New Amino Acid-Based Thiosemicarbazones and Hydrazones: Synthesis and Evaluation as Fluorimetric Chemosensors in Aqueous Mixtures
Abstract
:1. Introduction
2. Results and Discussion
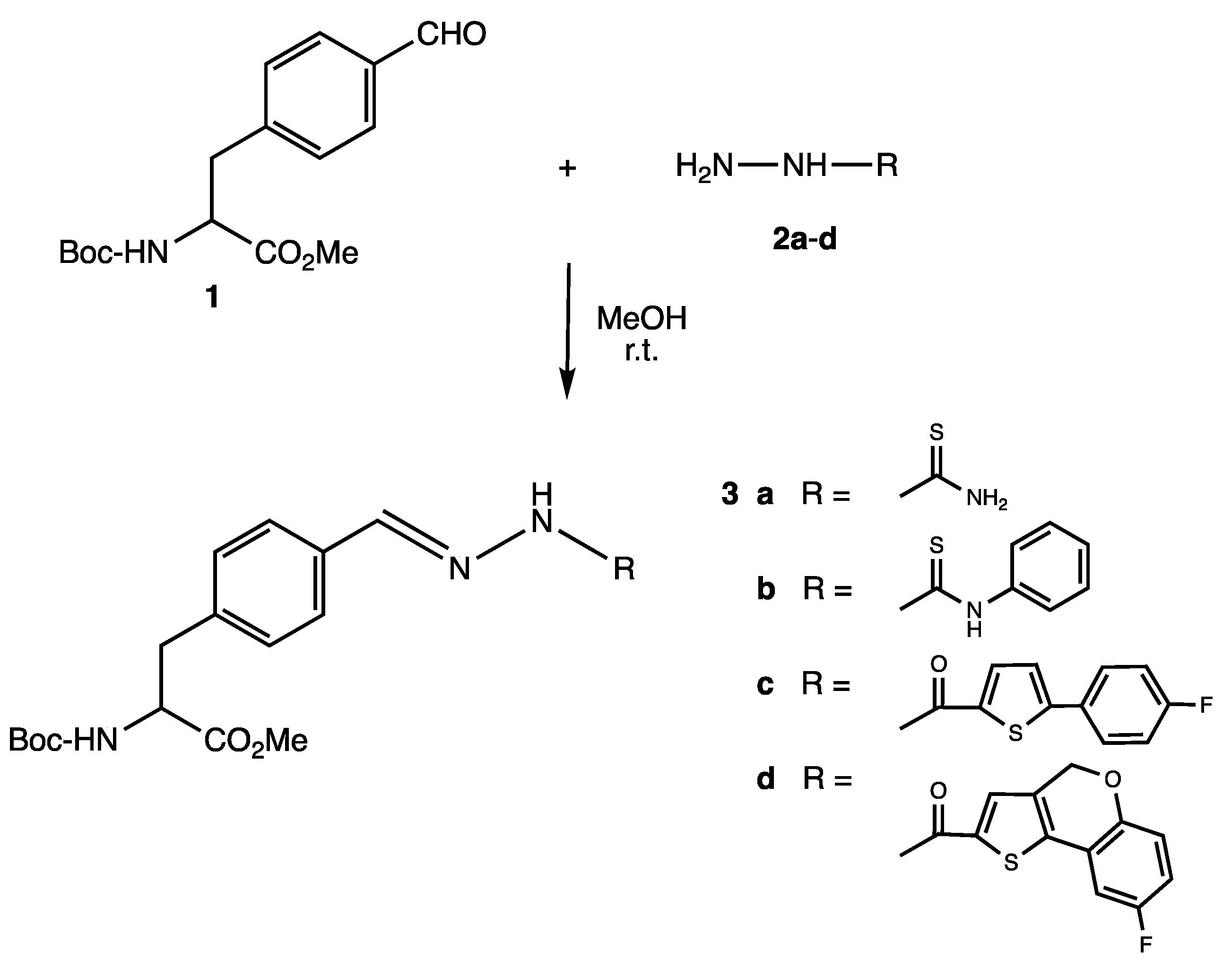
2.1. Synthesis of Phenylalanine Thiosemicarbazones and Hydrazones 3a–d
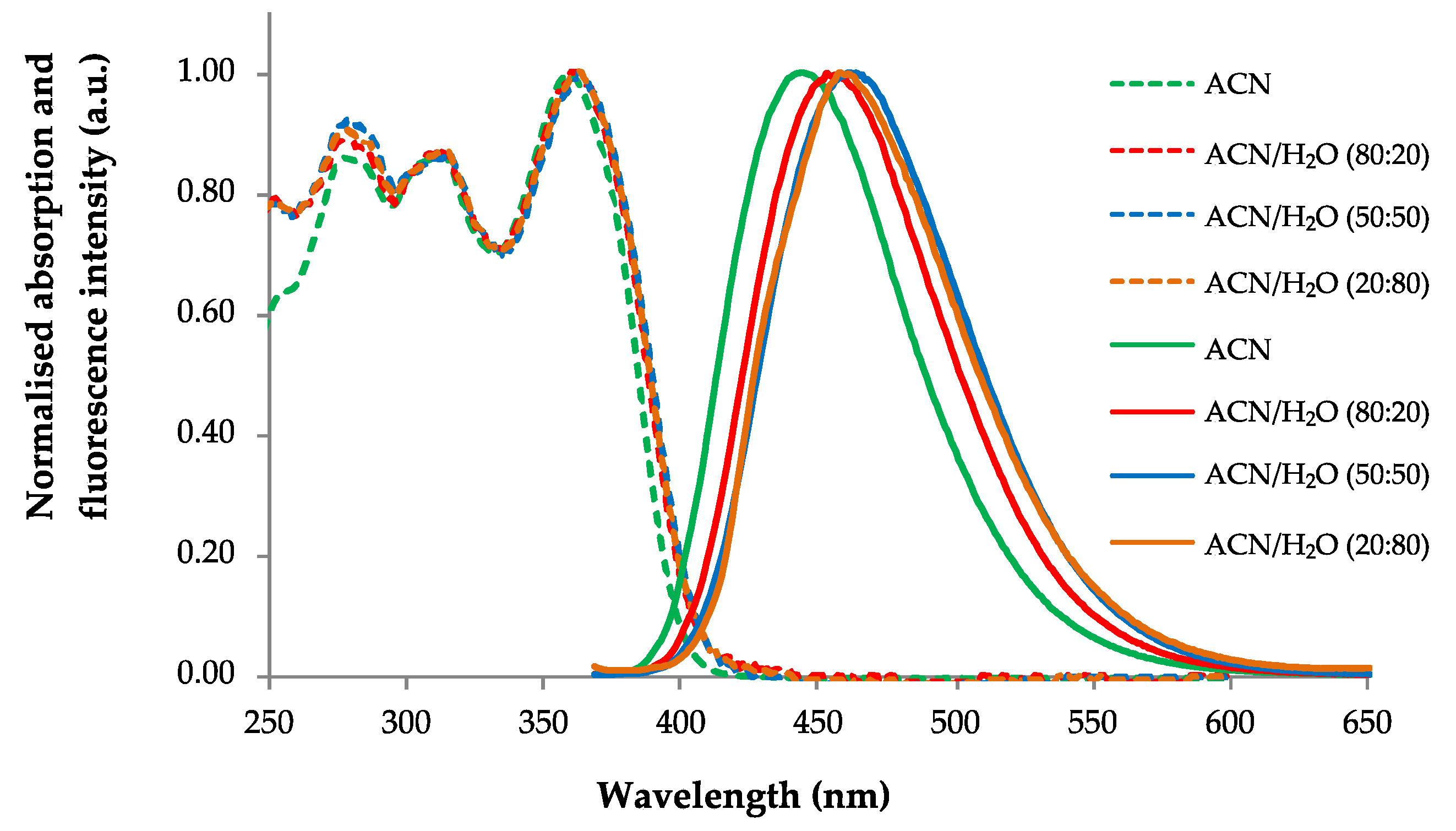
2.2. Photophysical Study of Phenylalanines 3a–d
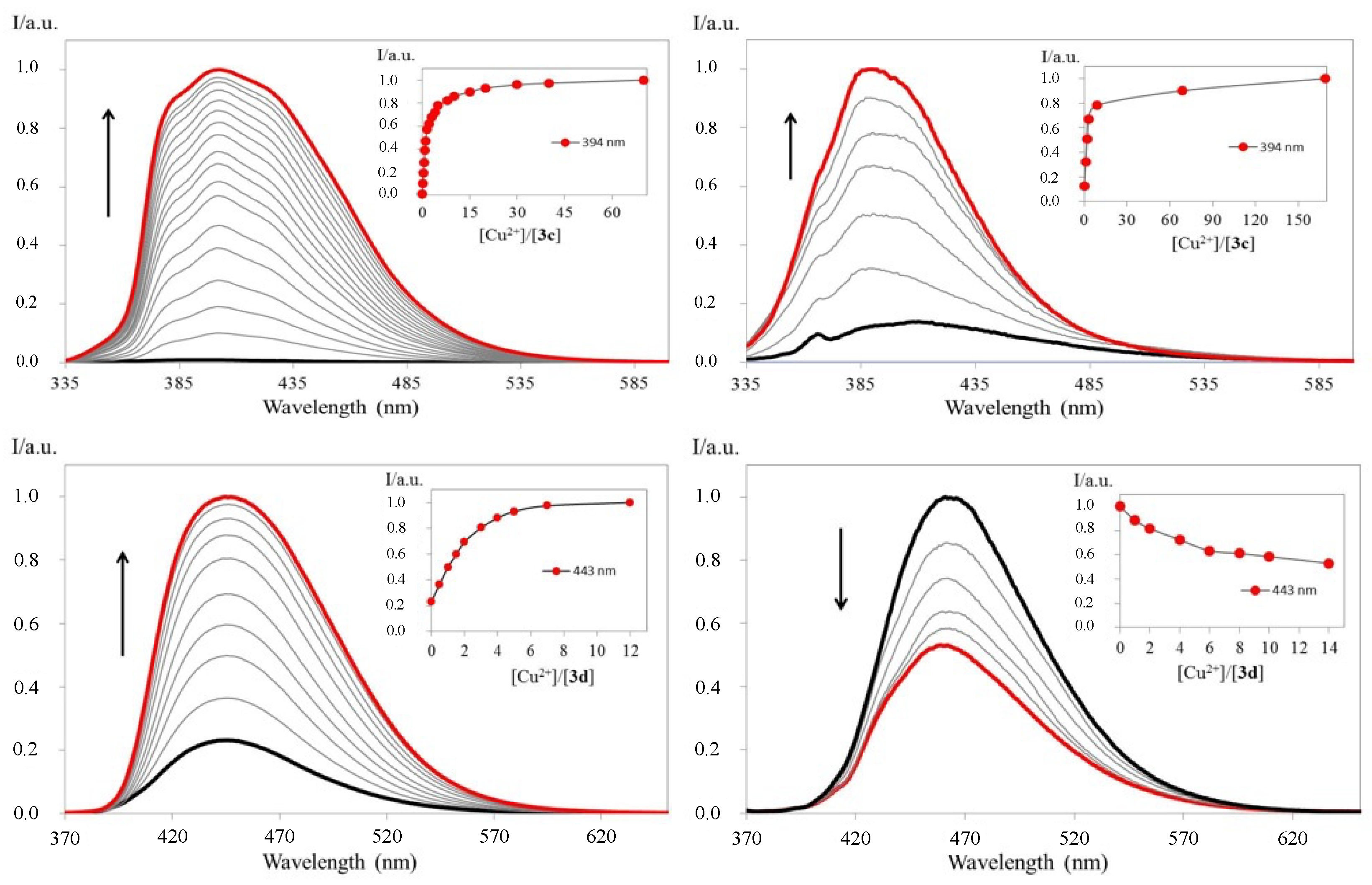
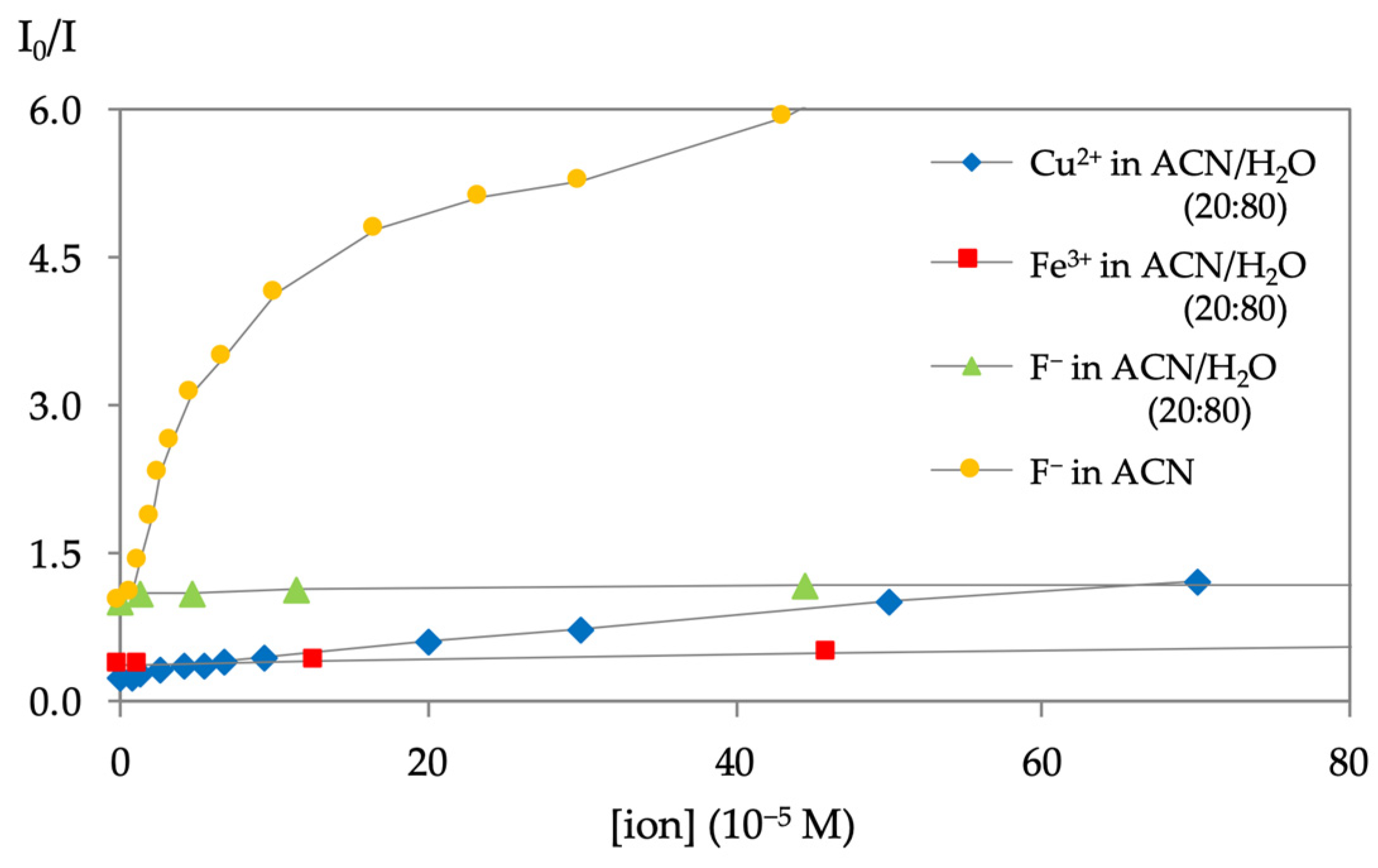
2.3. Preliminary Chemosensing Tests and Spectrofluorimetric Titrations of Phenylalanines 3a–d with Selected Ions
3. Materials and Methods
3.1. General
3.2. Synthesis of Phenylalanine Thiosemicarbazones and Hydrazones 3a–d
3.2.1. N-(tert-Butoxycarbonyl)-4-((2-carbamothioylhydrazono)methylene)-L-phenylalanine methyl ester 3a
3.2.2. N-(tert-Butoxycarbonyl)-4-((2-(phenylcarbamothioyl)hydrazono)methylene)-L-phenyl-alanine methyl ester 3b
3.2.3. N-(tert-Butoxycarbonyl)-4-((2-(5′-(4″-fluorophenyl)thiophene-2′-carbonyl)hydrazono) methylene)-L-phenylalanine methyl ester 3c
3.2.4. N-(tert-Butoxycarbonyl)-4-((2-(8′-fluoro-4H-[1]-benzopyrano [4,3-b]thiophene-2′-carbonyl)hydrazono)methylene)-L-phenylalanine methyl ester 3d
3.3. Stock Solutions
3.4. Preliminary Chemosensing Tests and Fluorimetric Titrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qian, X.; Xu, Z. Fluorescence imaging of metal ions implicated in diseases. Chem. Soc. Rev. 2015, 44, 4487–4493. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Takani, M.; Yamauchi, O. Metal complexes of amino acids and amino acid side chain groups. Structure and properties. Dalton Trans. 2009, 38, 7854–7869. [Google Scholar] [CrossRef]
- Kubik, S. Amino acid containing anion receptors. Chem. Soc. Rev. 2009, 38, 585–605. [Google Scholar] [CrossRef]
- Elia, N. Using unnatural amino acids to selectively label proteins for cellular imaging: A cell biologist viewpoint. FEBS J. 2021, 288, 1107–1117. [Google Scholar] [CrossRef]
- Won, Y.; Pagar, A.D.; Patil, M.D.; Dawson, P.E.; Yun, H. Recent advances in enzyme engineering through incorporation of unnatural amino acids. Biotech. Bioproc. Eng. 2019, 24, 592–604. [Google Scholar] [CrossRef]
- Pless, S.A.; Ahern, C.A. Unnatural amino acids as probes of ligand-receptor interactions and their conformational consequences. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Guo, J. Expanding the chemistry of fluorescent protein biosensors through genetic incorporation of unnatural amino acids. Mol. BioSyst. 2013, 9, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, Y.; Yufu, T.; Kato, T. The effect of a peptide substrate containing an unnatural branched amino acid on chymotrypsin activity. Processes 2021, 9, 242. [Google Scholar] [CrossRef]
- Nagarajan, R.; Vanjare, B.D.; Lee, K.H. The first tryptophan based turn-off chemosensor for Fe2+ ion detection. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 262, 120103. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ryu, K.; Park, J.; Subedi, S.; Lee, K.-H. Design and synthesis of fluorescent peptide-based probes with aggregation-induced emission characteristic for detecting CH3Hg+ and Hg2+ in aqueous environment: Tuning fluorescent detection for CH3Hg+ by replacing peptide receptors. Dyes Pigment. 2022, 204, 110461. [Google Scholar] [CrossRef]
- Kumar, V.; Rattan, G.; Tewatia, P.; Kaur, M.; Pathania, D.; Singhal, S.; Kaushik, A. Rice straw derived cellulose nanofibers modified with L-histidine for ultra-trace fluorometric assay of Cr(VI) and Hg(II) in aqueous medium. J. Clean. Prod. 2023, 391, 136106. [Google Scholar] [CrossRef]
- Swathy, S.; Pallam, G.S.; Kumar, K.G. Tryptophan capped gold–silver bimetallic nanoclusters-based turn-off fluorescence sensor for the determination of histamine. Talanta 2023, 256, 124321. [Google Scholar] [CrossRef]
- Alcay, Y.; Ozdemir, E.; Yildirim, M.S.; Ertugral, U.; Yavuz, O.; Aribuga, H.; Ozkilic, Y.; Tuzun, N.Ş.; Sert, A.B.O.; Kok, F.N.; et al. A methionine biomolecule-modified chromenylium-cyanine fluorescent probe for the analysis of Hg2+ in the environment and living cells. Talanta 2023, 259, 124471. [Google Scholar] [CrossRef]
- Drisya, V.; Shurooque, K.S.; Das, S.; Chakkumkumarath, L. Fmoc-phenylalanine-based fluorimetric and colorimetric dual-channel probes for the detection of Fe3+, Cu2+ and F−. J. Photochem. Photobiol. A Chem. 2023, 442, 114796. [Google Scholar] [CrossRef]
- Tamrakar, A.; Nigam, K.K.; Maddeshiya, T.; Pandey, M.D. Pyrene functionalized luminescent phenylalanine for selective detection of copper (II) ions in aqueous media. J. Fluoresc. 2023, 33, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Pandey, M.; Chakraborty, S.; Sen, S.; Halder, S. Development of colorimetric probe for the selective detection of HgII. Aust. J. Chem. 2023, 76, 581–589. [Google Scholar] [CrossRef]
- Qin, T.; Wang, J.; Liu, Y.; Guo, S. Carbon Quantum Dots based chemosensor array for monitoring multiple metal ions. Molecules 2022, 27, 3843. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzyński, L.; Makowska, J. A pentapeptide with tyrosine moiety as fluorescent chemosensor for selective nanomolar-level detection of copper(II) ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef] [PubMed]
- Immanuel David, C.; Prabakaran, G.; Nandhakumar, R. Recent approaches of 2HN derived fluorophores on recognition of Al3+ ions: A review for future outlook. Microchem. J. 2021, 169, 106590. [Google Scholar] [CrossRef]
- Prabakaran, G.; Immanuel David, C.; Nandhakumar, R. A review on pyrene based chemosensors for the specific detection on d-transition metal ions and their various applications. J. Environ. Chem. Eng. 2023, 11, 109701. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Patil, N.S.; Dhake, R.B.; Ahamed, M.I.; Fegade, U. A mini review on organic chemosensors for cation recognition (2013–2019). J. Fluoresc. 2020, 30, 1295–1330. [Google Scholar] [CrossRef] [PubMed]
- Wagay, S.A.; Khan, L.; Ali, R. Recent advancements in ion-pair receptors. Chem. Asian J. 2023, 18, e202201080. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, D.; Xie, X.; Ma, S.; Liu, Y.; Xu, Z.; Gao, Y.; Ye, Y. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B 2016, 224, 661–667. [Google Scholar] [CrossRef]
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, G.; Fegade, U.A.; Singh, A.; Sahoo, S.K.; Kuwar, A.S.; Singh, N. Anion sensing with chemosensors having multiple NH recognition units. Trends Anal. Chem. 2017, 95, 86–109. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, C.; Zhang, H.; Ai, T.; Kou, K. Hydrogen-bonded colorimetric and fluorescence chemosensor for fluoride anion with high selectivity and sensitivity: A review. Front. Chem. 2021, 9, 666450. [Google Scholar] [CrossRef]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; PonikvarSvet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical aspects of human and environmental overload with fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef]
- Pereira, T.M.; Kümmerle, A.E. Hydrazone-Based Small-Molecule Chemosensors. In Computational Biology and Chemistry; Behzadi, P., Bernabò, N., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Jabeen, M. A comprehensive review on analytical applications of hydrazone derivatives. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 663–698. [Google Scholar] [CrossRef]
- Chen, W.; Liang, H.; Wen, X.; Li, Z.; Xiong, H.; Tian, Q.; Yan, M.; Tan, Y.; Royal, G. Synchronous colorimetric determination of CN−, F−, and H2PO4− based on structural manipulation of hydrazone sensors. Inorg. Chim. Acta 2022, 532, 120760. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Khan, S. Recent advances in thiourea based colorimetric and fluorescent chemosensors for detection of anions and neutral analytes: A review. Crit. Rev. Anal. Chem. 2022, 52, 1–17. [Google Scholar] [CrossRef]
- Okudan, A.; Erdemir, S.; Kocyigit, O. Naked-eye detection of fluoride and acetate anions by using simple and efficient urea and thiourea based colorimetric sensors. J. Mol. Struct. 2013, 1048, 392–398. [Google Scholar] [CrossRef]
- Santos-Figueroa, L.E.; Moragues, M.E.; Raposo, M.M.M.; Batista, R.M.F.; Ferreira, R.C.M.; Costa, S.P.G.; Sancenón, F.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J. Synthesis and evaluation of thiosemicarbazones functionalized with furyl moieties as new chemosensors for anion recognition. Org. Biomol. Chem. 2012, 10, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.M.R.; Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Novel crown ether amino acids as fluorescent reporters for metal ions. Molecules 2023, 28, 3326. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New fluoroionophores for metal cations based on benzo[d]oxazol-5-yl-alanine bearing pyrrole and imidazole. Dyes Pigm. 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. New 2,4,5-triarylimidazoles based on a phenylalanine core: Synthesis, photophysical characterization and evaluation as fluorimetric chemosensors for ion recognition. Dyes Pigm. 2016, 134, 358–368. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. Non-canonical amino acids bearing thiophene and bithiophene: Synthesis by an Ugi multicomponent reaction and studies on ion recognition ability. Amino Acids 2017, 49, 921–930. [Google Scholar] [CrossRef]
- Morera, E.; Ortar, G.; Varani, A. An improved preparation of 4-hydroxymethyl-L-phenylalanine. Synth. Commun. 1998, 28, 4279–4285. [Google Scholar] [CrossRef]
- Shieh, W.-C.; Carlson, J.A. A simple asymmetric synthesis of 4-arylphenylalanines via palladium-catalyzed cross-coupling reaction of arylboronic acids with tyrosine triflate. J. Org. Chem. 1992, 57, 379–381. [Google Scholar] [CrossRef]
- Morris, J.V.; Mahaney, M.A.; Huber, J.R. Fluorescence quantum yield determinations-9,10-diphenylanthracene as a reference-standard in different solvents. J. Phys. Chem. 1976, 80, 969–974. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Diao, X.; Li, Y.; Su, Y.; Yang, J.; Shang, Z.; Liu, S.; Zhou, J.; Li, G.; et al. Mitochondria-targeted fluorescent nanoparticles with large stokes shift for long-term bioimaging. Molecules 2023, 28, 3962. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Costa, S.P.G.; Raposo, M.M.M.; Faza, O.N.; Lodeiro, C. Synthesis, characterization, fluorescence and computational studies of new Cu2+, Ni2+ and Hg2+ complexes with emissive thienylbenzoxazolyl-alanine ligands. Inorg. Chim. Acta 2011, 366, 154–160. [Google Scholar] [CrossRef]
- Oliveira, E.; Genovese, D.; Juris, R.; Zaccheroni, N.; Capelo, J.L.; Raposo, M.M.M.; Costa, S.P.G.; Prodi, L.; Lodeiro, C. Bioinspired systems for metal-ion sensing: New emissive peptide probes based on benzo[d]oxazole derivatives and their gold and silica nanoparticles. Inorg. Chem. 2011, 50, 8834–8849. [Google Scholar] [CrossRef]
- Marín-Hernández, C.; Santos-Figueroa, L.E.; El Sayed, S.; Pardo, T.; Raposo, M.M.M.; Batista, R.M.F.; Costa, S.P.G.; Sancenón, F.; Martínez-Máñez, R. Synthesis and evaluation of the chromo-fluorogenic recognition ability of imidazoquinoline derivatives toward ions. Dyes Pigm. 2015, 122, 50–58. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. Synthesis and evaluation of benzothiazolyl and benzimidazolyl asparagines as amino acid based selective fluorimetric chemosensors for Cu2+. Tetrahedron 2010, 66, 7479–7486. [Google Scholar] [CrossRef]
- Amendola, V.; Fabbrizzi, L.; Mosca, L.; Schmidtchen, F.-P. Urea-, squaramide-, and sulfonamide-based anion receptors: A thermodynamic study. Chem. Eur. J. 2011, 17, 5972–5981. [Google Scholar] [CrossRef]
- Pérez-Casas, C.; Yatsimirsky, A.K. Detailing hydrogen bonding and deprotonation equilibria between anions and urea/thiourea derivatives. J. Org. Chem. 2008, 73, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Mulas, A.; Isaia, F.; Lippolis, V.; Gale, P.A.; Light, M.A. Metal-induced pre-organisation for anion recognition in a neutral platinum-containing receptor. Chem. Commun. 2009, 7, 6279–6281. [Google Scholar] [CrossRef]
- Amendola, V.; Esteban-Gómez, D.; Fabbrizzi, L.; Licchelli, M. What anions do to N−H-containing receptors. Acc. Chem. Res. 2006, 39, 343–353. [Google Scholar] [CrossRef]
- Barišić, D.; Cindro, N.; Vidović, N.; Bregović, N.; Tomišić, V. Protonation and anion-binding properties of aromatic sulfonylurea derivatives. RSC Adv. 2021, 11, 23992–24000. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of program. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Nigam, K.K.; Tamrakar, A.; Mishra, G.; Pandey, M.D. A luminescent pyrene-valine conjugates for the detection of copper (II) ions in aqueous media. Mater. Lett. X 2023, 19, 100212. [Google Scholar] [CrossRef]
- Hao, C.; Li, Y.; Fan, B.; Zeng, G.; Zhang, D.; Bian, Z.; Wu, J. A new peptide-based chemosensor for selective imaging of copper ion and hydrogen sulfide in living cells. Microchem. J. 2020, 154, 104658. [Google Scholar] [CrossRef]
- Hao, C.; Guo, X.; Lai, Q.; Li, Y.; Fan, B.; Zeng, G.; He, Z.; Wu, J. Peptide-based fluorescent chemical sensors for the specific detection of Cu2+ and S2−. Inorg. Chim. Acta 2020, 513, 119943. [Google Scholar] [CrossRef]
- Pundi, A.; Chang, C.-J.; Chen, J.; Hsieh, S.-R.; Lee, M.-C. A dimedone-phenylalanine-based fluorescent sensor for the detection of iron (III), copper (II), L-cysteine, and L-tryptophan in solution and pharmaceutical samples. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 2022, 274, 121108. [Google Scholar] [CrossRef]
- Müller, L.K.; Duznovic, I.; Tietze, D.; Weber, W.; Ali, M.; Stein, V.; Ensinger, W.; Tietze, A.A. Ultrasensitive and selective copper(ii) detection: Introducing a bioinspired and robust sensor. Chem.—A Eur. J. 2020, 26, 8511–8517. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K. Fluorescence Quantum Yields: Methods of Determination and Standards. In Standardization and Quality Assurance in Fluorescence Measurements I.; Resch-Genger, U., Ed.; Springer Series on Fluorescence; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5. [Google Scholar] [CrossRef]


| Cpd. | Yield (%) | UV/Vis | Fluorescence | |||
|---|---|---|---|---|---|---|
| λabs | log ε | λem | Stokes′ Shift (cm−1) | ΦF | ||
| 3a | 93 | 316 | 4.21 | 399 | 6005 | 0.01 |
| 3b | 96 | 322 | 4.24 | 407 | 6486 | 0.01 |
| 3c | 95 | 325 | 4.24 | 394 | 5389 | 0.10 |
| 3d | 95 | 359 | 4.28 | 443 | 5282 | 0.86 |
| Cpd. | Solvent | UV/Vis | Fluorescence | |||
|---|---|---|---|---|---|---|
| λabs | log ε | λem | Stokes′ Shift (cm−1) | ΦF | ||
| 3a | ACN | 316 | 4.21 | 399 | 6005 | 0.01 |
| ACN/H2O (80:20) | 313 | 4.20 | 400 | 6949 | <0.01 | |
| ACN/H2O (50:50) | 312 | 4.20 | 400 | 7051 | <0.01 | |
| ACN/H2O (20:80) | 311 | 4.21 | 400 | 7154 | <0.01 | |
| 3b | ACN | 322 | 4.24 | 407 | 6486 | 0.01 |
| ACN/H2O (80:20) | 321 | 4.24 | 407 | 6583 | <0.01 | |
| ACN/H2O (50:50) | 319 | 4.24 | 407 | 6778 | <0.01 | |
| ACN/H2O (20:80) | 317 | 4.24 | 407 | 6976 | <0.01 | |
| 3c | ACN | 325 | 4.24 | 394 | 5389 | 0.10 |
| ACN/H2O (80:20) | 328 | 4.24 | 405 | 5796 | 0.06 | |
| ACN/H2O (50:50) | 329 | 4.23 | 400 | 5395 | 0.07 | |
| ACN/H2O (20:80) | 333 | 4.23 | 414 | 5875 | 0.08 | |
| 3d | ACN | 359 | 4.28 | 443 | 5282 | 0.86 |
| ACN/H2O (80:20) | 360 | 4.28 | 453 | 5703 | 0.85 | |
| ACN/H2O (50:50) | 363 | 4.28 | 456 | 5618 | 0.78 | |
| ACN/H2O (20:80) | 363 | 4.28 | 458 | 5714 | 0.71 | |
| Cpd. | Ion | log Kass | |
|---|---|---|---|
| ACN | ACN/H2O (20:80) | ||
| 3c | Cd2+ | 11.34 ± 0.04 | --- |
| Cr3+ | 5.08 ± 0.01 | --- | |
| Cu2+ | 12.306 ± 0.007 | 10.15 ± 0.01 | |
| Fe2+ | 10.14 ± 0.05 | --- | |
| Fe3+ | 11.97 ± 0.01 | 7.734 ± 0.008 | |
| Hg2+ | 10.05 ± 0.02 | --- | |
| Ni2+ | 9.958 ± 0.006 | --- | |
| Pb2+ | 10.145 ± 0.006 | --- | |
| Zn2+ | 10.173 ± 0.002 | --- | |
| 3d | F− | 13.25 ± 0.04 | --- |
| OH− | 12.9 ± 0.1 | --- | |
| Cu2+ | 13.88 ± 0.02 | 10.28 ± 0.04 | |
| Fe3+ | 13.482 ± 0.007 | 10.50 ± 0.06 | |
| Hg2+ | 12.93 ± 0.07 | --- | |
| Pb2+ | 12.6 ± 0.1 | --- | |
| Pd2+ | 7.443 ± 0.008 | --- | |
| Reported Chemosensor | Solvent | Fluorescence Variation | Log Kass | Ref. |
|---|---|---|---|---|
| Phenylalanine 3c | ACN/H2O (20:80) | increase | 10.15 | This work |
| Phenylalanine 3d | ACN/H2O (20:80) | quenching | 10.28 | This work |
| Pyrene-phenylalanine conjugate | H2O/DMSO (9:1) | quenching | 4.70 | [15] |
| Pyrene-valine conjugate | H2O/DMSO (9:1) | quenching | 3.00 | [55] |
| Fluorescein isothiocyanate-Ahx-His-Glu-Phe-His-NH2 | 10 mM HEPES buffer pH 7.4 | quenching | 5.20 | [56] |
| Fluorescein isothiocyanate-Ahx-His-Glu-Phe-Cys-NH2 | 10 mM HEPES buffer pH 7.4 | quenching | 11.82 | [57] |
| Dimedone-phenylalanine conjugate | H2O/DMSO (3:7) | quenching | 4.89 | [58] |
| 5,6-carboxyfluorescein-Dap-β-Ala-His | 100 mM MES buffer pH 6.5 | quenching | 6.80 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, C.I.C.; Raposo, M.M.M.; Costa, S.P.G. New Amino Acid-Based Thiosemicarbazones and Hydrazones: Synthesis and Evaluation as Fluorimetric Chemosensors in Aqueous Mixtures. Molecules 2023, 28, 7256. https://doi.org/10.3390/molecules28217256
Esteves CIC, Raposo MMM, Costa SPG. New Amino Acid-Based Thiosemicarbazones and Hydrazones: Synthesis and Evaluation as Fluorimetric Chemosensors in Aqueous Mixtures. Molecules. 2023; 28(21):7256. https://doi.org/10.3390/molecules28217256
Chicago/Turabian StyleEsteves, Cátia I. C., Maria Manuela M. Raposo, and Susana P. G. Costa. 2023. "New Amino Acid-Based Thiosemicarbazones and Hydrazones: Synthesis and Evaluation as Fluorimetric Chemosensors in Aqueous Mixtures" Molecules 28, no. 21: 7256. https://doi.org/10.3390/molecules28217256
APA StyleEsteves, C. I. C., Raposo, M. M. M., & Costa, S. P. G. (2023). New Amino Acid-Based Thiosemicarbazones and Hydrazones: Synthesis and Evaluation as Fluorimetric Chemosensors in Aqueous Mixtures. Molecules, 28(21), 7256. https://doi.org/10.3390/molecules28217256