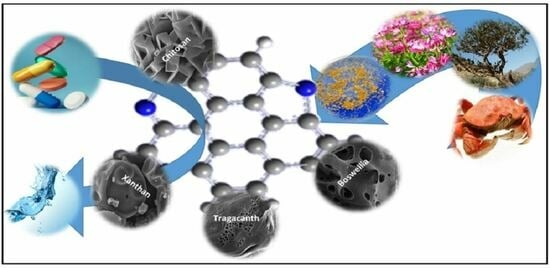
In Situ Synthesis of Doped Bio-Graphenes as Effective Metal-Free Catalysts in Removal of Antibiotics: Effect of Natural Precursor on Doping, Morphology, and Catalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Synthesized Bio-Graphenes
2.2. Catalytic Study
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of Bio-Graphenes
3.3. Catalytic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Larsson, D.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Ali, N.S.; Kalash, K.R.; Ahmed, A.N.; Albayati, T.M. Performance of a solar photocatalysis reactor as pretreatment for wastewater via UV, UV/TiO2, and UV/H2O2 to control membrane fouling. Sci. Rep. 2022, 12, 16782. [Google Scholar] [CrossRef]
- Jabbar, N.M.; Alardhi, S.M.; Mohammed, A.K.; Salih, I.K.; Albayati, T.M. Challenges in the implementation of bioremediation processes in petroleum-contaminated soils: A review. Environ. Nanotech. Manag. 2022, 18, 100694. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Eslami Moghadam, M.; Sadeghi, M.; Mansouri-Torshizi, H.; Saidifar, M. High cancer selectivity and improving drug release from mesoporous silica nanoparticles in the presence of human serum albumin in cisplatin, carboplatin, oxaliplatin, and oxalipalladium treatment. Eur. J. Pharm. Sci. 2023, 187, 106477. [Google Scholar] [CrossRef] [PubMed]
- Eslami Moghadam, M.; Rezaeisadat, M.; Mansouri-Torshizi, H.; Hosseinzadeh, S.; Daneshyar, H. New anticancer potential Pt complex with tertamyl dithiocarbamate ligand: Synthesis, DNA targeting behavior, molecular dynamic, and biological activity. J. Mol. Liq. 2023, 379, 121651. [Google Scholar] [CrossRef]
- Abbood, N.S.; Ali, N.S.; Khader, E.H.; Majdi, H.S.; Albayati, T.M.; Saady, N. Photocatalytic degradation of cefotaxime pharmaceutical compounds onto a modified nanocatalyst. Res. Chem. Intermed. 2022, 49, 43–56. [Google Scholar] [CrossRef]
- Shimizu, Y.; Okuno, Y.-I.; Uryu, K.; Ohtsubo, S.; Watanabe, A. Filtration characteristics of hollow fiber microfiltration membranes used in membrane bioreactor for domestic wastewater treatment. Water Res. 1996, 30, 2385–2392. [Google Scholar] [CrossRef]
- Afsharpour, M.; Khomad, E. Synthesis of bio-inspired porous silicon carbides using Cortaderia selloana and Equisetum arvense grasses as remarkable sulfur adsorbents. Intern. J. Environ. Sci. Technol. 2019, 16, 3125–3134. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Zhu, G.; Lu, Y.; AL-Falahi, A.H.; Lu, Y.; Huang, S.; Wan, Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical Technology: Current knowledge and future perspectives. Environ. Eng. Res. 2021, 26, 190405. [Google Scholar] [CrossRef]
- Jiang, F.; Qiu, B.; Sun, D. Advanced degradation of refractory pollutants in incineration leachate by UV/Peroxymonosulfate. Chem. Eng. J. 2018, 349, 338–346. [Google Scholar] [CrossRef]
- Gomi, L.S.; Afsharpour, M. Porous MoO3@SiC hallow nanosphere composite as an efficient oxidative desulfurization catalyst. Appl. Organometal. Chem. 2019, 33, e4830. [Google Scholar]
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, K.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [CrossRef]
- Gomi, L.S.; Afsharpour, M.; Lianos, P. Novel Porous SiO2@SiC Core-Shell Nanospheres Functionalized with an Amino Hybrid of WO3 as an Oxidative Desulfurization Catalyst. J. Indust. Eng. Chem. 2020, 89, 448–457. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef]
- Saviano, L.; Brouziotis, A.A.; Suarez, E.G.P.; Siciliano, A.; Spampinato, M.; Guida, M.; Trifuoggi, M.; Bianco, D.D.; Carotenuto, M.; Spica, V.R.; et al. Catalytic Activity of Rare Earth Elements (REEs) in Advanced Oxidation Processes of Wastewater Pollutants: A Review. Molecules 2023, 28, 6185. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Brigante, M.; Mailhot, G. Enhanced Degradation of Paracetamol by the Fe(III)-Sulfite System under UVA Irradiation. Molecules 2022, 27, 2248. [Google Scholar] [CrossRef]
- Moratalla, A.; Cotillas, S.; Lacasa, E.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Electrochemical Technologies to Decrease the Chemical Risk of Hospital Wastewater and Urine. Molecules 2021, 26, 6813. [Google Scholar] [CrossRef]
- Gomi, L.S.; Afsharpour, M.; Ghasemzadeh, M.; Lianos, P. Bio-inspired N, S-doped siligraphenes as novel metal-free catalysts for removal of dyes in the dark. J. Mol. Liq. 2019, 295, 111657. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.; Wang, S.; Wang, Y.; Wang, X.; Li, X.; Niu, C.; Wang, D. Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: Mineralization efficiency and mechanism. ACS Appl. Mater. Interfaces 2016, 8, 32887–32900. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Yang, X.; Li, F.; Li, A.; Liu, Y.; Zhang, J.; Zhou, Z.; Ni, L. Fabricating carbon quantum dots doped ZnIn2S4 nanoflower composites with broad spectrum and enhanced photocatalytic Tetracycline hydrochloride degradation. Mater. Res. Bull. 2018, 97, 158–168. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Pei, J.; Liu, F.; Li, D. Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalin. Water Treat. 2016, 57, 19981–19987. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Wang, Y. Visible active N-doped TiO2/reduced graphene oxide for the degradation of tetracycline hydrochloride. Chem. Phys. Lett. 2018, 691, 408–414. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Z.; Liu, C.; Li, J.; Zhou, M.; Guan, Q.; Lv, P.; Huo, P.; Yan, Y. Visible light driven Ag/Ag3PO4/AC photocatalyst with highly enhanced photodegradation of tetracycline antibiotics. Appl. Surf. Sci. 2015, 353, 391–399. [Google Scholar] [CrossRef]
- Ma, N.; Xu, J.; Bian, Z.; Yang, Y.; Zhang, L.; Wang, H. BiVO4 plate with Fe and Ni oxyhydroxide cocatalysts for the photodegradation of sulfadimethoxine antibiotics under visible-light irradiation. Chem. Eng. J. 2020, 389, 123426. [Google Scholar] [CrossRef]
- Rana, A.; Kumar, A.; Sharma, G.; Naushad, M.; Bathula, C.; Stadler, F.J. Pharmaceutical pollutant as sacrificial agent for sustainable synergistic water treatment and hydrogen production via novel Z- scheme Bi7O9I3/B4C heterojunction photocatalysts. J. Mol. Liq. 2021, 343, 17652. [Google Scholar] [CrossRef]
- Ivan, R.; Popescu, C.; Antohe, V.A.; Antohe, S.; Negrila, C.; Logofatu, C.; del Pino, A.P.; György, E. Iron oxide/hydroxide–nitrogen doped graphene-like visible-light active photocatalytic layers for antibiotics removal from wastewater. Sci. Rep. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Song, Y.; Tian, J.; Gao, S.; Shao, P.; Qi, J.; Cui, F. Photodegradation of sulfonamides by gC3N4 under visible light irradiation: Effectiveness, mechanism and pathways. Appl. Catal. B Environ. 2017, 210, 88–96. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Feng, Y.; Xie, Z.; Zhang, Q.; Jin, X.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. Facile synthesis of carbon quantum dots loaded with mesoporous gC3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics. Dalton Trans. 2018, 47, 1284–1293. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Huang, W.; Zheng, S. Facile synthesis of g-C3N4/montmorillonite composite with enhanced visible light photodegradation of rhodamine B and tetracycline. J. Taiwan Inst. Chem. Eng. 2016, 66, 363–371. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, B.; Yang, j.; Yuan, Y.; Zhang, J. Persulfate-enhanced degradation of ciprofloxacin with SiC/g-C3N4 photocatalyst under visible light irradiation. Chemosphere 2021, 276, 130217. [Google Scholar] [CrossRef]
- Afsharpour, M.; Behtooei, H.R.; Shakiba, M.; Martí, V. Novel N,P,S co-doped graphenic SiC layers (g-SiC) in visible-light photodegradation of antibiotics and inactivating the bacteria. Process Saf. Environ. Prot. 2022, 166, 704–717. [Google Scholar] [CrossRef]
- Darvishi-Farash, S.; Afsharpour, M.; Heidarian, J. Novel siligraphene/g-C3N4 composites with enhanced visible light photocatalytic degradations of dyes and drugs. Environ. Sci. Pollut. Res. 2021, 28, 5938–5952. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.H.; Homaeigohar, S.; Rezaei, A.; Shekari, H. Bi/SnO2/TiO2-graphene nanocomposite photocatalyst for solar visible light–induced photodegradation of pentachlorophenol. Environ. Sci. Pollut. Res. 2021, 28, 15236–15247. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Wang, S. Catalytic degradation of antibiotics by metal-free catalysis over nitrogen-doped grapheme. Catal. Today 2020, 357, 341–349. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Zhang, X.; Yang, K.; Li, Y. Simultaneous degradation of trace antibiotics in water by adsorption and catalytic oxidation induced by N-doped reduced graphene oxide (N-rGO): Synergistic mechanism. Mater. Res. Express 2022, 9, 065601. [Google Scholar] [CrossRef]
- Siddiqui, A.S.; Ahmad, M.A.; Nawaz, M.H.; Hayat, A.; Nasir, M. Nitrogen-doped graphene oxide as a catalyst for the oxidation of Rhodamine B by hydrogen peroxide: Application to a sensitive fluorometric assay for hydrogen peroxide. Microchim. Acta 2020, 187, 47. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and Nitrogen Co-Doped Graphene for Metal-Free Catalytic Oxidation Reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Xie, X.; Xu, J.; Gu, Q.; Chen, L.; Wang, X. Nitrogen-Doped Graphene Nanosheets as Metal-Free Catalysts for Aerobic Selective Oxidation of Benzylic Alcohols. ACS Catal. 2012, 2, 622–631. [Google Scholar] [CrossRef]
- Luo, E.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Selectively doping pyridinic and pyrrolic nitrogen into a 3D porous carbon matrix through template-induced edge engineering: Enhanced catalytic activity towards the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 21709–21714. [Google Scholar] [CrossRef]
- Wang, X.X.; Zou, B.; Du, X.X.; Wang, J.N. N-doped carbon nanocages with high catalytic activity and durability for oxygen reduction. J. Mater. Chem. A 2015, 3, 12427–12435. [Google Scholar] [CrossRef]
- Afsharpour, M.; Gomi, L.S.; Elyasi, M. Novel metal-free N-doped bio-graphenes and their MoO3 bifunctional catalysts for ultra-deep oxidative desulfurization of heavy fuel. Separ. Purif. Technol. 2021, 274, 119014. [Google Scholar] [CrossRef]
- Miao, H.; Li, S.; Wang, Z.; Sun, S.; Kuang, M.; Liu, Z.; Yuan, J. Enhancing the pyridinic N content of Nitrogen-doped graphene and improving its catalytic activity for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 28298–28308. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Al-Musawi, T.J.; Kareem, S.L.; Zarrabi, M.; Al-Ma’abreh, A.M. Simultaneous adsorption of tetracycline, amoxicillin, and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles. Arab. J. Chem. 2020, 13, 4629–4643. [Google Scholar] [CrossRef]
- Hsu, L.C.; Liu, Y.T.; Syu, C.H.; Huang, M.H.; Tzou, Y.M.; Teah, H.Y. Adsorption of tetracycline on Fe (hydr)oxides: Effects of pH and metal cation (Cu2+, Zn2+ and Al3+) addition in various molar ratios. R. Soc. Open Sci. 2018, 5, 171941. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsharpour, M.; Radmanesh, L.; Yang, C. In Situ Synthesis of Doped Bio-Graphenes as Effective Metal-Free Catalysts in Removal of Antibiotics: Effect of Natural Precursor on Doping, Morphology, and Catalytic Activity. Molecules 2023, 28, 7212. https://doi.org/10.3390/molecules28207212
Afsharpour M, Radmanesh L, Yang C. In Situ Synthesis of Doped Bio-Graphenes as Effective Metal-Free Catalysts in Removal of Antibiotics: Effect of Natural Precursor on Doping, Morphology, and Catalytic Activity. Molecules. 2023; 28(20):7212. https://doi.org/10.3390/molecules28207212
Chicago/Turabian StyleAfsharpour, Maryam, Lugain Radmanesh, and Chuanxi Yang. 2023. "In Situ Synthesis of Doped Bio-Graphenes as Effective Metal-Free Catalysts in Removal of Antibiotics: Effect of Natural Precursor on Doping, Morphology, and Catalytic Activity" Molecules 28, no. 20: 7212. https://doi.org/10.3390/molecules28207212
APA StyleAfsharpour, M., Radmanesh, L., & Yang, C. (2023). In Situ Synthesis of Doped Bio-Graphenes as Effective Metal-Free Catalysts in Removal of Antibiotics: Effect of Natural Precursor on Doping, Morphology, and Catalytic Activity. Molecules, 28(20), 7212. https://doi.org/10.3390/molecules28207212