Antimony in Polyethylene Terephthalate-Bottled Beverages: The Migration Puzzle
Abstract
:1. Introduction
2. Results
2.1. Determination of Total Sb in Juices
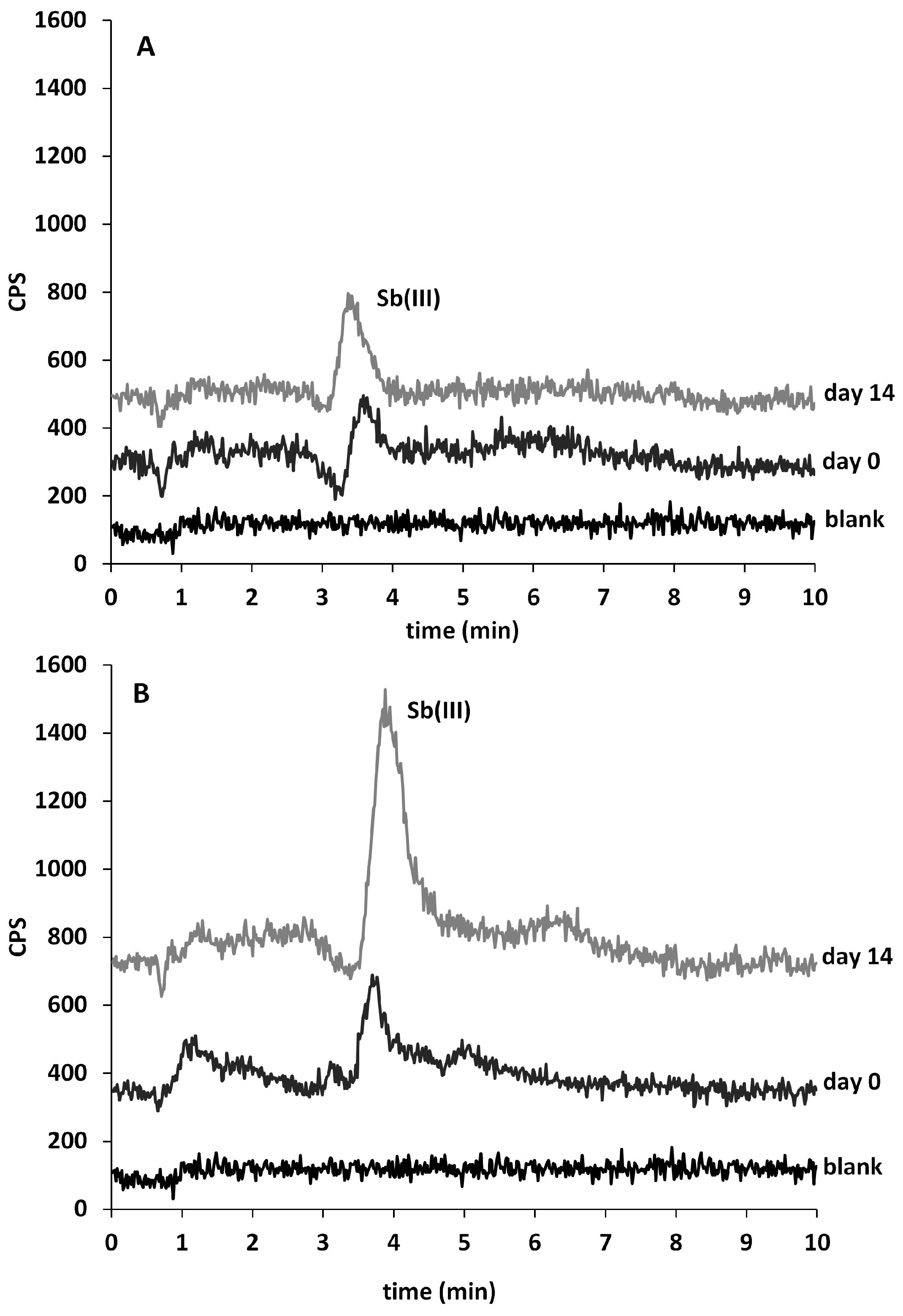
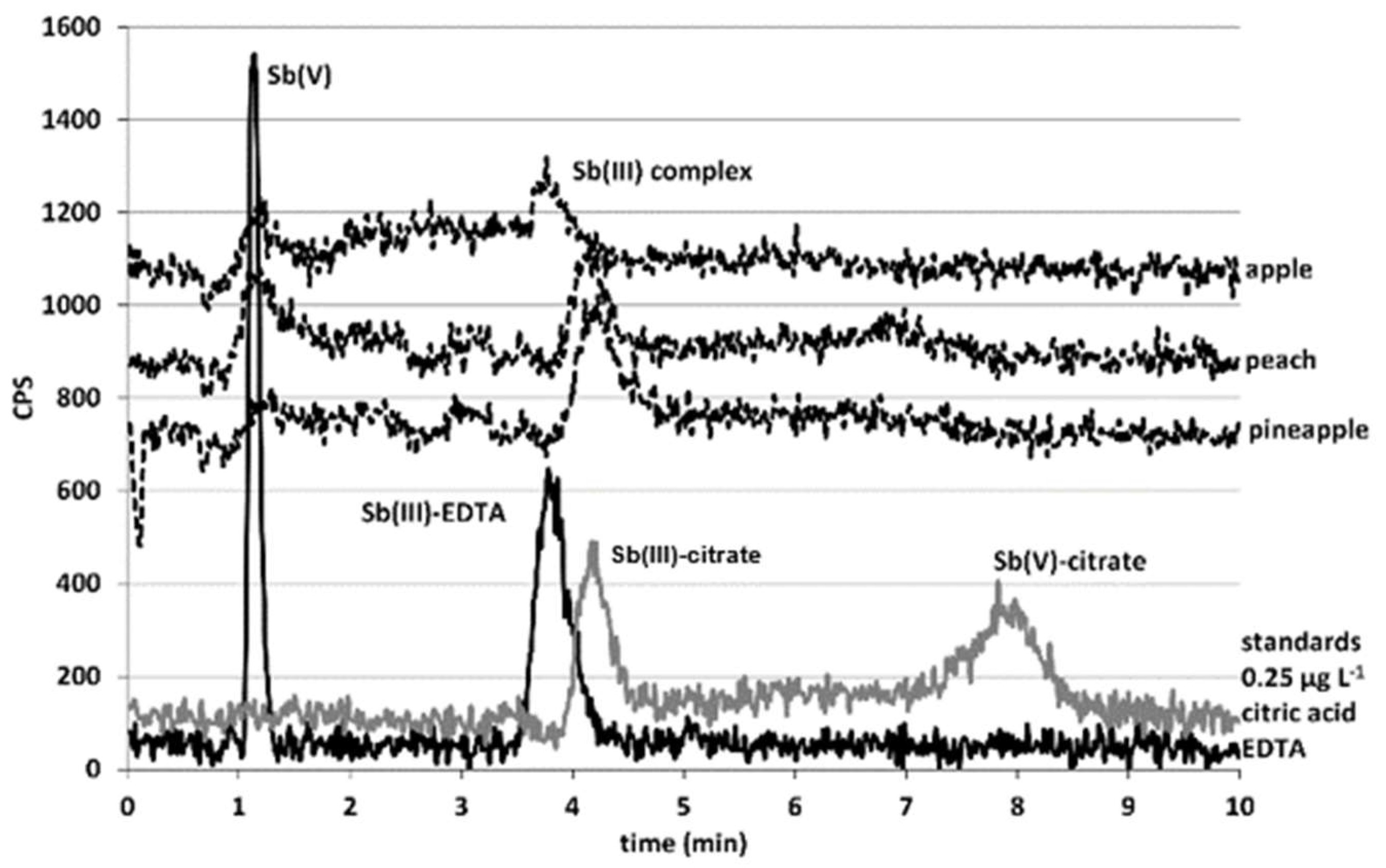
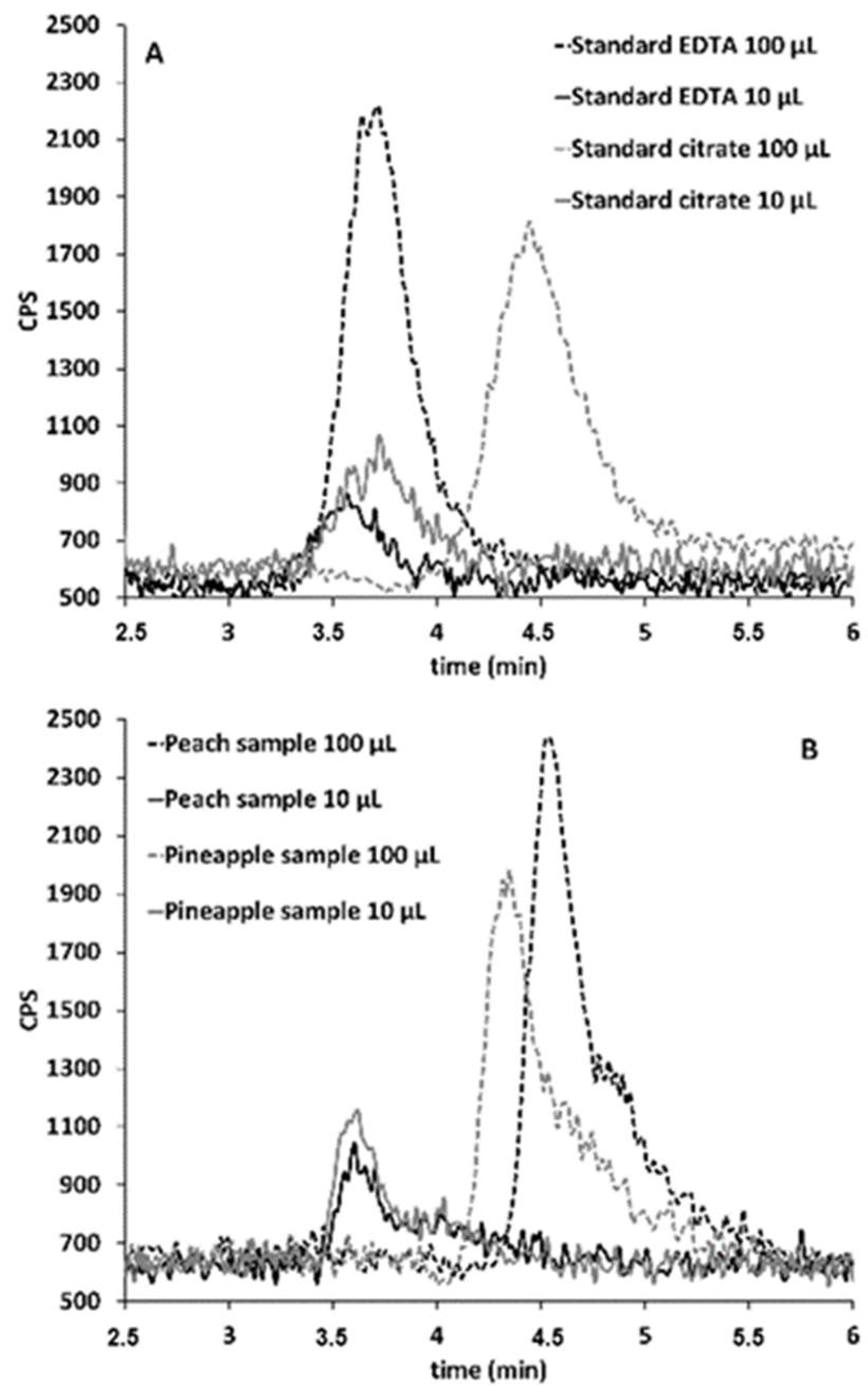
2.2. Sb Speciation in Juices
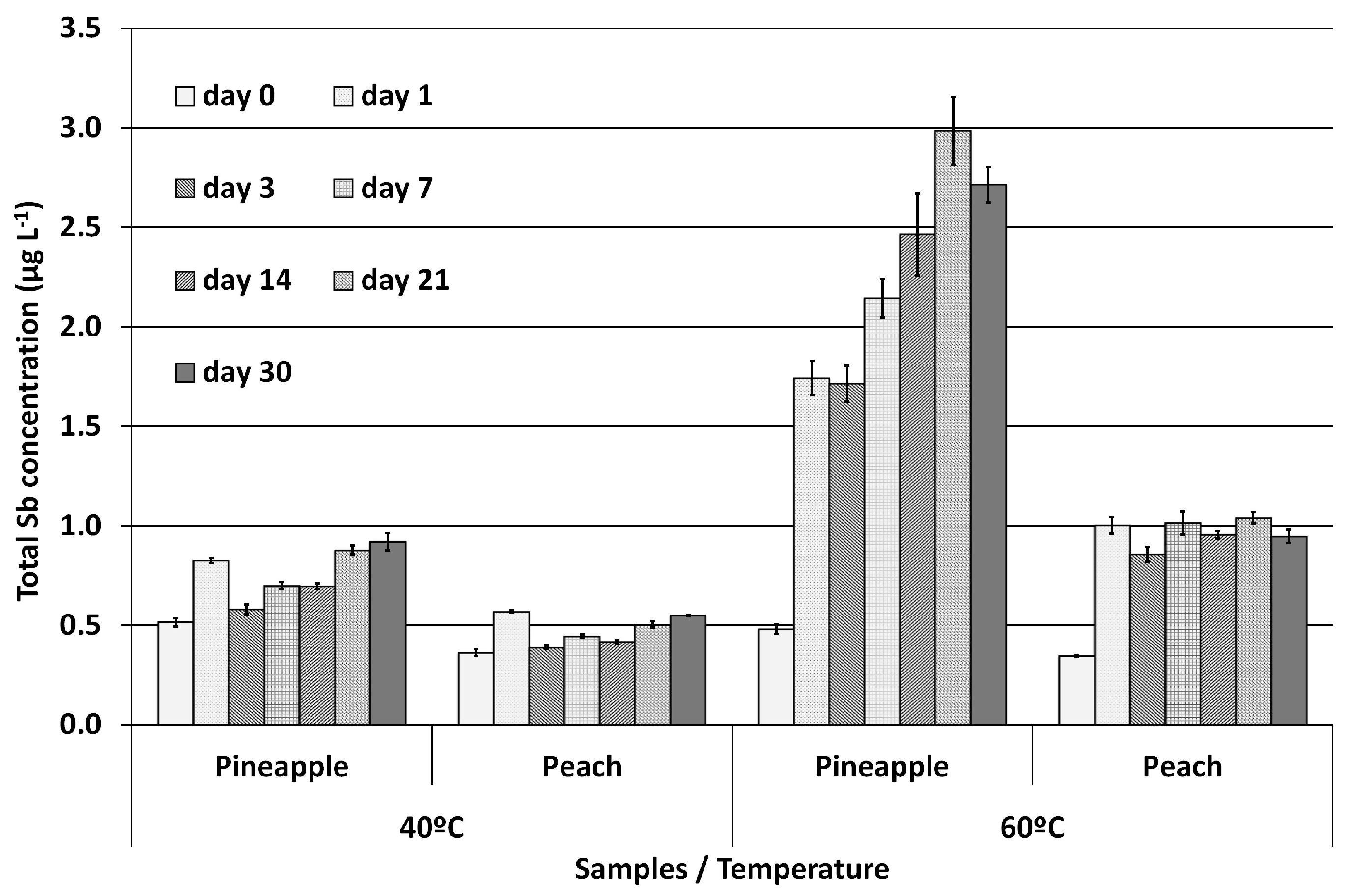
2.3. Migration Experiments
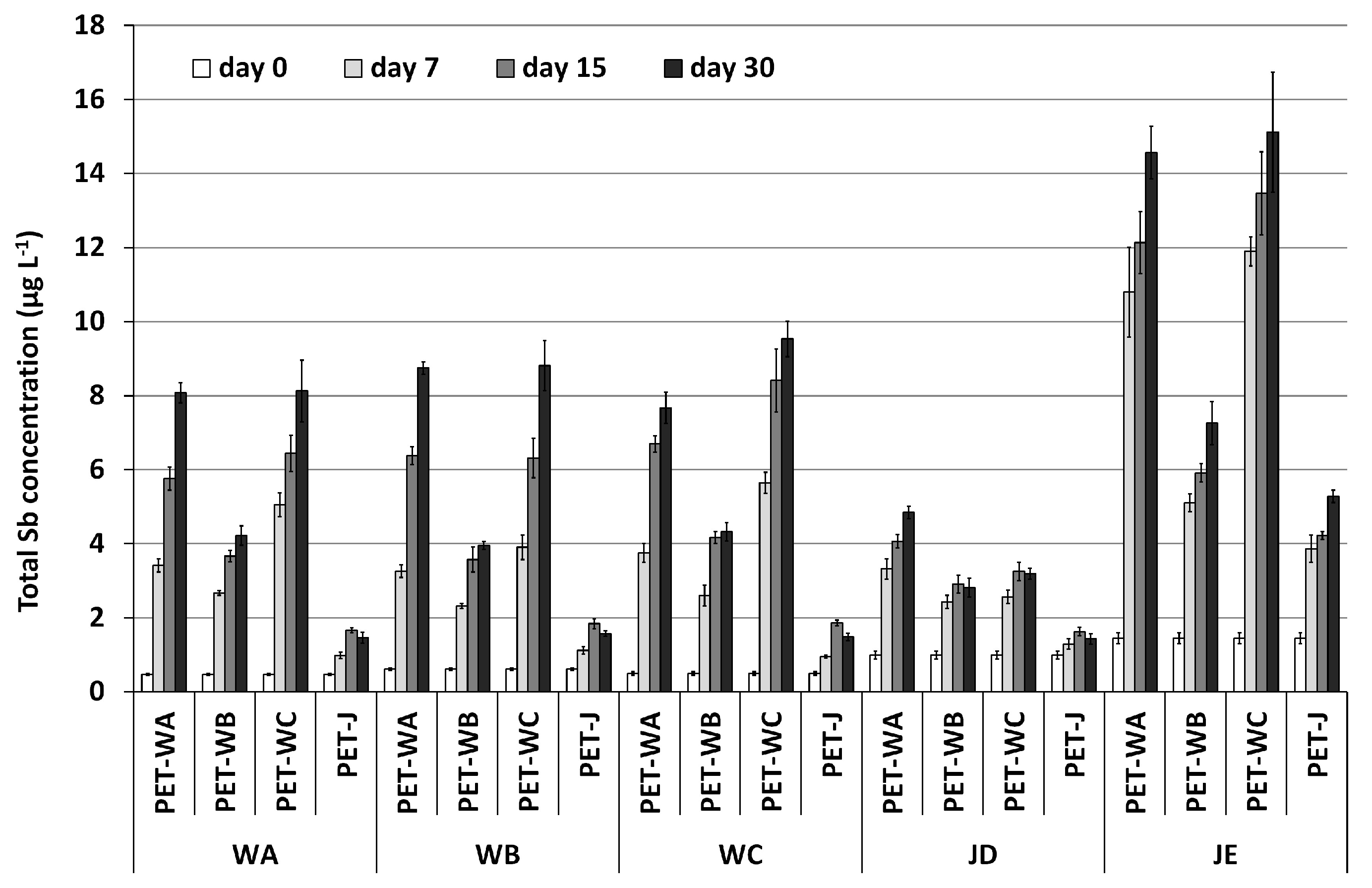
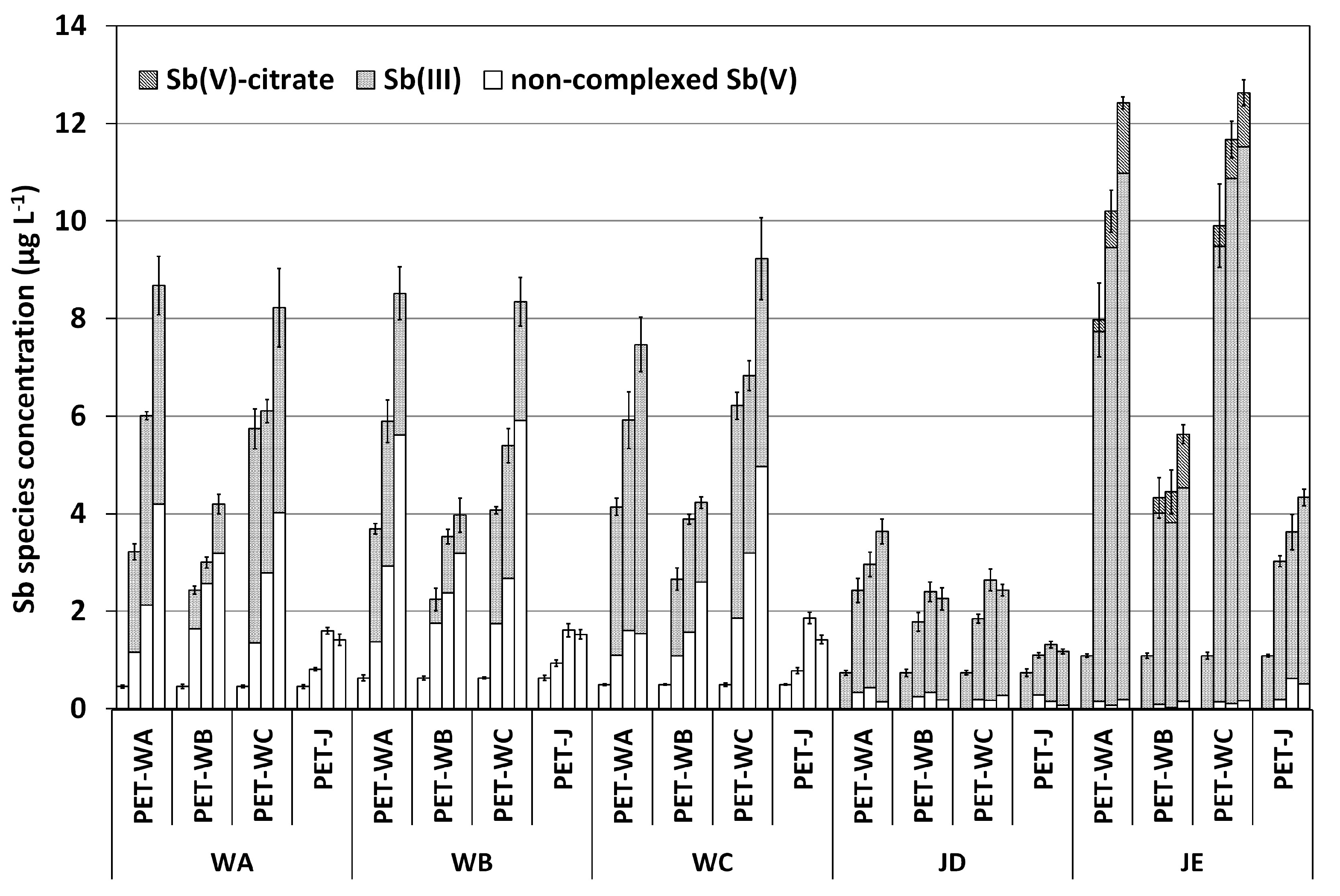
2.4. Cross-Migration Experiments
3. Discussion
3.1. Determination of Total Sb in Juices
3.2. Sb Speciation in Juices: Optimisation of the Analytical Method
3.3. Sb Speciation in Juices
3.4. Migration Experiments
3.5. Cross-Migration Experiments
4. Materials and Methods
4.1. Instrumentation
4.2. Reagents and Standards
4.3. Selection of Samples
4.4. Migration and Cross-Migration Experiments
4.5. Determination of Antimony by ICP-MS
4.5.1. Total Analysis
4.5.2. Speciation Analysis
4.5.3. Structure Characterization by HRMS
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Duh, B. Effect of antimony catalyst on solid-state polycondensation of poly(ethylene terephthalate). Polymers 2002, 43, 3147–3154. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sakuma, K.; Itai, T.; Zheng, G.; Mitsunobu, S. Speciation of antimony in PET bottles produced in Japan and China by X-ray absorption fine structure spectroscopy. Environ. Sci. Technol. 2008, 42, 9045–9050. [Google Scholar] [CrossRef]
- Filella, M. Antimony and PET bottles: Checking facts. Chemosphere 2020, 261, 127732. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Lett, M.C. Antimony in the environment: A review focused on natural waters III. Microbiota relevant interactions. Earth-Sci. Rev. 2007, 80, 195–217. [Google Scholar] [CrossRef]
- Poon, R.; Chu, I.; Lacavalier, P.; Valli, V.; Foster, W.; Gupta, S.; Thomas, B. Effects of Antimony on Rats Following 90-Day Exposure via Drinking Water. Food Chem. Toxicol. 1998, 36, 21–35. [Google Scholar] [CrossRef] [PubMed]
- EPA (Environmental Protection Agency). National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganic (accessed on 25 April 2023).
- EU (Commission of the European Communities, Official Journal of the European Union). Directive 2003/40/EC. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0040&from=EN (accessed on 25 April 2023).
- Shotyk, W.; Krachler, M.; Chen, B. Contamination of Canadian and European bottled waters with antimony from PET containers. J. Environ. Monit. 2006, 8, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Shotyk, W.; Krachler, M. Contamination of bottled waters with antimony leaching from polyethylene terephthalate (PET) increases upon storage. Environ Sci. Technol. 2007, 41, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef]
- Keresztes, S.; Tatár, E.; Mihucz, V.G.; Virág, I.; Majdik, C.; Záray, G. Leaching of antimony from polyethylene terephthalate (PET) bottles into mineral water. Sci. Total Environ. 2009, 407, 4731–4735. [Google Scholar] [CrossRef]
- Welle, F.; Franz, R. Migration of antimony from PET bottles into beverages: Determination of the activation energy of diffusion and migration modelling compared with literature data. Food Addit. Contam. 2011, 28, 115–126. [Google Scholar] [CrossRef]
- Tukur, A.; Sharp, L.; Stern, B.; Tizaoui, C.; Benkreira, H. PET bottle use patterns and antimony migration into bottled water and soft drinks: The case of British and Nigerian bottles. J. Environ. Monit. 2012, 14, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Rungchang, S.; Numthuam, S.; Qiu, X.; Li, Y.; Satake, T. Diffusion coefficient of antimony leaching from polyethylene terephthalate bottles into beverages. J. Food Eng. 2013, 115, 322–329. [Google Scholar] [CrossRef]
- Carneado, S.; Hernández-Nataren, E.; López-Sánchez, J.F.; Sahuquillo, A. Migration of antimony from polyethylene terephthalate used in mineral water bottles. Food Chem. 2015, 166, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Kishi, E.; Ozaki, A.; Ooshima, T.; Abe, Y.; Mutsuga, M.; Yamaguchi, Y.; Yamano, T. Determination of various constituent elements of polyethylene terephthalate bottles used for beverages in Japan. Packag. Technol. Sci. 2020, 33, 183–193. [Google Scholar] [CrossRef]
- Ristić, M.; Popović, I.; Pocajt, V.; Antanasijević, D.; Perić-Grujić, A. Concentrations of selected trace elements in mineral and spring bottled waters on the Serbian market. Food Addit. Contam. Part B Surveill. 2011, 4, 6–14. [Google Scholar] [CrossRef]
- Andra, S.S.; Makris, K.C.; Shine, J.P.; Lu, C. Co-leaching of brominated compounds and antimony from bottled water. Environ. Int. 2012, 38, 45–53. [Google Scholar] [CrossRef]
- Magana-Maldonado, L.M.; Wrobel, K.; Espinoza Cruz, T.L.; Yanez Barrientos, E.; Corrales Escobosa, A.R.; Wrobel, K. Application of hydride generation—Microwave plasma—Atomic emission spectrometry and partial least squares regression for the determination of antimony directly in water and in PET after alkaline methanolysis. Chemosphere 2023, 313, 137316. [Google Scholar] [CrossRef]
- Reimann, C.; Birke, M.; Filzmoser, P. Bottled drinking water: Water contamination from bottle materials (glass, hard PET, soft PET), the influence of colour and acidification. Appl. Geochem. 2010, 25, 1030–1046. [Google Scholar] [CrossRef]
- Reimann, C.; Birke, M.; Filzmoser, P. Temperature-dependent leaching of chemical elements from mineral water bottle materials. Appl. Geochem. 2012, 27, 1492–1498. [Google Scholar] [CrossRef]
- Bach, C.; Dauchy, D.; Severin, I.; Munoz, J.F.; Etienne, S.; Chagnon, M.C. Effect of temperature on the release of intentionally and non-intentionally added substances from polyethylene terephthalate (PET) bottles into water: Chemical analysis and potential toxicity. Food Chem. 2013, 139, 672–680. [Google Scholar] [CrossRef]
- Aghaee, E.M.; Alimohammadi, M.; Nabizadeh, R.; Khaniki, G.J.; Naseri, S.; Mahvi, A.H.; Yaghmaeian, K.; Aslani, H.; Nazmara, S.; Mahmoudi, B.; et al. Effects of storage time and temperature on the antimony and some trace element release from polyethylene terephthalate (PET) into the bottled drinking water. J. Environ. Sci. Health Eng. 2014, 12, 133. [Google Scholar] [CrossRef]
- Dogan, C.E.; Cebi, N. Investigation of antimony, cobalt, and acetaldehyde migration into the drinking water in Turkey. Packag. Technol. Sci. 2019, 32, 239–246. [Google Scholar] [CrossRef]
- Chapa-Martínez, C.A.; Hinojosa-Reyes, L.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.; Maya-Treviño, L.; Guzmán-Mar, J.L. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci. Total Environ. 2016, 565, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Rowell, C.; Kuiper, N.; Preud’Homme, H. Is container type the biggest predictor of trace element and BPA leaching from drinking water bottles? Food Chem. 2016, 202, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Gerassimidou, S.; Lanska, P.; Hahladakis, J.N.; Lovat, E.; Vanzetto, S.; Geueke, B.; Groh, K.J.; Muncke, J.; Maffini, M.; Martin, O.V.; et al. Unpacking the complexity of the PET drink bottles value chain: A chemicals perspective. J. Hazard. Mater. 2022, 430, 128410. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, A.; Dessuy, M.B.; Huber, C.S.; Zmozinski, A.V.; Duarte, A.T.; Vale, M.G.R.; Andrade, J.B. Determination of antimony in PET containers by direct analysis of solid samples using graphite furnace atomic absorption spectrometry and leaching studies. Microchem. J. 2016, 124, 222–227. [Google Scholar] [CrossRef]
- Shakerian, F.; Dadfarnia, S.; Haji Shabani, A.M.; Nili Ahmad Abadi, M. Synthesis and characterisation of nano-pore antimony imprinted polymer and its use in the extraction and determination of antimony in water and fruit juice sample. Food Chem. 2014, 145, 571–577. [Google Scholar] [CrossRef]
- Hansen, C.; Tsirigotaki, A.; Bak, S.A.; Pergantis, S.A.; Stürup, S.; Gammelgaard, B.; Hansen, H.R. Elevated antimony concentrations in commercial juices. J. Environ. Monit. 2010, 12, 822–824. [Google Scholar] [CrossRef]
- Hansen, H.R.; Pergantis, S.A. Investigating the formation of an Sb(V)–citrate complex by HPLC-ICP-MS and HPLC-ES-MS(/MS). J. Anal. At. Spectrom. 2006, 21, 1240–1248. [Google Scholar] [CrossRef]
- Hansen, H.R.; Pergantis, S.A. Identification of Sb(V) complexes in biological and food matrixes and their stibine formation efficiency during hydride generation with ICPMS detection. Anal. Chem. 2007, 79, 5304–5311. [Google Scholar] [CrossRef]
- Sánchez-Martínez, M.; Pérez-Corona, T.; Cámara, C.; Madrid, Y. Migration of antimony from PET containers into regulated EU food simulants. Food Chem. 2013, 141, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Carneado, S.; López-Sánchez, J.F.; Sahuquillo, A.; Klontzas, E.; Froudakis, G.E.; Pergantis, S.A. Antimony speciation in spirits stored in PET bottles: Identification of a novel antimony complex. J. Anal. At. Spectrom. 2017, 32, 1109–1118. [Google Scholar] [CrossRef]
- Hansen, H.R.; Pergantis, S.A. Detection of antimony species in citrus juices and drinking water stored in PET containers. J. Anal. At. Spectrom. 2006, 21, 731–733. [Google Scholar] [CrossRef]
- Welna, M.; Szymczycha-Madeja, A. Effect of sample preparation procedure for the determination of As, Sb and Se in fruit juices by HG-ICP-OES. Food Chem. 2014, 159, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; May, P.M. Critical appraisal of available thermodynamic data for the complexation of antimony(III) and antimony(V) by low molecular mass organic ligands. J. Environ. Monit. 2005, 7, 1226–1237. [Google Scholar] [CrossRef]
- Romao, W.; Franco, M.F.; Corilo, Y.E.; Eberlin, M.N.; Spinacé, M.A.S.; De Paoli, M. Poly(ethylene terephthalate) thermo-mechanical and thermo-oxidative degradation mechanisms. Polym. Degrad. Stab. 2009, 94, 1849–1859. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Zheng, J.L.; Ren, J.H.; Luo, J.; Cui, X.Y.; Ma, L.Q. Effects of storage temperature and duration on release of antimony and bisphenol A from polyethylene terephthalate drinking water bottles of China. Environ. Pollut. 2014, 192, 113–120. [Google Scholar] [CrossRef]
| Juice | Concentration (µg L−1) | RSD (%) |
|---|---|---|
| Apple | 0.343 ± 0.002 | 0.7 |
| Grape | 0.937 ± 0.018 | 1.9 |
| Pear | 0.522 ± 0.008 | 1.6 |
| Pineapple | 0.338 ± 0.002 | 0.5 |
| Peach | 0.273 ± 0.009 | 3.2 |
| Orange | 0.364 ± 0.005 | 1.3 |
| Tomato | 0.166 ± 0.007 | 4.2 |
| Sample | Total Sb (µg L−1) | Sb(V) (µg L−1) | Sb (III) (µg L−1) |
|---|---|---|---|
| WA | 0.47 ± 0.03 (6.9%) | 0.46 ± 0.02 (5.0%) | <LOD |
| WB | 0.61 ± 0.04 (6.2%) | 0.63 ± 0.06 (8.9%) | <LOD |
| WC | 0.49 ± 0.05 (9.8%) | 0.50 ± 0.05 (9.2%) | <LOD |
| JD | 0.99 ± 0.11 (10.7%) | <LOQ | 0.74 ± 0.04 (5.7%) |
| JE | 1.45 ± 0.15 (10.1%) | <LOQ | 1.09 ± 0.03 (3.0%) |
| ICP-MS Parameters | |
|---|---|
| RF power | 1550 W |
| RF matching | 1.66 V |
| Peristaltic pump speed | 0.1 rps |
| Stabilization delay | 30 s |
| Sampler and skimmer cones | Nickel |
| Nebulizer | BURGENER Ari Mist HP |
| Number of replicates | 3 |
| Spray chamber (type and temperature) | Scott-type and 15 °C |
| Carrier gas flow, Ar | 0.75 L min−1 |
| Make up gas flow, Ar | 0.33 L min−1 |
| Sampling depth | 7.5 mm |
| Cell exit | −36 V |
| Quadrupole/Octopole bias difference | 3 V |
| Mass to charge ratio | m/z 121 (121Sb) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneado, S.; López-Sánchez, J.F.; Sahuquillo, Á. Antimony in Polyethylene Terephthalate-Bottled Beverages: The Migration Puzzle. Molecules 2023, 28, 7166. https://doi.org/10.3390/molecules28207166
Carneado S, López-Sánchez JF, Sahuquillo Á. Antimony in Polyethylene Terephthalate-Bottled Beverages: The Migration Puzzle. Molecules. 2023; 28(20):7166. https://doi.org/10.3390/molecules28207166
Chicago/Turabian StyleCarneado, Sergio, José Fermín López-Sánchez, and Ángeles Sahuquillo. 2023. "Antimony in Polyethylene Terephthalate-Bottled Beverages: The Migration Puzzle" Molecules 28, no. 20: 7166. https://doi.org/10.3390/molecules28207166
APA StyleCarneado, S., López-Sánchez, J. F., & Sahuquillo, Á. (2023). Antimony in Polyethylene Terephthalate-Bottled Beverages: The Migration Puzzle. Molecules, 28(20), 7166. https://doi.org/10.3390/molecules28207166






