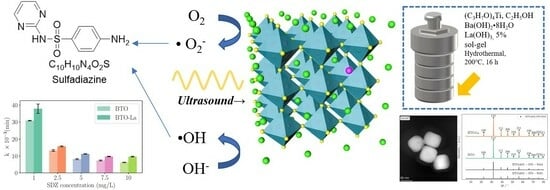
The Piezocatalytic Degradation of Sulfadiazine by Lanthanum-Doped Barium Titanate
Abstract
:1. Introduction
2. Results and Discussion
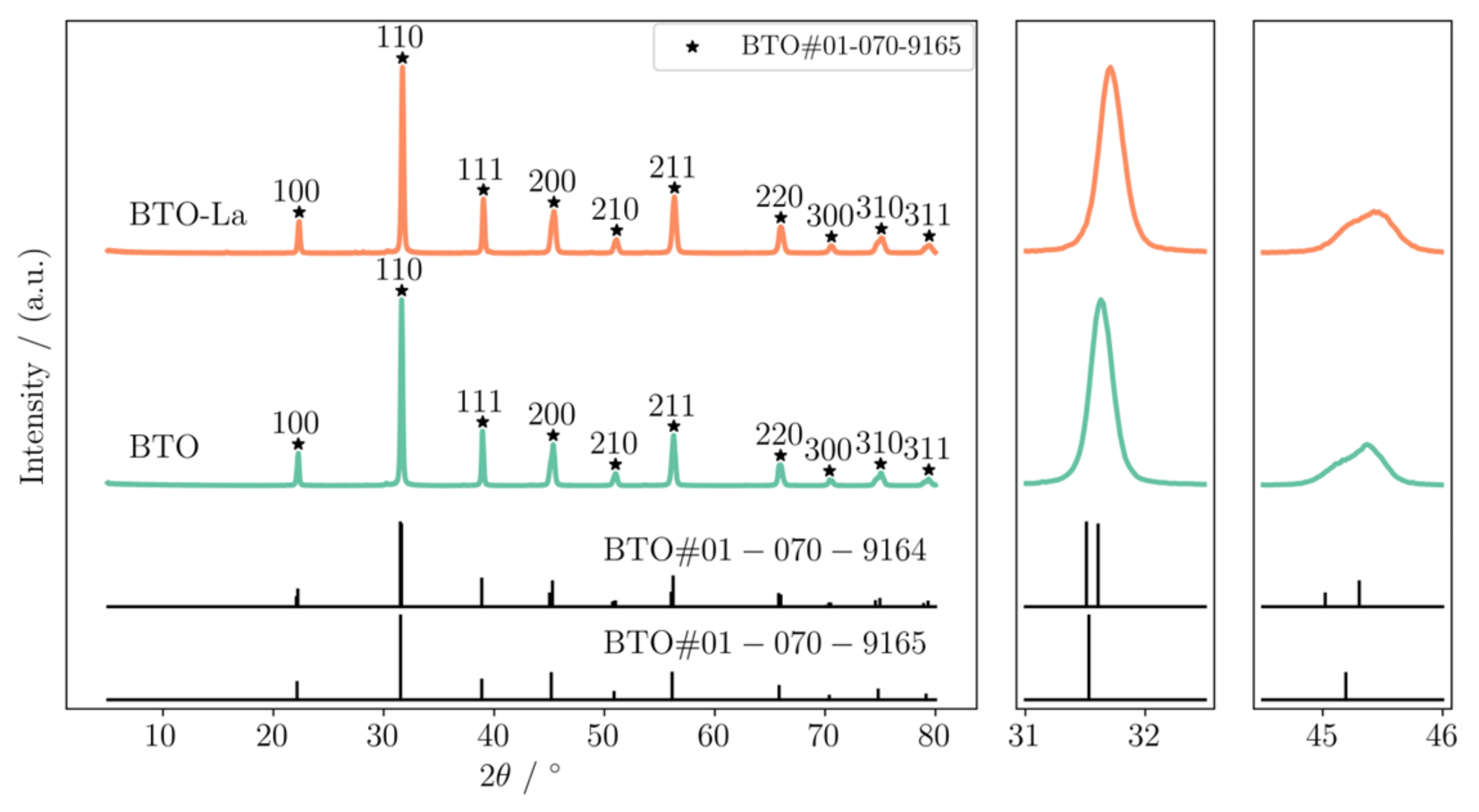
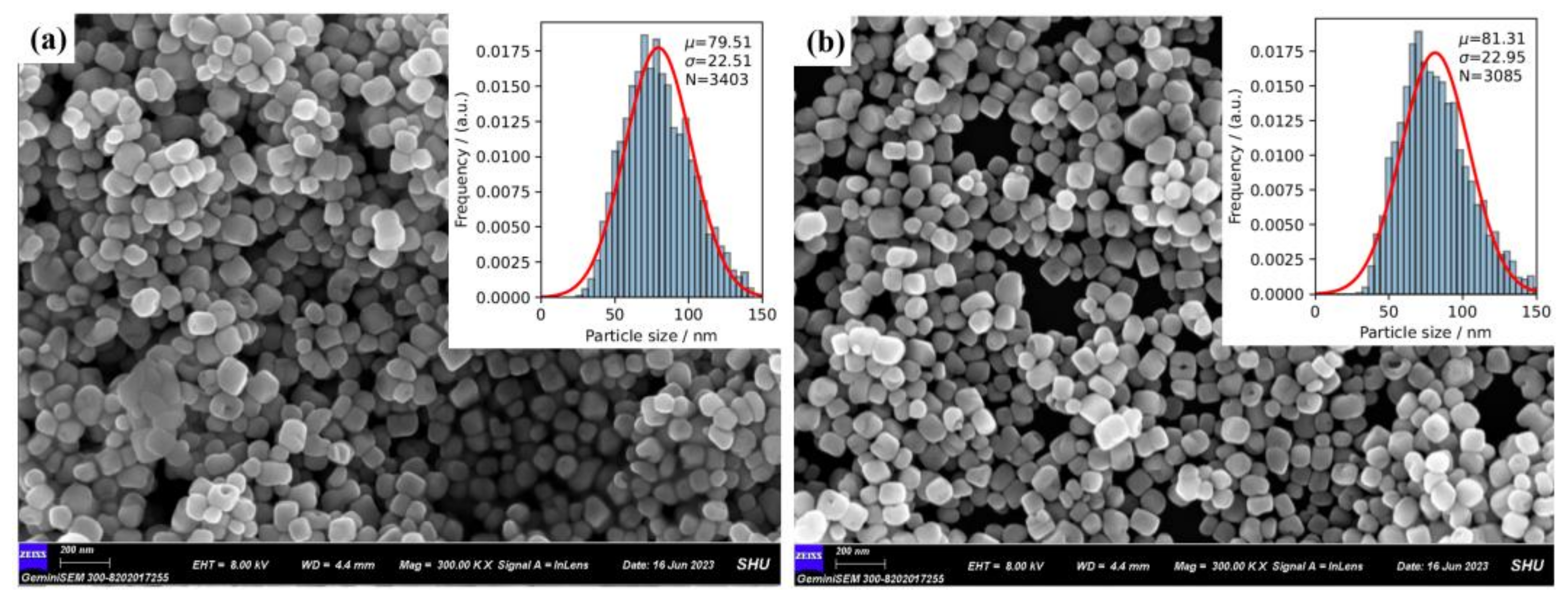
2.1. Characterization of BTO and BTO-La
2.2. Effects of the Ultrasound and Recycle Tests
2.3. Effects of the Catalysis Dosage
2.4. Effects of the Initial Concentration of SDZ
2.5. Effects of the Background Conditions
2.6. Possible Piezocatalytic Degradation Mechanisms
3. Conclusions
4. Materials and Methods
4.1. Synthesis of BTO
4.2. Characterization
4.3. Piezocatalytic Degradation Experiment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the Presence of Sulfonamides in the Environment and Their Influence on Human Health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Lv, K.; Deng, C.; Yu, Z.; Shi, J.; Johnson, A.C. Persistence and Migration of Tetracycline, Sulfonamide, Fluoroquinolone, and Macrolide Antibiotics in Streams Using a Simulated Hydrodynamic System. Environ. Pollut. 2019, 252, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Shi, J.; Zhang, X.; Qin, L.; Jiang, Z.; Wang, J.; Li, Y.; Liu, B. Occurrence and Bioaccumulation of Sulfonamide Antibiotics in Different Fish Species from Hangbu-Fengle River, Southeast China. Environ. Sci. Pollut. Res. 2021, 28, 44111–44123. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of Sulfonamide Residues along the Ebro River Basin: Removal in Wastewater Treatment Plants and Environmental Impact Assessment. Environ. Int. 2011, 37, 462–473. [Google Scholar] [CrossRef]
- Cheong, M.S.; Seo, K.H.; Chohra, H.; Yoon, Y.E.; Choe, H.; Kantharaj, V.; Lee, Y.B. Influence of Sulfonamide Contamination Derived from Veterinary Antibiotics on Plant Growth and Development. Antibiotics 2020, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ouyang, W.; Lin, C.; Wang, J.; Cui, X.; Li, Y.; Guo, Z.; Zhu, W.; He, M. Occurrence, Distribution, and Potential Ecological Risks of Antibiotics in a Seasonal Freeze-Thaw Basin. J. Hazard. Mater. 2023, 459, 132301. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, K.; Wilk, J.; Felis, E.; Bajkacz, S. Application of UHPLC-MS/MS Method to Study Occurrence and Fate of Sulfonamide Antibiotics and Their Transformation Products in Surface Water in Highly Urbanized Areas. Chemosphere 2021, 283, 131189. [Google Scholar] [CrossRef]
- Hanamoto, S.; Minami, Y.; Hnin, S.S.T.; Yao, D. Localized Pollution of Veterinary Antibiotics in Watersheds Receiving Treated Effluents from Swine Farms. Sci. Total Environ. 2023, 902, 166211. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Ji, X.; Wu, H.; Yang, H.; Zhang, H.; Zhang, X.; Li, Z.; Ni, X.; Qian, M. Determination of Veterinary Drug/Pesticide Residues in Livestock and Poultry Excrement Using Selective Accelerated Solvent Extraction and Magnetic Material Purification Combined with Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1617, 460808. [Google Scholar] [CrossRef]
- Zhao, K.; Li, C.; Wang, Q.; Lu, H. Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter. Sustainability 2022, 14, 7301. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Feng, W.; Yuan, M.; Li, J.; Xu, H.; Zheng, X.; Zhang, W. Sulfonamides-Induced Oxidative Stress in Freshwater Microalga Chlorella Vulgaris: Evaluation of Growth, Photosynthesis, Antioxidants, Ultrastructure, and Nucleic Acids. Sci. Rep. 2020, 10, 8243. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xu, C.; Huang, Y.; Nie, H.; Wang, J. Tetracyclines, Sulfonamides and Quinolones and Their Corresponding Resistance Genes in the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 631–632, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Alsolami, E.S.; Mkhalid, I.A.; Shawky, A.; Hussein, M.A. Efficient Visible-Light Photooxidation of Ciprofloxacin Antibiotic over CoTiO3-Impregnated 2D CeO2 Nanocomposites Synthesized by a Sol-Gel-Based Process. Mater. Sci. Semicond. Process. 2023, 162, 107487. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, X.; Li, Y.; Shao, W.; Fu, J.; Lin, Q.; Tan, J.; Gao, S.; Zhang, Y.; Ye, W. Bi5Ti3FeO15 Nanofibers for Highly Efficient Piezocatalytic Degradation of Mixed Dyes and Antibiotics. ACS Appl. Nano Mater. 2023, 6, 5602–5612. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, U.; Kushwaha, H.S. ZnO Hollow Pitchfork: Coupled Photo-Piezocatalytic Mechanism for Antibiotic and Pesticide Elimination. Catal. Sci. Technol. 2022, 12, 812–822. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into Electro-Fenton and Photo-Fenton for the Degradation of Antibiotics: Mechanism Study and Research Gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Zhu, L.; Santiago-Schübel, B.; Xiao, H.; Hollert, H.; Kueppers, S. Electrochemical Oxidation of Fluoroquinolone Antibiotics: Mechanism, Residual Antibacterial Activity and Toxicity Change. Water Res. 2016, 102, 52–62. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous Ozonation of Urban Wastewater: Removal of Antibiotics, Antibiotic-Resistant Escherichia coli and Antibiotic Resistance Genes and Phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Dong, C.; Chen, X.; Cai, H.; Chen, X.; Jiang, X.; Zhang, Y.; Peng, A.; Badsha, M.A.H. Review of Piezocatalysis and Piezo-Assisted Photocatalysis in Environmental Engineering. Crystals 2023, 13, 1382. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, M.; Huo, B.; Wang, J.; Yang, L.; Ma, W.; Qi, J.; Wang, Y.; Zhu, Z.; Meng, F. A Novel ZnO/CQDs/PVDF Piezoelectric System for Efficiently Degradation of Antibiotics by Using Water Flow Energy in Pipeline: Performance and Mechanism. Nano Energy 2023, 107, 108162. [Google Scholar] [CrossRef]
- Wu, E.; Yu, Y.; Hu, J.; Ren, G.; Zhu, M. Piezoelectric-Channels in MoS2-Embedded Polyvinylidene Fluoride Membrane to Activate Peroxymonosulfate in Membrane Filtration for Wastewater Reuse. J. Hazard. Mater. 2023, 458, 131885. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, I.C.; Hadj Youssef, A.; Kolhatkar, G.; Rawach, D.; Gomez-Yañez, C.; Claverie, J.P.; Sun, S.; Ruediger, A. Ultrafast Microwave-Assisted Hydrothermal Synthesis and Photocatalytic Behaviour of Ferroelectric Fe3+-Doped BaTiO3 Nanoparticles under Simulated Sunlight. Catal. Today 2021, 360, 90–98. [Google Scholar] [CrossRef]
- Huang, T.-H.; Espino, F.K.C.; Tian, X.-Y.; Widakdo, J.; Austria, H.F.M.; Setiawan, O.; Hung, W.-S.; Pamintuan, K.R.S.; Leron, R.B.; Chang, C.-Y.; et al. Piezocatalytic Property of PVDF/Graphene Self-Assembling Piezoelectric Membrane for Environmental Remediation. Chem. Eng. J. 2024, 487, 150569. [Google Scholar] [CrossRef]
- Mamba, G.; Mafa, P.J.; Muthuraj, V.; Mashayekh-Salehi, A.; Royer, S.; Nkambule, T.I.T.; Rtimi, S. Heterogeneous Advanced Oxidation Processes over Stoichiometric ABO3 Perovskite Nanostructures. Mater. Today Nano 2022, 18, 100184. [Google Scholar] [CrossRef]
- Mi, L.; Zhang, Q.; Wang, H.; Wu, Z.; Guo, Y.; Li, Y.; Xiong, X.; Liu, K.; Fu, W.; Ma, Y.; et al. Synthesis of BaTiO3 Nanoparticles by Sol-Gel Assisted Solid Phase Method and Its Formation Mechanism and Photocatalytic Activity. Ceram. Int. 2020, 46, 10619–10633. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, Y.; Lian, X.; Fan, B.; Liu, X.; Xiao, P.; Zhang, Y. Hydroxylation Mechanism of Phase Regulation of Nanocrystal BaTiO3 Synthesized by a Hydrothermal Method. Ceram. Int. 2022, 48, 2281–2288. [Google Scholar] [CrossRef]
- Sewify, G.H.; Shawky, A. Solvothermal-Based Synthesis of Barium Stannate Nanosheets Coupled with Copper Manganate Nanoparticles for Efficient Photooxidation of Tetracycline under Visible Light. J. Colloid Interface Sci. 2023, 648, 348–356. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, H.; Peng, F.; Zhang, W.; Su, C.; Guo, Q.; Yao, Z.; Cao, M.; Liu, H. Defect Evolution and Effect on Structure and Electric Properties of A/B Site Sm Doped BaTiO3 Sintered in Different Atmospheres. J. Alloys Compd. 2023, 945, 169211. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Q.; Lin, E.; Yuan, B.; Qin, N.; Thatikonda, S.K.; Bao, D. Insights into the Role of Ferroelectric Polarization in Piezocatalysis of Nanocrystalline BaTiO3. ACS Appl. Mater. Interfaces 2018, 10, 17842–17849. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective Enhancement of Piezocatalytic Activity of BaTiO3 Nanowires under Ultrasonic Vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Lv, Y.; Sui, M.; Lv, X.; Xie, J. Piezoelectric Catalytic Performance of BaTiO3 for Sulfamethoxazole Degradation. Environ. Sci. Water Res. Technol. 2022, 8, 3007–3018. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Kim, D.; Franco, C.; Terzopoulou, A.; Veciana, A.; Puigmartí-Luis, J.; Chen, X.-Z.; Nelson, B.J.; Pané, S. Enhanced Piezocatalytic Performance of BaTiO3 Nanosheets with Highly Exposed 001 Facets. Adv. Funct. Mater. 2022, 32, 2202180. [Google Scholar] [CrossRef]
- Yu, C.; He, J.; Tan, M.; Hou, Y.; Zeng, H.; Liu, C.; Meng, H.; Su, Y.; Qiao, L.; Lookman, T.; et al. Selective Enhancement of Photo-Piezocatalytic Performance in BaTiO3 via Heterovalent Ion Doping. Adv. Funct. Mater. 2022, 32, 2209365. [Google Scholar] [CrossRef]
- Liu, X.; Shen, L.; Xu, W.; Kang, W.; Yang, D.; Li, J.; Ge, S.; Liu, H. Low Frequency Hydromechanics-Driven Generation of Superoxide Radicals via Optimized Piezotronic Effect for Water Disinfection. Nano Energy 2021, 88, 106290. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, X.; Wen, J.; Zhu, J.; Bian, Z. Piezo-Promoted the Generation of Reactive Oxygen Species and the Photodegradation of Organic Pollutants. Appl. Catal. B Environ. 2019, 258, 118024. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.; Sun, J.; Zhang, L.; Zhong, L. Effect of the Mn Doping Concentration on the Dielectric and Ferroelectric Properties of Different-Routes-Fabricated BaTiO3-Based Ceramics. J. Alloys Compd. 2016, 670, 48–54. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Na, E.-S.; Paik, U.; Lee, J.; Kim, J. A Study on the Phase Transition and Characteristics of Rare Earth Elements Doped BaTiO3. Mater. Res. Bull. 2002, 37, 1633–1640. [Google Scholar] [CrossRef]
- Siemek, K.; Olejniczak, A.; Korotkov, L.N.; Konieczny, P.; Belushkin, A.V. Investigation of Surface Defects in BaTiO3 Nanopowders Studied by XPS and Positron Annihilation Lifetime Spectroscopy. Appl. Surf. Sci. 2022, 578, 151807. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Chen, T.C.S. Surface Chemical States of Barium Titanate: Influence of Sample Processing. J. Mater. Res. 1995, 10, 1502–1507. [Google Scholar] [CrossRef]
- Li, P.-H.; Song, Z.-Y.; Yang, M.; Chen, S.-H.; Xiao, X.-Y.; Duan, W.; Li, L.-N.; Huang, X.-J. Electrons in Oxygen Vacancies and Oxygen Atoms Activated by Ce3+/Ce4+ Promote High-Sensitive Electrochemical Detection of Pb(II) over Ce-Doped α-MoO3 Catalysts. Anal. Chem. 2020, 92, 16089–16096. [Google Scholar] [CrossRef]
- Quasim Khan, M.; Alharthi, F.A.; Ahmad, K.; Kim, H. Hydrothermal Synthesis of BaTiO3 Perovskite for H2O2 Sensing. Chem. Phys. Lett. 2022, 805, 139950. [Google Scholar] [CrossRef]
- Lan, S.; Feng, J.; Xiong, Y.; Tian, S.; Liu, S.; Kong, L. Performance and Mechanism of Piezo-Catalytic Degradation of 4-Chlorophenol: Finding of Effective Piezo-Dechlorination. Environ. Sci. Technol. 2017, 51, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhuang, W.; Zhao, M.; Zhang, J.; Song, Y.; Liu, S.; Zheng, H.; Zhao, C. Role of Driven Approach on the Piezoelectric Ozonation Processes: Comparing Ultrasound with Hydro-Energy as Driving Forces. J. Hazard. Mater. 2021, 418, 126392. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lan, S.; Cheng, S.; Zeng, L.; Zhu, M. Ba Substituted SrTiO3 Induced Lattice Deformation for Enhanced Piezocatalytic Removal of Carbamazepine from Water. J. Hazard. Mater. 2022, 424, 127440. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jin, C.; Shan, F.; He, J.; Wang, F. Synthesizing BaTiO3 Nanostructures to Explore Morphological Influence, Kinetics, and Mechanism of Piezocatalytic Dye Degradation. ACS Appl. Mater. Interfaces 2020, 12, 17443–17451. [Google Scholar] [CrossRef]
- Ji, M.; Kim, J.H.; Ryu, C.-H.; Lee, Y.-I. Synthesis of Self-Modified Black BaTiO3−x Nanoparticles and Effect of Oxygen Vacancy for the Expansion of Piezocatalytic Application. Nano Energy 2022, 95, 106993. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Li, J.; Qiu, J.; Sunarso, J.; Liu, S. The Mechanism of Piezocatalysis: Energy Band Theory or Screening Charge Effect? Angew. Chem. 2022, 134, e202110429. [Google Scholar] [CrossRef]
- Liu, Q.; Zhan, F.; Luo, H.; Luo, X.; Yi, Q.; Sun, Q.; Xiao, Z.; Zhang, Y.; Zhang, D.; Bowen, C.R. Na-Sm Bimetallic Regulation and Band Structure Engineering in CaBi2Nb2O9 to Enhance Piezo-Photo-Catalytic Performance. Adv. Funct. Mater. 2023, 33, 2303736. [Google Scholar] [CrossRef]
- Zhu, M.; Liao, B.; Tang, Y.; Chen, X.; Ma, R.; Li, L.; Fan, X. The Superior Piezocatalytic Performance of SrBi2Ta2O9 Nanoflower: Mechanism of Screening Effect and Energy Band Theory. Appl. Surf. Sci. 2023, 628, 157366. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, L.; Xu, Y.; Feng, W.; Qiu, X. Impact of Oxygen Vacancy on Piezo-Photocatalytic Catalytic Activity of Barium Titanate. Appl. Surf. Sci. 2023, 619, 156794. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Fan, S.; Chen, X.; Qin, M.; Long, D.; Tadé, M.O.; Liu, S. Impact of Oxygen Vacancy Occupancy on Piezo-Catalytic Activity of BaTiO3 Nanobelt. Appl. Catal. B Environ. 2020, 279, 119340. [Google Scholar] [CrossRef]
- Gan, J.; Lu, X.; Wu, J.; Xie, S.; Zhai, T.; Yu, M.; Zhang, Z.; Mao, Y.; Wang, S.C.I.; Shen, Y.; et al. Oxygen Vacancies Promoting Photoelectrochemical Performance of In2O3 Nanocubes. Sci. Rep. 2013, 3, 1021. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, D.; Xiang, Y.; Yang, Z.; Yuan, H.; Tang, L.; Li, S. The Piezocatalytic Degradation of Sulfadiazine by Lanthanum-Doped Barium Titanate. Molecules 2024, 29, 1719. https://doi.org/10.3390/molecules29081719
Meng D, Xiang Y, Yang Z, Yuan H, Tang L, Li S. The Piezocatalytic Degradation of Sulfadiazine by Lanthanum-Doped Barium Titanate. Molecules. 2024; 29(8):1719. https://doi.org/10.3390/molecules29081719
Chicago/Turabian StyleMeng, Daijun, Yuqi Xiang, Ziwei Yang, Hao Yuan, Liang Tang, and Shiyang Li. 2024. "The Piezocatalytic Degradation of Sulfadiazine by Lanthanum-Doped Barium Titanate" Molecules 29, no. 8: 1719. https://doi.org/10.3390/molecules29081719