Hydroxyethyl Cellulose-Based Hydrogels as Controlled Release Carriers for Amorphous Solid Dispersion of Bioactive Components of Radix Paeonia Alba
Abstract
:1. Introduction
2. Results and Discussion
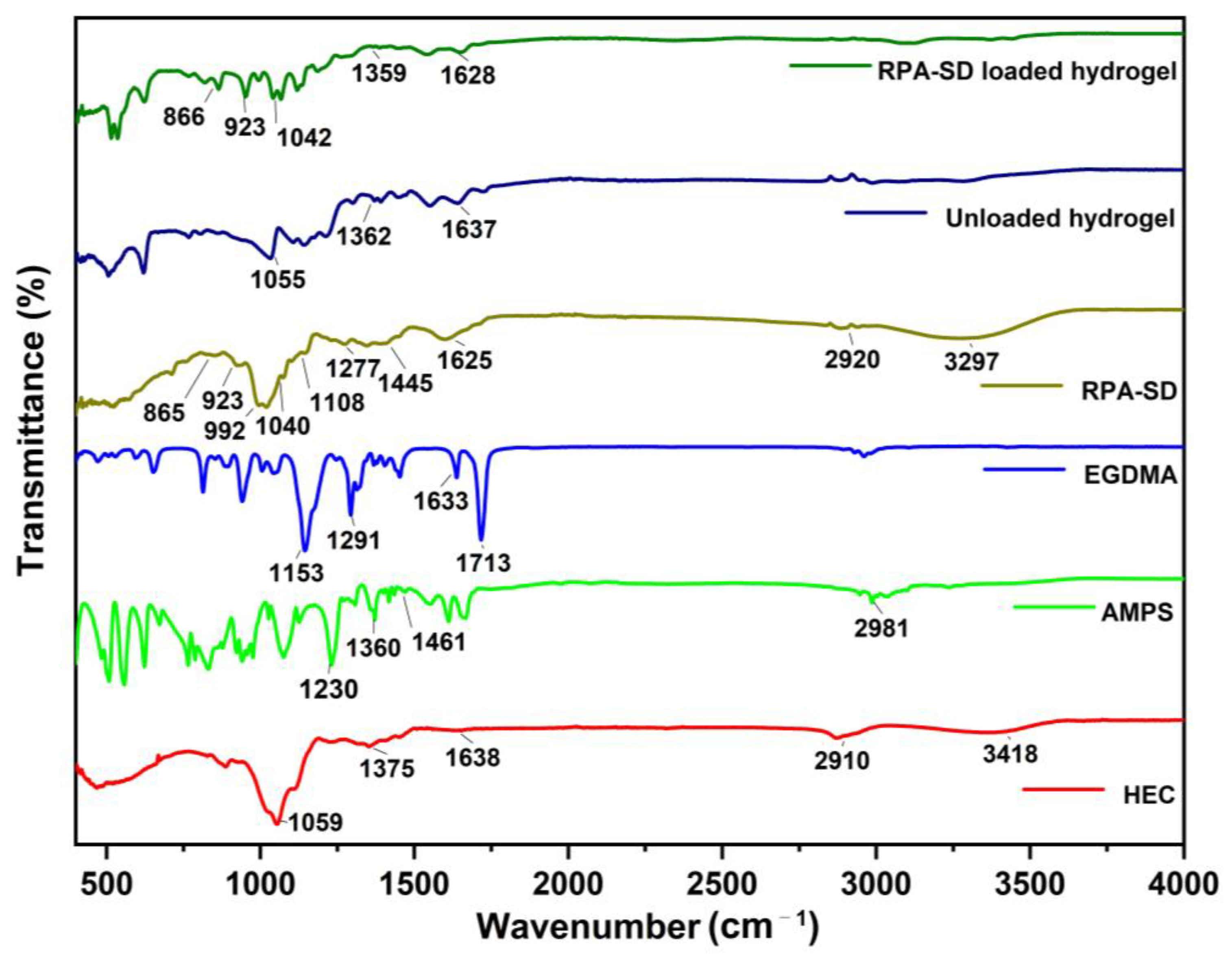
2.1. FTIR Spectral Analysis
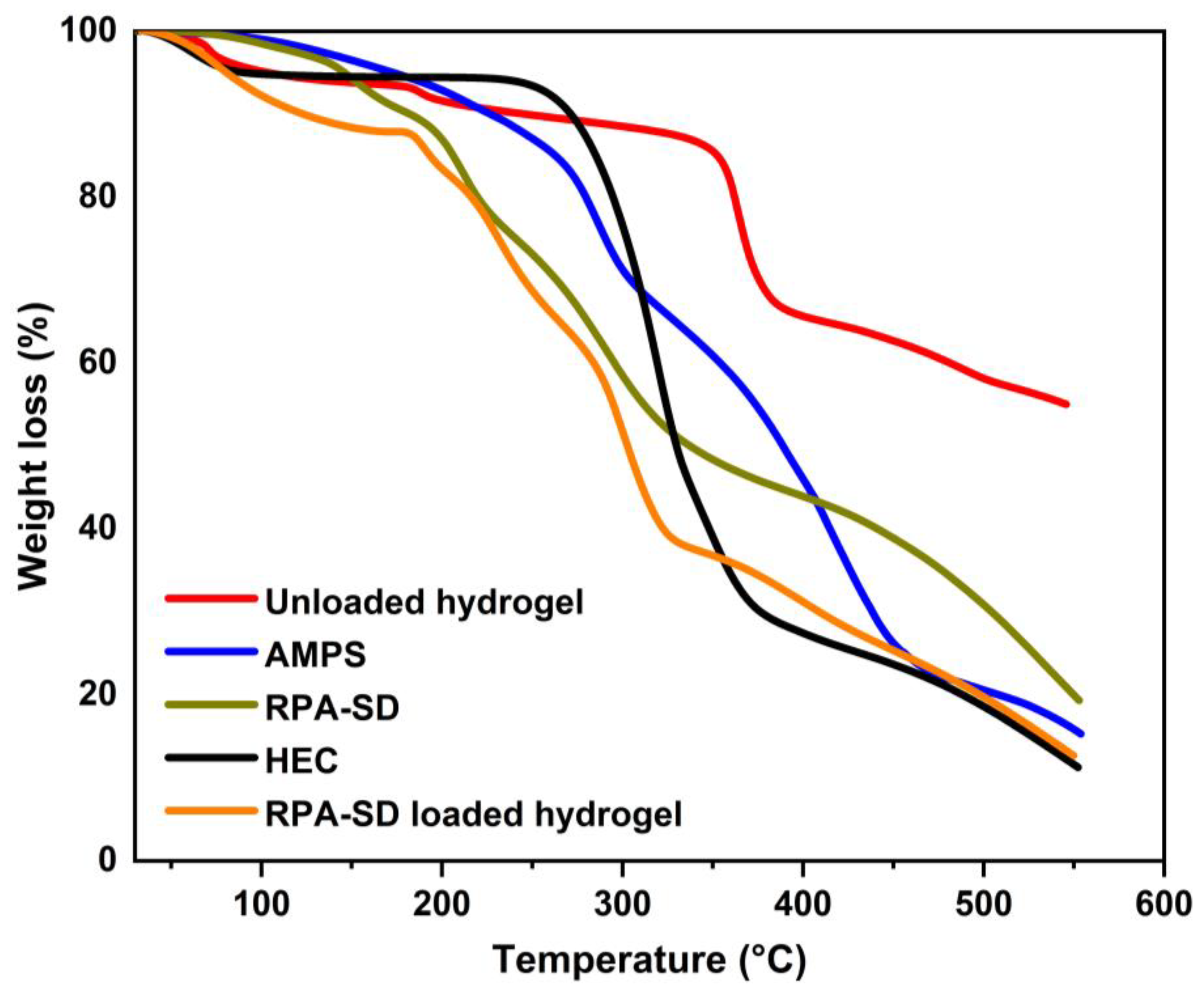
2.2. TGA Thermograms
2.3. DSC Study
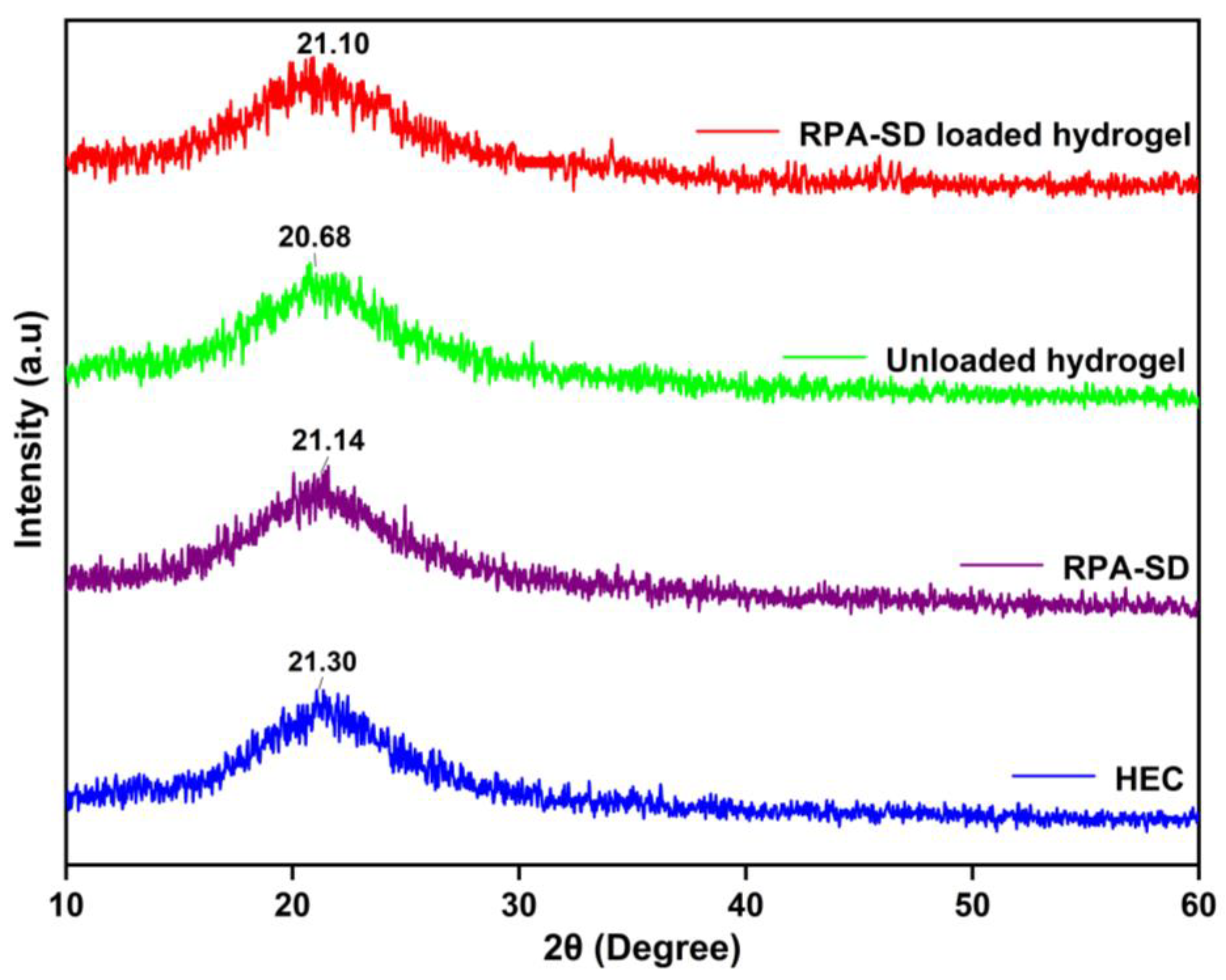
2.4. XRD Studies
2.5. Morphological Analysis
2.6. Mechanical Properties Evaluation
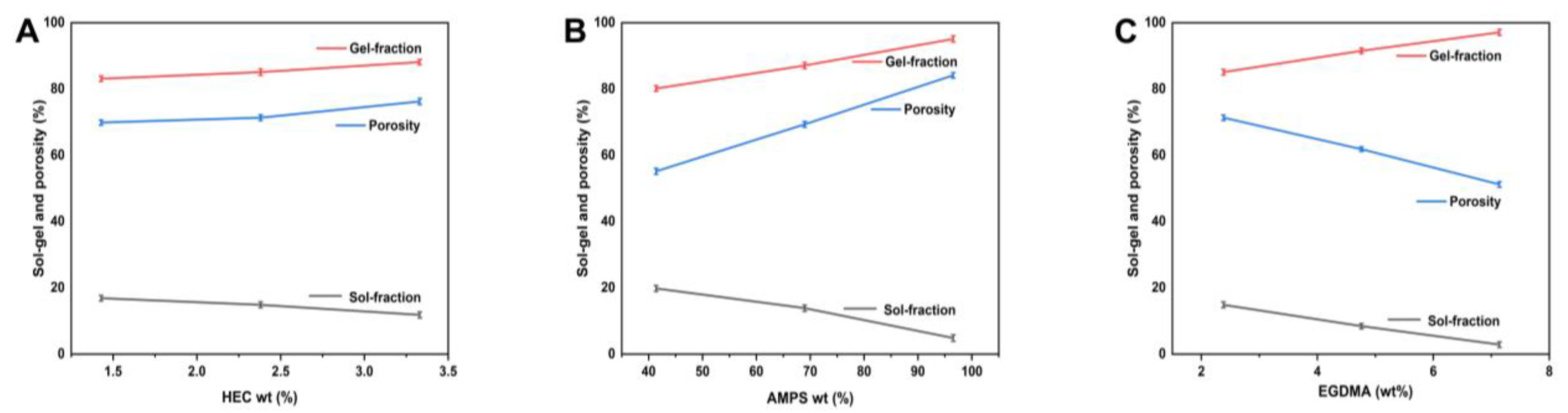
2.7. Sol-Gel Study
2.8. Porosity Evaluation
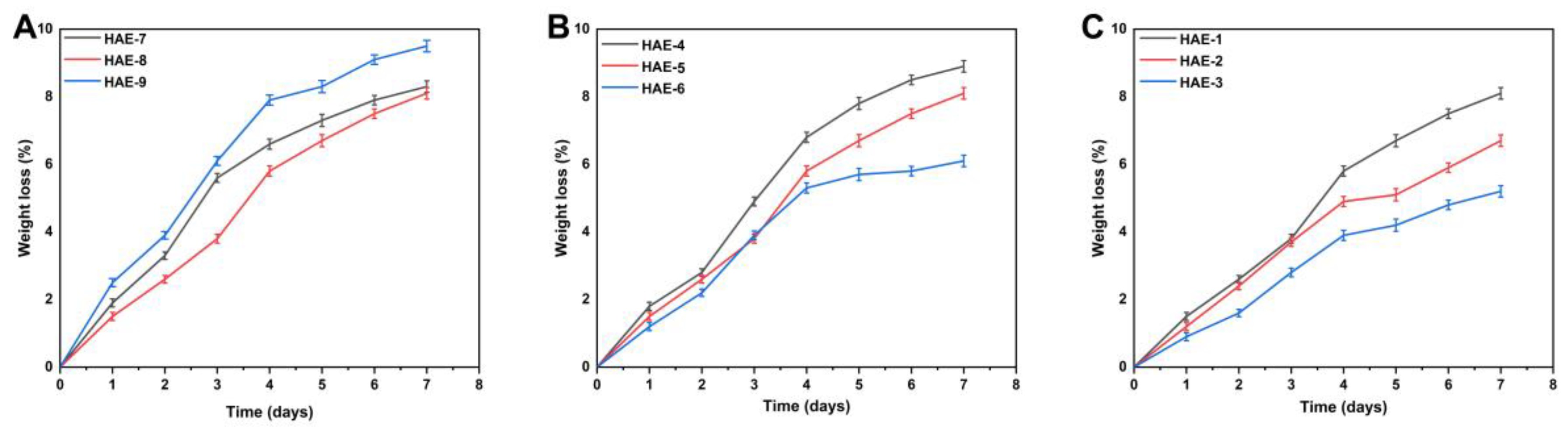
2.9. Biodegradation of Synthesized Hydrogels
2.10. Structural Characteristics of Hydrogels
2.11. Swelling Behavior of Hydrogels
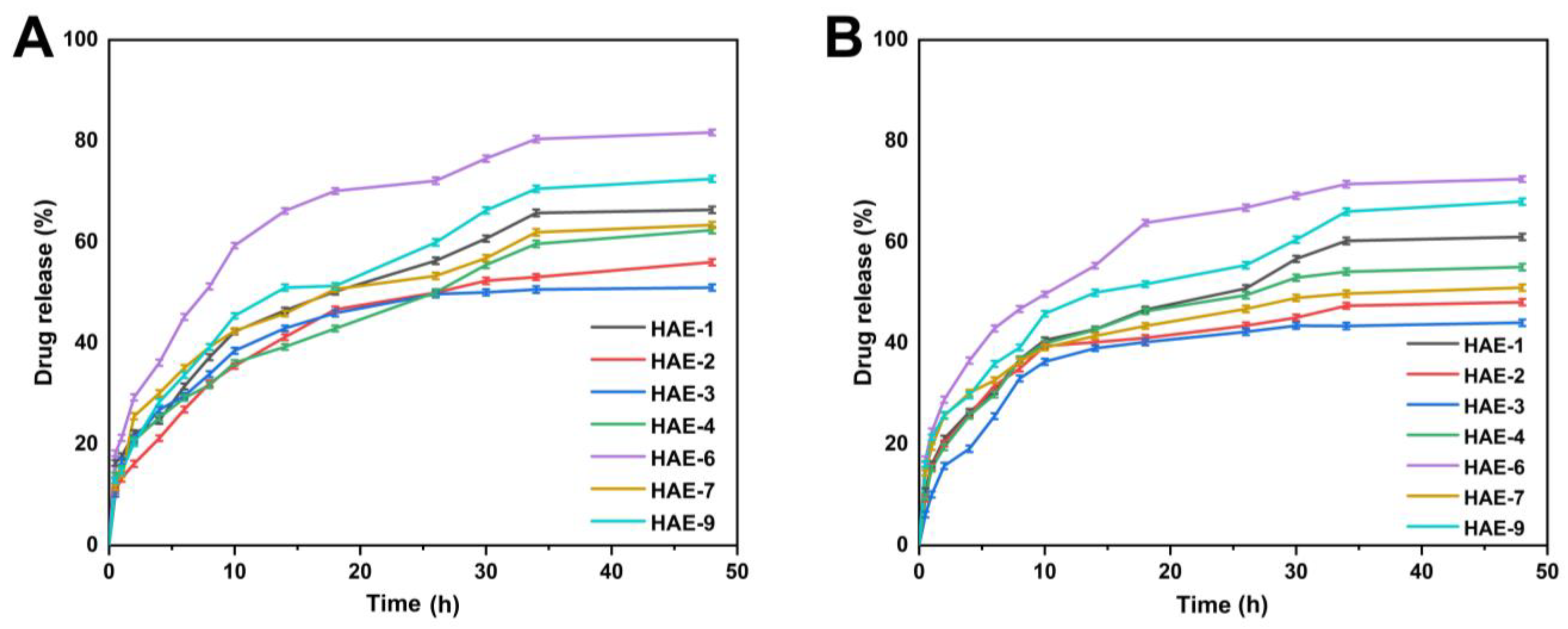
2.12. Release and Kinetic Modelling
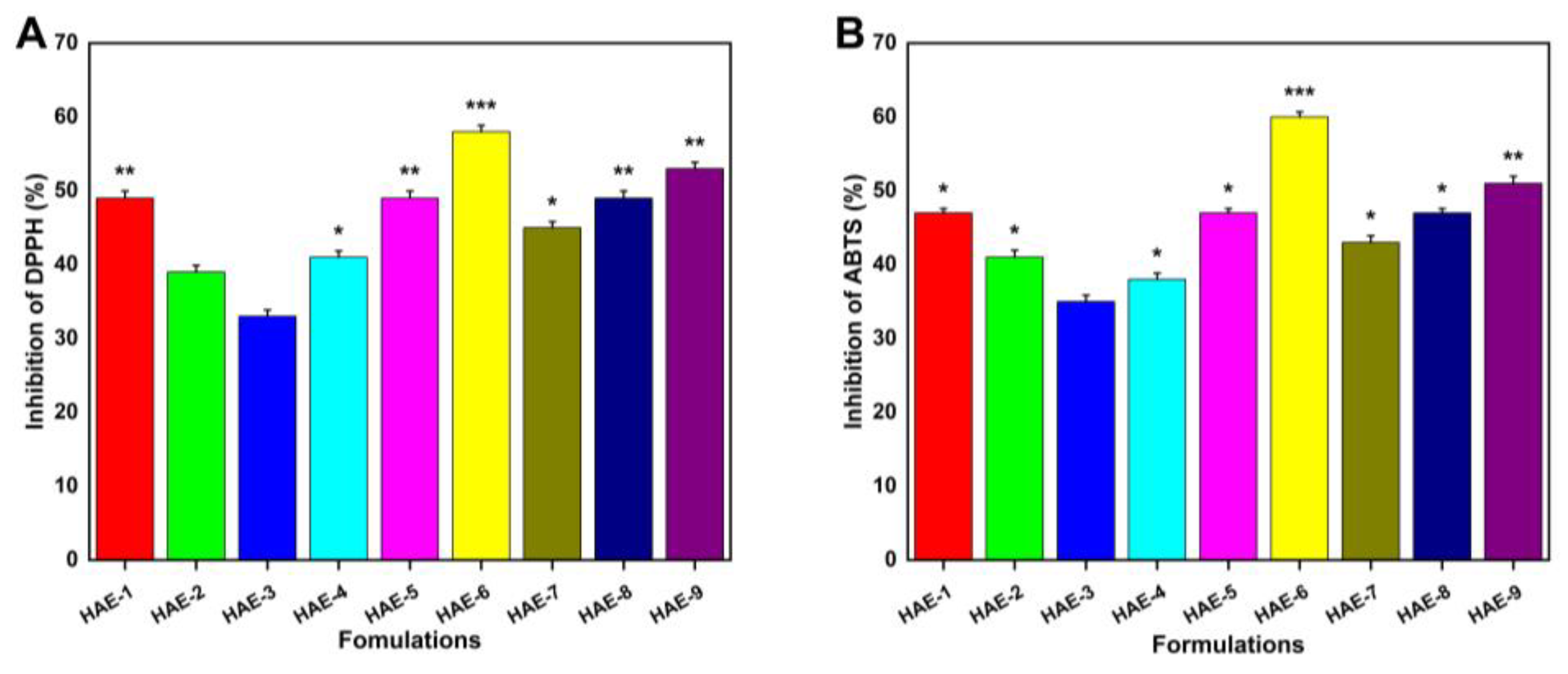
2.13. Antioxidant Effects of Hydrogels
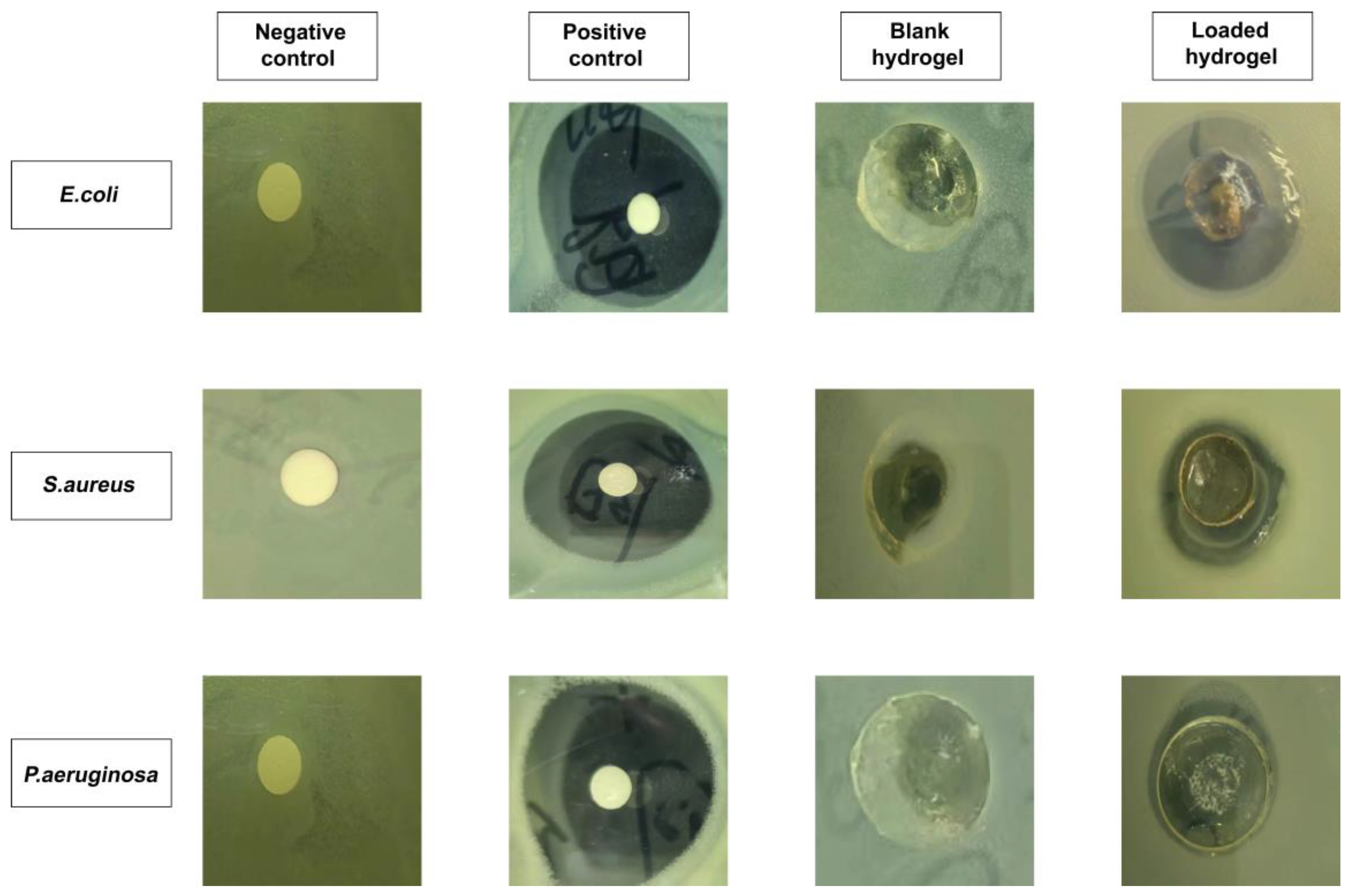
2.14. Antibacterial Properties Evaluation
3. Materials and Methods
3.1. Materials
3.2. Development of Radix Paeonia Alba Amorphous Solid Dispersion (RPA–SD)
Identification of Compounds in RPA-SD Using the UHPLC-Q-TOF-MS Method
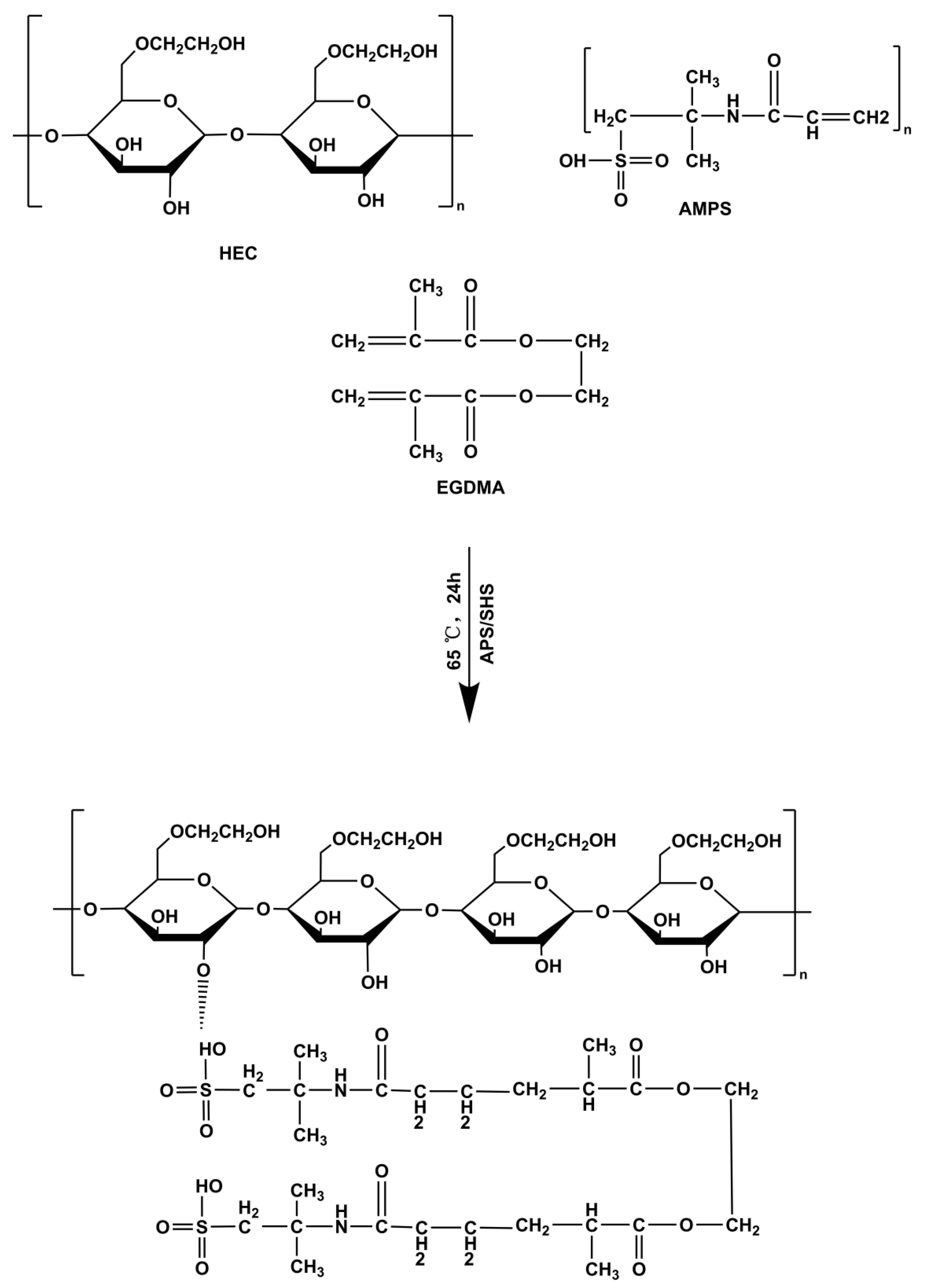
3.3. Preparation of HEC-g-AMPS Hydrogels
RPA–SD Loading into Hydrogels
3.4. In Vitro Solid-State Characterization
3.4.1. Fourier-Transform Infrared (FTIR) Study
3.4.2. Thermal Studies
3.4.3. X-ray Diffraction (XRD) Studies
3.4.4. Morphological Characteristics of Hydrogels
3.4.5. Tensile Behavior of Hydrogels
3.4.6. Sol-Gel Fraction Investigation
3.4.7. Porosity of Hydrogels
3.4.8. Equilibrium Swelling Ratio (ESR)
3.4.9. In Vitro Release Study and Kinetic Data Modelling
3.5. Structural Parameters Affecting Hydrogel Networks
3.5.1. Diffusion Coefficient (D)
3.5.2. Volume Fraction of Polymer (V2,s)
3.5.3. Distribution of Molecular Weights between Crosslinks (Mc)
3.5.4. Solvent Interaction Factor (χ)
3.5.5. Crosslink Repeating Units (N)
3.6. In Vitro Biodegradation of Hydrogels
3.7. Potential Antioxidant Effects of the Hydrogels
3.7.1. DPPH Assay
3.7.2. ABTS Assay
3.8. Antibacterial Effects of Hydrogels
3.9. Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Byeon, J.C.; Ahn, J.B.; Jang, W.S.; Lee, S.-E.; Choi, J.-S.; Park, J.-S. Recent formulation approaches to oral delivery of herbal medicines. J. Pharm. Investig. 2019, 49, 17–26. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, J.; Wang, C.; Fitzgerald, M.; Hu, W.; Zhou, Y.; Zhang, H.; Chen, S. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm. Sin. B 2020, 10, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [PubMed]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Weathers, P.J.; Rich, S.M. Dried whole-plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proc. Natl. Acad. Sci. USA 2015, 112, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.-H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar]
- Weathers, P.J.; Arsenault, P.R.; Covello, P.S.; McMickle, A.; Teoh, K.H.; Reed, D.W. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem. Rev. 2011, 10, 173–183. [Google Scholar]
- Jürgenliemk, G.; Nahrstedt, A. Dissolution, solubility and cooperativity of phenolic compounds from Hypericum perforatum L. in aqueous systems. Die Pharm.-Int. J. Pharm. Sci. 2003, 58, 200–203. [Google Scholar]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef]
- Loureiro Damasceno, J.P.; Silva da Rosa, H.; Silva de Araújo, L.; Jacometti Cardoso Furtado, N.A. Andrographis paniculata formulations: Impact on diterpene lactone oral bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Tchicaillat-Landou, M.; Petit, J.; Gaiani, C.; Miabangana, E.S.; Kimbonguila, A.; Nzikou, J.-M.; Scher, J.; Matos, L. Ethnobotanical study of medicinal plants used by traditional healers for the treatment of oxidative stress-related diseases in the Congo Basin. J. Herb. Med. 2018, 13, 76–90. [Google Scholar]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [PubMed]
- Taylor, L.S.; Zhang, G.G. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016, 101, 122–142. [Google Scholar]
- Tan, Y.-Q.; Chen, H.-W.; Li, J.; Wu, Q.-J. Efficacy, Chemical Constituents, and Pharmacological Actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Dong, Y.; Sun, G.; Jiang, A.; Li, Y. Cleavage rules of mass spectrometry fragments and rapid identification of chemical components of Radix Paeoniae Alba using UHPLC-Q-TOF-MS. Phytochem. Anal. 2021, 32, 836–849. [Google Scholar] [CrossRef]
- Yan, B.; Shen, M.; Fang, J.; Wei, D.; Qin, L. Advancement in the chemical analysis of Paeoniae Radix (Shaoyao). J. Pharm. Biomed. Anal. 2018, 160, 276–288. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar]
- Zhang, X.; Zhao, J.; Xie, P.; Wang, S. Biomedical Applications of Electrets: Recent Advance and Future Perspectives. J. Funct. Biomater. 2023, 14, 320. [Google Scholar]
- Bernhard, S.; Tibbitt, M.W. Supramolecular engineering of hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2021, 171, 240–256. [Google Scholar]
- Tang, J.; Yi, W.; Yan, J.; Chen, Z.; Fan, H.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Highly absorbent bio-sponge based on carboxymethyl chitosan/poly-γ-glutamic acid/platelet-rich plasma for hemostasis and wound healing. Int. J. Biol. Macromol. 2023, 247, 125754. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels based drug delivery synthesis, characterization and administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, T.; Liu, L.; Li, G. Electrochemical characteristics of heme proteins in hydroxyethylcellulose film. Sens. Actuators B Chem. 2006, 113, 106–111. [Google Scholar] [CrossRef]
- Cerrato, A.; Cavaliere, C.; Montone, C.M.; Piovesana, S. New hydrophilic material based on hydrogel polymer for the selective enrichment of intact glycopeptides from serum protein digests. Anal. Chim. Acta 2023, 1245, 340862. [Google Scholar] [CrossRef] [PubMed]
- Biglione, C.; Neumann-Tran, T.M.P.; Kanwal, S.; Klinger, D. Amphiphilic micro- and nanogels: Combining properties from internal hydrogel networks, solid particles, and micellar aggregates. J. Polym. Sci. 2021, 59, 2665–2703. [Google Scholar] [CrossRef]
- Kong, M.; Liu, H.-H.; Wu, J.; Shen, M.-Q.; Wang, Z.-G.; Duan, S.-M.; Zhang, Y.-B.; Zhu, H.; Li, S.-L. Effects of sulfur-fumigation on the pharmacokinetics, metabolites and analgesic activity of Radix Paeoniae Alba. J. Ethnopharmacol. 2018, 212, 95–105. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Sohail, M.; Badshah, S.F.; Abdullah, O.; Khan, S.; Munir, A.; Suhail, M. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev. Ind. Pharm. 2021, 47, 465–476. [Google Scholar] [CrossRef]
- Tokgöz, S.; Kara, A.; Peksoz, A. Synthesis and characterization of poly (EGDMA-co-VPCA)/SWCNT composite films by surface polymerization method. Mater. Sci. Semicond. Process. 2020, 116, 105144. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, W.; Zhao, J.; Qiu, X.; Xiong, H.; Liang, Y.; Ye, X.; Lei, Z.; Chen, D. Preparation and anti-leakage properties of hydroxyethyl cellulose-g-poly (butyl acrylate-co-vinyl acetate) emulsion. Carbohydr. Polym. 2021, 255, 117467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, Y.; Zhao, J.; Zhang, Y.; Guo, D.; Gao, C.; Duan, J.; Li, P. Immunoregulation and antioxidant activities of a novel acidic polysaccharide from Radix Paeoniae Alba. Glycoconj. J. 2020, 37, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Enawgaw, H.; Tesfaye, T.; Yilma, K.T.; Limeneh, D.Y. Synthesis of a cellulose-co-amps hydrogel for personal hygiene applications using cellulose extracted from corncobs. Gels 2021, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf. Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- Tronci, G.; Ajiro, H.; Russell, S.J.; Wood, D.J.; Akashi, M. Tunable drug-loading capability of chitosan hydrogels with varied network architectures. Acta Biomater. 2014, 10, 821–830. [Google Scholar] [CrossRef]
- Owusu-Ware, S.K.; Boateng, J.; Jordan, D.; Portefaix, S.; Tasseto, R.; Ramano, C.D.; Antonijević, M.D. Molecular mobility of hydroxyethyl cellulose (HEC) films characterised by thermally stimulated currents (TSC) spectroscopy. Int. J. Pharm. 2016, 497, 222–227. [Google Scholar] [CrossRef]
- Ashames, A.; Ullah, K.; Al-Tabakha, M.; Khan, S.A.; Hassan, N.; Mannan, A.; Ikram, M.; Buabeid, M.; Murtaza, G. Development, characterization and In-vitro evaluation of guar gum based new polymeric matrices for controlled delivery using metformin HCl as model drug. PLoS ONE 2022, 17, e0271623. [Google Scholar] [CrossRef]
- Farid-ul-Haq, M.; Amin, M.; Hussain, M.A.; Sher, M.; Khan, T.A.; Kausar, F.; Amin, H.M. Comparative isoconversional thermal analysis of Artemisia vulgaris hydrogel and its acetates; a potential matrix for sustained drug delivery. Int. J. Polym. Anal. Charact. 2020, 25, 529–538. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A. Novel biocompatible and antimicrobial supramolecular O-carboxymethyl chitosan biguanidine/zinc physical hydrogels. Int. J. Biol. Macromol. 2020, 163, 649–656. [Google Scholar] [CrossRef]
- Salawi, A.; Khan, A.; Zaman, M.; Riaz, T.; Ihsan, H.; Butt, M.H.; Aman, W.; Khan, R.; Majeed, I.; Almoshari, Y. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14, 2369. [Google Scholar] [CrossRef] [PubMed]
- Hosary, R.E.; El-Mancy, S.M.; El Deeb, K.S.; Eid, H.H.; Tantawy, M.E.E.; Shams, M.M.; Samir, R.; Assar, N.H.; Sleem, A.A. Efficient wound healing composite hydrogel using Egyptian Avena sativa L. polysaccharide containing β-glucan. Int. J. Biol. Macromol. 2020, 149, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, F.H.; Hussain, F.S.J.; Harun, W.; Yusoff, M.M. Highly porous of hydroxyethyl cellulose biocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019, 122, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Stoyneva, V.; Momekova, D.; Kostova, B.; Petrov, P. Stimuli sensitive super-macroporous cryogels based on photo-crosslinked 2-hydroxyethylcellulose and chitosan. Carbohydr. Polym. 2014, 99, 825–830. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef]
- Xing, Z.; Lu, H.; Hossain, M. Renormalized Flory-Huggins lattice model of physicochemical kinetics and dynamic complexity in self-healing double-network hydrogel. J. Appl. Polym. Sci. 2021, 138, 50304. [Google Scholar] [CrossRef]
- Jalil, A.; Khan, S.; Naeem, F.; Haider, M.S.; Sarwar, S.; Riaz, A.; Ranjha, N.M. The structural, morphological and thermal properties of grafted pH-sensitive interpenetrating highly porous polymeric composites of sodium alginate/acrylic acid copolymers for controlled delivery of diclofenac potassium. Des. Monomers Polym. 2017, 20, 308–324. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Ferrero, C.; Massuelle, D.; Doelker, E. Towards elucidation of the drug release mechanism from compressed hydrophilic matrices made of cellulose ethers. II. Evaluation of a possible swelling-controlled drug release mechanism using dimensionless analysis. J. Control. Release 2010, 141, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef]
- Hanna, D.H.; Lotfy, V.F.; Basta, A.H.; Saad, G.R. Comparative evaluation for controlling release of niacin from protein-and cellulose-chitosan based hydrogels. Int. J. Biol. Macromol. 2020, 150, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Danshiitsoodol, N.; Sugimoto, S.; Noda, M.; Sugiyama, M. Anti-oxidant and anti-inflammatory substance generated newly in Paeoniae Radix Alba extract fermented with plant-derived Lactobacillus brevis 174A. Antioxidants 2021, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.S.; Hélary, C.; Mebert, A.M.; Wang, X.; Coradin, T.; Desimone, M.F. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660–4670. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, C.; Zang, Z.; Wan, X.; Naeem, A.; Zhang, R.; Zhu, W. Chitosan/xanthan gum-based (Hydroxypropyl methylcellulose-co-2-Acrylamido-2-methylpropane sulfonic acid) interpenetrating hydrogels for controlled release of amorphous solid dispersion of bioactive constituents of Pueraria lobatae. Int. J. Biol. Macromol. 2023, 224, 380–395. [Google Scholar] [CrossRef]
- Luo, N.; Li, Z.; Qian, D.; Qian, Y.; Guo, J.; Duan, J.-A.; Zhu, M. Simultaneous determination of bioactive components of Radix Angelicae Sinensis–Radix Paeoniae Alba herb couple in rat plasma and tissues by UPLC–MS/MS and its application to pharmacokinetics and tissue distribution. J. Chromatogr. B 2014, 963, 29–39. [Google Scholar] [CrossRef]
- Yu, C.; Chen, X.; Zhu, W.; Li, L.; Peng, M.; Zhong, Y.; Naeem, A.; Zang, Z.; Guan, Y. Synthesis of Gallic Acid-Loaded Chitosan-Grafted-2-Acrylamido-2-Methylpropane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery: In Vitro Biodegradation, Antioxidant, and Antibacterial Effects. Gels 2022, 8, 806. [Google Scholar] [CrossRef]
- Naeem, A.; Yu, C.; Zhu, W.; Zang, Z.; Guan, Y. Study of Hydroxypropyl β-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery. Gels 2023, 9, 246. [Google Scholar] [CrossRef]
- Naeem, A.; Yu, C.; Zhu, W.; Chen, X.; Wu, X.; Chen, L.; Zang, Z.; Guan, Y. Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization. Molecules 2022, 27, 8397. [Google Scholar] [CrossRef]
- Hanna, D.H.; Saad, G.R. Encapsulation of ciprofloxacin within modified xanthan gum-chitosan based hydrogel for drug delivery. Bioorg. Chem. 2019, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.M.; Iqbal, S.; Nasir, B.; Hassan, W.; Ahmed, H.; Iftikhar, S.Y. Formulation of captopril-loaded hydrogel by microwave-assisted free radical polymerization and its evaluation. Polym. Bull. 2022, 79, 7613–7633. [Google Scholar] [CrossRef]
- Kesharwani, P.; Fatima, M.; Singh, V.; Sheikh, A.; Almalki, W.H.; Gajbhiye, V.; Sahebkar, A. Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis. Biomimetics 2022, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; D/O Segar Singh, S.K.; Chetty Annan, N.; Bhattamisra, S.K.; Gorain, B.; Mohd Amin, M.C.I. Budesonide-loaded pectin/polyacrylamide hydrogel for sustained delivery: Fabrication, characterization and in vitro release kinetics. Molecules 2021, 26, 2704. [Google Scholar] [CrossRef] [PubMed]
- Ilgin, P.; Ozay, H.; Ozay, O. A new dual stimuli responsive hydrogel: Modeling approaches for the prediction of drug loading and release profile. Eur. Polym. J. 2019, 113, 244–253. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodriguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or κ-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C 2019, 96, 583–590. [Google Scholar] [CrossRef]
- Naeem, A.; Yu, C.; Zang, Z.; Zhu, W.; Deng, X.; Guan, Y. Synthesis and Evaluation of Rutin–Hydroxypropyl β-Cyclodextrin Inclusion Complexes Embedded in Xanthan Gum-Based (HPMC-g-AMPS) Hydrogels for Oral Controlled Drug Delivery. Antioxidants 2023, 12, 552. [Google Scholar] [CrossRef]
- Hegab, R.A.; Pardue, S.; Shen, X.; Kevil, C.; Peppas, N.A.; Caldorera-Moore, M.E. Effect of network mesh size and swelling to the drug delivery from pH responsive hydrogels. J. Appl. Polym. Sci. 2020, 137, 48767. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, C.; Li, H.; Dong, X.; Zhang, X. Studies on the physicochemical properties, gelling behavior and drug release performance of agar/κ-carrageenan mixed hydrogels. Int. J. Biol. Macromol. 2020, 154, 878–887. [Google Scholar] [CrossRef]
- Zang, Z.; Zhao, S.; Yang, M.; Yu, C.; Ouyang, H.; Chen, L.; Zhu, W.; Liao, Z.-G.; Naeem, A.; Guan, Y. Blood chemical components analysis of honeysuckle and formulation of xanthan gum/starch-based (PVA-co-AA) hydrogels for controlled release. Arab. J. Chem. 2022, 15, 104312. [Google Scholar] [CrossRef]
- Peppas, N.A.; Barr-Howell, B.D. Characterization of the cross-linked structure of hydrogels. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 2019; pp. 27–56. [Google Scholar]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Esposito, L.; Barbosa, A.I.; Moniz, T.; Costa Lima, S.; Costa, P.; Celia, C.; Reis, S. Design and characterization of sodium alginate and poly (vinyl) alcohol hydrogels for enhanced skin delivery of quercetin. Pharmaceutics 2020, 12, 1149. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Buljeta, I.; Šimunović, J.; Kopjar, M. Carboxymethylcellulose hydrogels: Effect of its different amount on preservation of tart cherry anthocyanins and polyphenols. Curr. Plant Biol. 2021, 28, 100222. [Google Scholar] [CrossRef]
- Khattak, S.; Qin, X.-T.; Huang, L.-H.; Xie, Y.-Y.; Jia, S.-R.; Zhong, C. Preparation and characterization of antibacterial bacterial cellulose/chitosan hydrogels impregnated with silver sulfadiazine. Int. J. Biol. Macromol. 2021, 189, 483–493. [Google Scholar] [CrossRef]




| Peak No. | Retention Time/min | Compounds | Formula | Experimental m/z | Theoretical m/z | Error (ppm) | Mode |
|---|---|---|---|---|---|---|---|
| 1 | 1.26 | Sucrose | C12H22O11 | 341.10921 | 341.10894 | 0.8 | − |
| 2 | 3.41 | Desbenzoyl paeoniflorin | C16H24O10 | 375.12996 | 375.12967 | 0.5 | − |
| 3 | 4.16 | 6-O-galloylglucose | C13H16O10 | 331.06762 | 331.06707 | 1.7 | − |
| 4 | 4.35 | Pyrogallol | C6H6O3 | 127.03939 | 127.03897 | 3.3 | + |
| 5 | 4.93 | Moutonone-1-O-β-D-glucoside or isomer | C16H24O9 | 359.13561 | 359.13476 | 2.4 | − |
| 6 | 5.45 | 1’-0-galloylsucrose or isomer | C19H26O15 | 493.12034 | 493.11989 | 0.9 | − |
| 7 | 7.07 | Paeonol | C9H10O3 | 167.07021 | 167.07027 | −0.4 | + |
| 8 | 8.09 | Methyl gallate | C8H8O5 | 185.04433 | 185.04445 | −0.6 | + |
| 9 | 8.30 | Mudanpioside F | C16H24O8 | 343.14057 | 343.13984 | 2.1 | − |
| 10 | 10.47 | Oxypaeoniflorin | C23H28O12 | 495.15169 | 495.1508 | 1.8 | − |
| 11 | 10.47 | Catechin | C15H14O6 | 289.07331 | 289.07176 | 5.4 | − |
| 12 | 10.49 | Salicylicacid | C7H6O3 | 139.03915 | 139.03897 | 1.3 | + |
| 13 | 11.97 | Paeonoside | C15H20O8 | 327.10858 | 327.10854 | 0.1 | − |
| 14 | 12.84 | 6-O-β-d-glucosyl pyran-paeoniolide | C16H26O9 | 361.15151 | 361.15041 | 3.1 | − |
| 15 | 13.59 | Paeonilactone B | C10H12O4 | 197.0807 | 197.08084 | −0.7 | + |
| 16 | 14.34 | Cortex moutan i | C23H28O11 | 479.15633 | 479.15589 | 0.9 | − |
| 17 | 14.35 | Cortex moutan e | C24H30O13 | 525.16158 | 525.16137 | 0.4 | − |
| 18 | 14.36 | Lactiflorin | C23H26O8 | 463.15977 | 463.15987 | −0.2 | + |
| 19 | 14.38 | Paeoniflorin | C23H28O11 | 479.15633 | 479.15589 | 0.9 | − |
| 20 | 14.42 | Albiflorin | C23H28O11 | 479.15633 | 479.15589 | 0.9 | − |
| 21 | 14.42 | Agnuside | C22H26O11 | 465.12014 | 465.14024 | −1.2 | − |
| 22 | 17.44 | Galloylpaeoniflorin or isomer | C30H32O15 | 631.16802 | 631.16684 | 1.9 | − |
| 23 | 17.56 | Galloylpaeoniflorin | C30H32O15 | 631.16802 | 631.16684 | 1.9 | − |
| 24 | 17.8 | Benzoicacid | C7H6O2 | 123.04448 | 123.04406 | 3.4 | + |
| 25 | 18.02 | 1,2,3,4,6-O-pentagalloylglu-cose | C41H32O26 | 939.11402 | 939.11091 | 3.3 | − |
| 26 | 20.11 | Mudanpioside D | C24H30O12 | 509.16701 | 509.16645 | 1.1 | − |
| 27 | 21.17 | 3’,6’-di-O-galloylpaeoniflorin | C37H36O19 | 783.18044 | 783.1778 | 3.4 | − |
| 28 | 21.89 | Benzoyloxypaeoniflorin | C30H32O13 | 599.17816 | 599.17702 | 1.9 | − |
| 29 | 21.89 | Mudanpioside H | C30H32O14 | 615.17351 | 615.17193 | 2.6 | − |
| 30 | 21.99 | Mudanpioside C | C30H32O13 | 599.17816 | 599.17702 | 1.9 | − |
| 31 | 25.51 | Benzoylpaeoniflorin | C30H32O12 | 583.18289 | 583.1821 | 1.4 | − |
| 32 | 25.59 | Mudanpioside J | C31H34O14 | 629.18919 | 629.18758 | 2.6 | − |
| 33 | 25.59 | Mudanpioside B | C31H34O14 | 629.18919 | 629.18758 | 2.6 | − |
| 34 | 25.88 | Benzoylpaeoniflorin | C30H32O12 | 583.18289 | 583.1821 | 1.4 | − |
| 35 | 28.49 | Formononetin | C16H12O4 | 269.08087 | 269.08084 | 0.1 | + |
| 36 | 29.49 | Palbinone | C22H30O4 | 357.20833 | 357.20713 | 3.6 | − |
| 37 | 35.60 | 30-norheder-agenin | C29H44O4 | 455.31751 | 455.31668 | 1.8 | − |
| 38 | 38.24 | Betulinic acid | C30H46O3 | 455.35235 | 455.35197 | 0.8 | + |
| 39 | 41.42 | Dibutylphthalate | C16H22O4 | 279.15918 | 279.15909 | 0.3 | + |
| 40 | 43.92 | Hederagenol | C30H48O4 | 473.36044 | 473.36254 | −4.4 | + |
| 41 | 44.03 | 2,3-hydroxybetulinicacid | C30H48O4 | 473.36044 | 473.36254 | −4.4 | + |
| 42 | 50.28 | Palmitic acid | C16H32O2 | 255.23399 | 255.23295 | 4.1 | − |
| 43 | 54.33 | Ethyl palmitate | C18H36O2 | 283.26519 | 283.26425 | 3.3 | − |
| F. Codes | Thickness (mm) | TS (N/m2) | EAB (%) | RPA-SD Loaded/1 g Hydrogel (g) |
|---|---|---|---|---|
| HAE-1 | 1.15 | 0.677 | 36.9 | 0.488 |
| HAE-2 | 1.27 | 0.824 | 69.7 | 0.411 |
| HAE-3 | 1.26 | 1.124 | 75.8 | 0.356 |
| HAE-4 | 0.98 | 0.716 | 55.7 | 0.465 |
| HAE-5 | 1.15 | 0.677 | 36.9 | 0.488 |
| HAE-6 | 1.26 | 0.414 | 31.5 | 0.571 |
| HAE-7 | 1.18 | 0.635 | 32.5 | 0.481 |
| HAE-8 | 1.15 | 0.677 | 36.9 | 0.488 |
| HAE-9 | 1.19 | 0.713 | 60.8 | 0.491 |
| F. Codes | V2,s | χ | Mc | Mr | N | D × 10−5 (cm2 s−1) |
|---|---|---|---|---|---|---|
| HAE-1 | 0.020 | 0.506 | 4080.781 | 219.380 | 37.202 | 0.017 |
| HAE-2 | 0.029 | 0.509 | 3971.142 | 218.883 | 34.640 | 0.016 |
| HAE-3 | 0.033 | 0.511 | 1013.333 | 218.409 | 9.279 | 0.023 |
| HAE-4 | 0.027 | 0.509 | 2203.846 | 227.000 | 19.417 | 0.024 |
| HAE-5 | 0.020 | 0.506 | 4080.781 | 219.380 | 37.202 | 0.017 |
| HAE-6 | 0.018 | 0.506 | 5730.769 | 215.965 | 53.071 | 0.011 |
| HAE-7 | 0.024 | 0.508 | 3285.714 | 214.413 | 30.648 | 0.019 |
| HAE-8 | 0.020 | 0.506 | 4080.781 | 219.380 | 37.202 | 0.017 |
| HAE-9 | 0.022 | 0.507 | 1222.727 | 224.254 | 10.904 | 0.016 |
| F. Codes | pH | Zero Order | First Order | Higuchi Model | Korsmeyer-Peppas Model | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ko (h−1) | r2 | K1 (h−1) | r2 | K2 (h−1) | r2 | r2 | n | ||
| HAE-1 | 1.2 | 1.837 | 0.9446 | 0.032 | 0.9888 | 10.406 | 0.9879 | 0.9878 | 0.527 |
| 7.4 | 1.404 | 0.9105 | 0.021 | 0.9539 | 8.155 | 0.9825 | 0.9861 | 0.426 | |
| HAE-2 | 1.2 | 1.484 | 0.9604 | 0.022 | 0.9878 | 8.398 | 0.9967 | 0.9969 | 0.521 |
| 7.4 | 0.920 | 0.9483 | 0.012 | 0.9660 | 5.309 | 0.9979 | 0.9983 | 0.428 | |
| HAE-3 | 1.2 | 1.036 | 0.9232 | 0.014 | 0.9504 | 5.989 | 0.9882 | 0.9906 | 0.439 |
| 7.4 | 0.824 | 0.9226 | 0.010 | 0.9421 | 4.821 | 0.9913 | 0.9962 | 0.382 | |
| HAE-4 | 1.2 | 1.243 | 0.9509 | 0.017 | 0.9760 | 7.109 | 0.9963 | 0.9963 | 0.473 |
| 7.4 | 0.972 | 0.9469 | 0.012 | 0.9651 | 5.616 | 0.9981 | 0.9992 | 0.419 | |
| HAE-5 | 1.2 | 1.837 | 0.9446 | 0.032 | 0.9888 | 10.406 | 0.9879 | 0.9878 | 0.527 |
| 7.4 | 1.404 | 0.9105 | 0.021 | 0.9539 | 8.155 | 0.9825 | 0.9861 | 0.426 | |
| HAE-6 | 1.2 | 1.740 | 0.9540 | 0.029 | 0.9906 | 9.823 | 0.9921 | 0.9922 | 0.539 |
| 7.4 | 1.393 | 0.9672 | 0.020 | 0.9899 | 7.867 | 0.9964 | 0.9971 | 0.528 | |
| HAE-7 | 1.2 | 1.553 | 0.8964 | 0.024 | 0.9209 | 8.851 | 0.9376 | 0.9377 | 0.485 |
| 7.4 | 1.039 | 0.9565 | 0.014 | 0.9733 | 5.953 | 0.9989 | 0.9990 | 0.455 | |
| HAE-8 | 1.2 | 1.837 | 0.9446 | 0.032 | 0.9888 | 10.406 | 0.9879 | 0.9878 | 0.527 |
| 7.4 | 1.404 | 0.9105 | 0.021 | 0.9539 | 8.155 | 0.9825 | 0.9861 | 0.426 | |
| HAE-9 | 1.2 | 1.440 | 0.9542 | 0.022 | 0.9814 | 8.230 | 0.9979 | 0.9980 | 0.472 |
| 7.4 | 1.178 | 0.9566 | 0.016 | 0.9780 | 0.730 | 0.9985 | 0.9985 | 0.473 | |
| Formulation Codes | HEC (g) | AMPS (g) | APS/SHS (g) | EGDMA (g) |
|---|---|---|---|---|
| HAE-1 | 0.5 | 20 | 0.3/0.3 | 0.5 |
| HAE-2 | 0.5 | 20 | 0.3/0.3 | 1 |
| HAE-3 | 0.5 | 20 | 0.3/0.3 | 1.5 |
| HAE-4 | 0.5 | 12 | 0.3/0.3 | 0.5 |
| HAE-5 | 0.5 | 20 | 0.3/0.3 | 0.5 |
| HAE-6 | 0.5 | 28 | 0.3/0.3 | 0.5 |
| HAE-7 | 0.3 | 20 | 0.3/0.3 | 0.5 |
| HAE-8 | 0.5 | 20 | 0.3/0.3 | 0.5 |
| HAE-9 | 0.7 | 20 | 0.3/0.3 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, A.; Yu, C.; Wang, X.; Peng, M.; Liu, Y.; Liu, Y. Hydroxyethyl Cellulose-Based Hydrogels as Controlled Release Carriers for Amorphous Solid Dispersion of Bioactive Components of Radix Paeonia Alba. Molecules 2023, 28, 7320. https://doi.org/10.3390/molecules28217320
Naeem A, Yu C, Wang X, Peng M, Liu Y, Liu Y. Hydroxyethyl Cellulose-Based Hydrogels as Controlled Release Carriers for Amorphous Solid Dispersion of Bioactive Components of Radix Paeonia Alba. Molecules. 2023; 28(21):7320. https://doi.org/10.3390/molecules28217320
Chicago/Turabian StyleNaeem, Abid, Chengqun Yu, Xiaoli Wang, Mingyan Peng, Yi Liu, and Yali Liu. 2023. "Hydroxyethyl Cellulose-Based Hydrogels as Controlled Release Carriers for Amorphous Solid Dispersion of Bioactive Components of Radix Paeonia Alba" Molecules 28, no. 21: 7320. https://doi.org/10.3390/molecules28217320
APA StyleNaeem, A., Yu, C., Wang, X., Peng, M., Liu, Y., & Liu, Y. (2023). Hydroxyethyl Cellulose-Based Hydrogels as Controlled Release Carriers for Amorphous Solid Dispersion of Bioactive Components of Radix Paeonia Alba. Molecules, 28(21), 7320. https://doi.org/10.3390/molecules28217320






