.F.; Sander, K.M.; Patterson, G.M.; Boyd, M.R. AIDS antiviral sulfolipids from cyanobacteria (blue-green algae). J. Natl. Cancer Inst. 1989, 81, 1254–1258. [Google Scholar] [CrossRef]
- Loya, S.; Reshef, V.; Mizrachi, E.; Silberstein, C.; Rachamim, Y.; Carmeli, S.; Hizi, A. The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from cyanobacteria: Contribution of different moieties to their high potency. J. Nat. Prod. 1998, 61, 891–895. [Google Scholar] [CrossRef]
- Singh, I.P.; Milligan, K.E.; Gerwick, W.H. Tanikolide, a toxic and antifungal lactone from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1999, 62, 1333–1335. [Google Scholar] [CrossRef]
- Kanada, R.M.; Taniguchi, T.; Ogasawara, K. The first synthesis of (+)-tanikolide, a toxic and antifungal lactone from the marine cyanobactetrium Lyngbya majuscula. Synlett 2000, 7, 1019–1021. [Google Scholar]
- Levert, A.; Alvarino, R.; Bornancin, L.; Mansour, E.A.; Burja, A.M.; Geneviere, A.; Bonnard, I.; Alonso, E.; Botana, L.M.; Banaigs, B. Structures and activities of tiahuramides AeC, cyclic depsipeptides from a Tahitian collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301e1310. [Google Scholar] [CrossRef]
- Falch, B.S.; König, G.M.; Wright, A.D.; Sticher, O.; Angerhofer, C.K.; Pezzuto, J.M.; Bachmann, H. Biological activities of cyanobacteria: Evaluation of extracts and pure compounds. Planta Med. 1995, 61, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Zerbe, O.; Heilmann, J.; Sticher, O. Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195). J. Nat. Prod. 2001, 64, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Kale, A.J.; Fenley, A.T.; Byrum, T.; Debonsi, H.M.; Gilson, M.K.; Valeriote, F.A.; Moore, B.S.; Gerwick, W.H. The carmaphycins: New proteasome inhibitors exhibiting an α, β-epoxyketone warhead from a marine cyanobacterium. ChemBioChem 2012, 13, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A. Antiparasitic Potential of Natural Marine Compounds. Dissertação de Mestrado em Biologia e Gestão da Qualidade da Água, Faculdade de Ciência da Universidade do Porto. 2016. Available online: https://repositorio-aberto.up.pt/handle/10216/102563 (accessed on 14 July 2023).
- O’Donoghue, A.J.; Bibo-Verdugo, B.; Miyamoto, Y.; Wang, S.C.; Yang, J.Z.; Zuill, D.E.; Matsuka, S.; Jiang, Z.; Almaliti, J.; Caffrey, C.R.; et al. 20S proteasome as a drug target in Trichomonas vaginalis. Antimicrob. Agents Chemother. 2019, 63, e00448-19. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, L.; M’sakni, N.H.; Ben Ouada, H.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess. Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).