Emodin-8-O-Glucoside—Isolation and the Screening of the Anticancer Potential against the Nervous System Tumors
Abstract
:1. Introduction
2. Results
2.1. Composition of the Extract
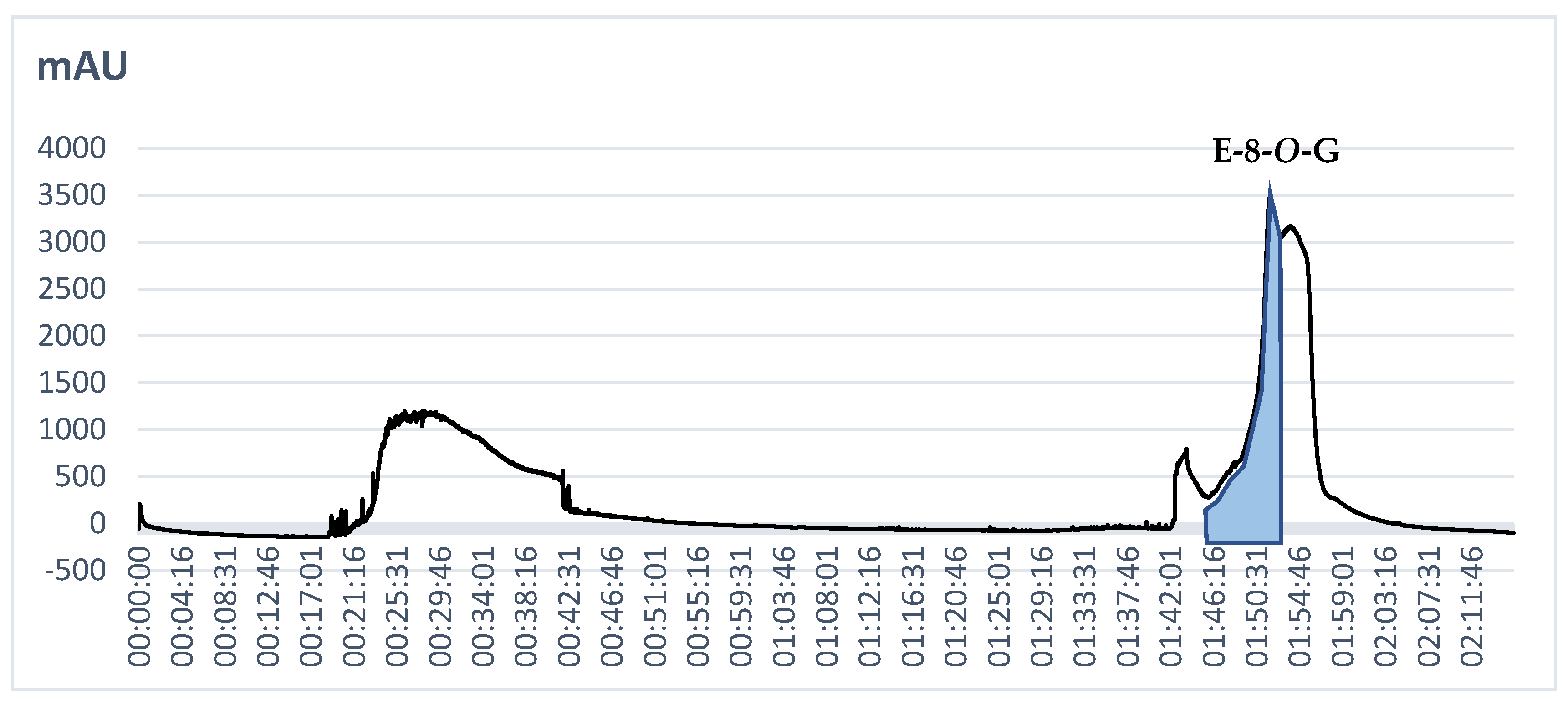
2.2. The Fractionation of the Extract by CPC and Preparative HPLC
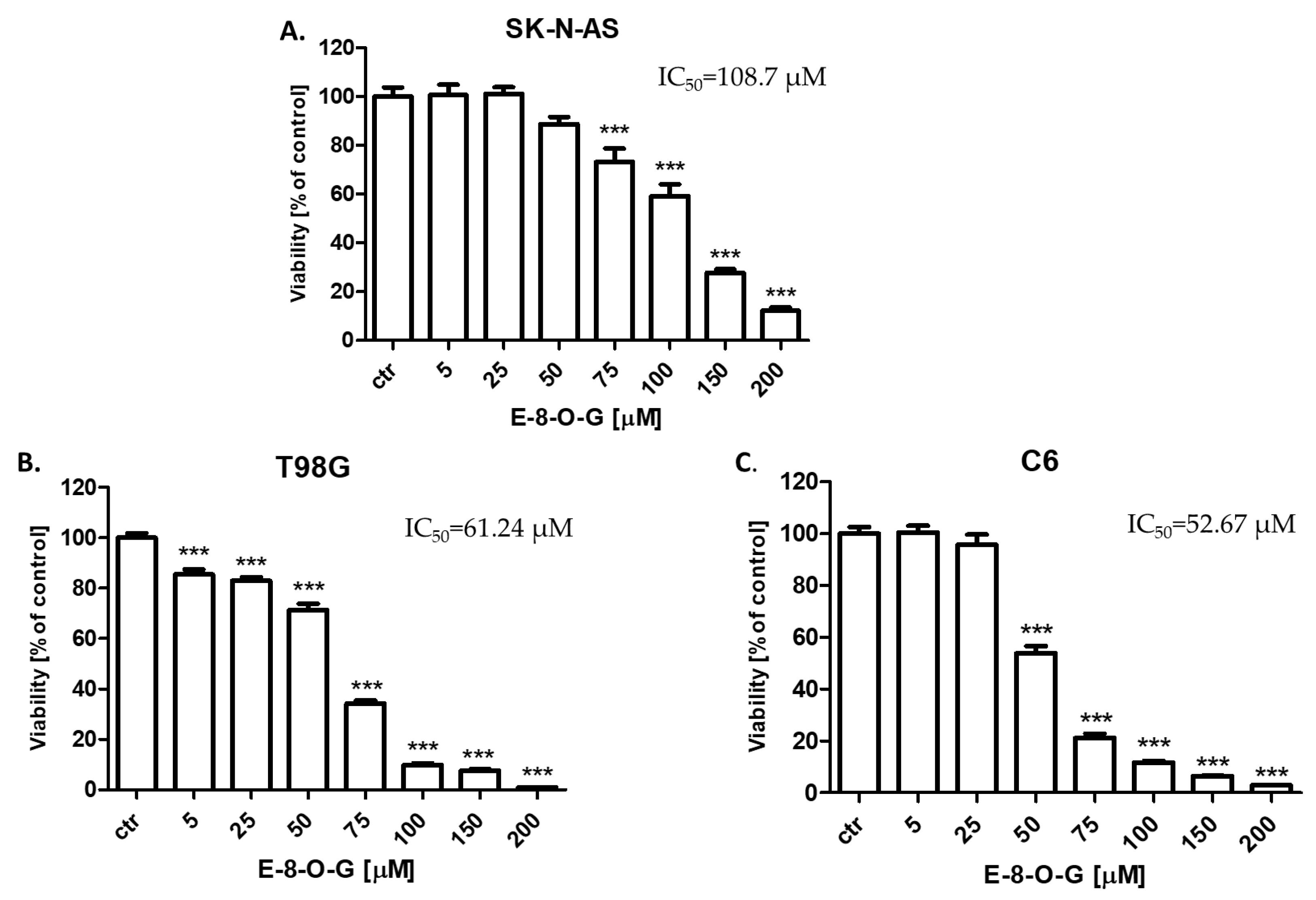
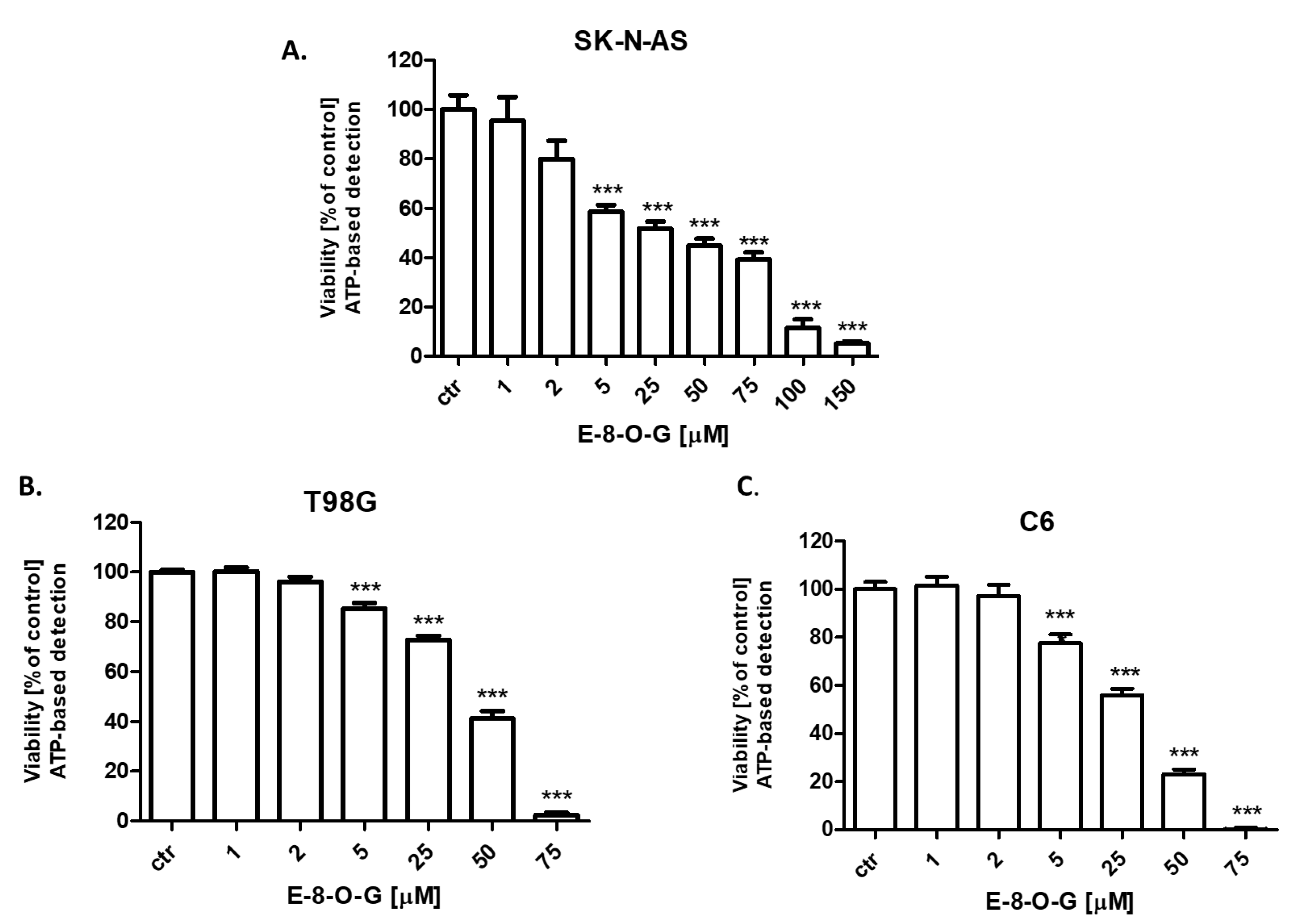
2.3. Cytotoxic Activity of the E-8-O-G
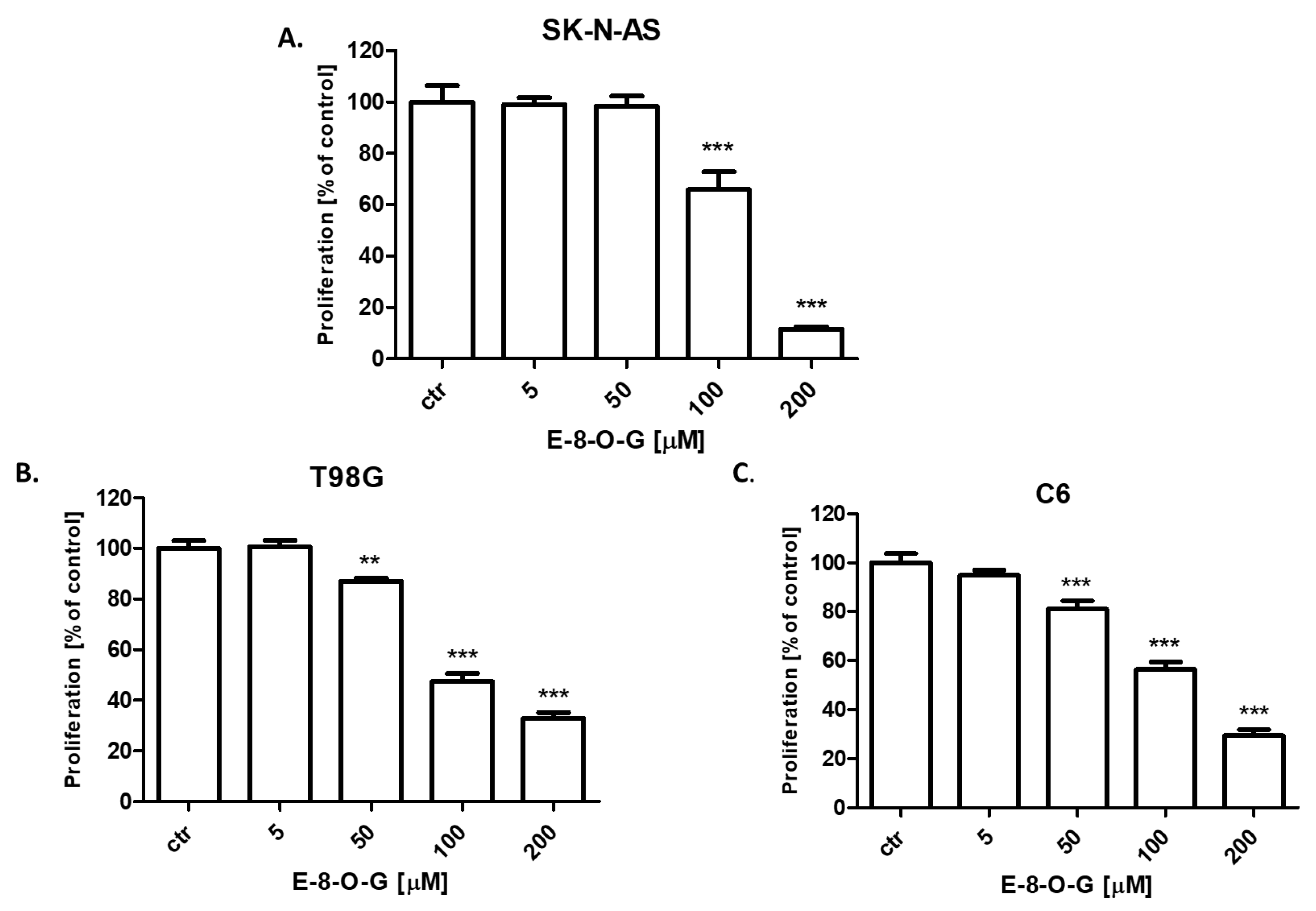
2.4. Antiproliferative Activity of the E-8-O-G
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material and Extraction of Reynoutria japonica Herb
4.3. Fingerprinting of the Extract by HPLC-ESI-QTOF-MS/MS
4.4. Fractionation of the Extract by Centrifugal Partition Chromatography (CPC)
4.5. Preparative HPLC Separation
4.6. Cell Lines
4.7. MTT Cell Viability Assay
4.8. CellTiter-Glo® Cell Viability Assay
4.9. BrdU ELISA Cell Proliferation Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gielecińska, A.; Kciuk, M.; Mujwar, S.; Celik, I.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kontek, R. Substances of Natural Origin in Medicine: Plants vs. Cancer. Cells 2023, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients 2023, 15, 3136. [Google Scholar] [CrossRef] [PubMed]
- Nelson, V.K.; Nuli, M.V.; Mastanaiah, J.; Saleem, T.S.M.; Birudala, G.; Jamous, Y.F.; Alshargi, O.; Kotha, K.K.; Sudhan, H.H.; Mani, R.R.; et al. Reactive oxygen species mediated apoptotic death of colon cancer cells: Therapeutic potential of plant derived alkaloids. Front. Endocrinol. 2023, 14, 1201198. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Al-Maktoum, A.; Amin, A. Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer. Cancers 2023, 15, 3900. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Perlak, M.; Ziółkowski, P.; Woźniak, M. A promising natural anthraquinones mediated by photodynamic therapy for anti-cancer therapy. Phytomedicine 2023, 119, 155035. [Google Scholar] [CrossRef]
- Malik, E.M.; Müller, C.E. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Das, A.; Dutta, S. Binding Studies of Aloe-Active Compounds with G-Quadruplex Sequences. ACS Omega 2021, 6, 18344–18351. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, L.J.; Huang, T.; Ying, J.Q.; Li, J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin. J. Nat. Med. 2020, 18, 425–435. [Google Scholar] [CrossRef]
- Horvat, M.; Avbelj, M.; Durán-Alonso, M.B.; Banjanac, M.; Petković, H.; Iskra, J. Antiviral Activities of Halogenated Emodin Derivatives against Human Coronavirus NL63. Molecules 2021, 26, 6825. [Google Scholar] [CrossRef]
- Li, T.; Lu, Y.; Zhang, H.; Wang, L.; Beier, R.C.; Jin, Y.; Wang, W.; Li, H.; Hou, X. Antibacterial Activity and Membrane-Targeting Mechanism of Aloe-Emodin Against Staphylococcus Epidermidis. Front. Microbiol. 2021, 12, 621866. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Q.; Yang, Y.; Ai, X.; Chen, S.; Song, Y. Synthesis and anti-inflammatory effects of novel emodin derivatives bearing azole moieties. Arch. Pharm. 2020, 353, e1900264. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Emodin—A natural anthraquinone derivative with diverse pharmacological activities. Phytochemistry 2021, 190, 112854. [Google Scholar] [CrossRef]
- Bai, J.; Wu, J.; Tang, R.; Sun, C.; Ji, J.; Yin, Z.; Ma, G.; Yang, W. Emodin, a natural anthraquinone, suppresses liver cancer in vitro and in vivo by regulating VEGFR2 and miR-34a. Investig. New Drugs 2020, 38, 229–245. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Li, R.Z.; Xu, C.; Fan, X.X.; Li, J.X.; Meng, W.Y.; Wang, X.R.; Liang, T.L.; Guan, X.X.; Pan, H.D.; et al. Emodin induces apoptosis and suppresses non-small-cell lung cancer growth via downregulation of sPLA2-IIa. Phytomedicine 2022, 95, 153786. [Google Scholar] [CrossRef]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Chen, H.; Yan, J.; Cheng, H. Emodin exerts antitumor effects in ovarian cancer cell lines by preventing the development of cancer stem cells via epithelial mesenchymal transition. Oncol. Lett. 2022, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.J.; Nguyen, T.T.H.; Kim, S.C.; Ree, J.; Choi, T.G.; Sohng, J.K.; Park, Y. Il Emodin 8-O-glucoside primes macrophages more strongly than emodin aglycone via activation of phagocytic activity and TLR-2/MAPK/NF-κB signalling pathway. Int. Immunopharmacol. 2020, 88, 106936. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, D.; Ma, H.; Liu, J. Neuroprotective effects of emodin-8-O-beta-D-glucoside in vivo and in vitro. Eur. J. Pharmacol. 2007, 577, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef]
- Szczeblewski, P.; Wróblewski, M.; Borzyszkowska-Bukowska, J.; Bairamova, T.; Górska, J.; Laskowski, T.; Samulewicz, A.; Kosno, M.; Sobiech, Ł.; Polit, J.T.; et al. The role of centrifugal partition chromatography in the removal of β-asarone from Acorus calamus essential oil. Sci. Rep. 2022, 12, 22217. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Slusarczyk, S.; Granica, S.; Hadzik, J.; Matkowski, A. Phytochemical Diversity in Rhizomes of Three Reynoutria Species and their Antioxidant Activity Correlations Elucidated by LC-ESI-MS/MS Analysis. Molecules 2019, 24, 1136. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Kruk-Słomka, M.; Stępnik, K.; Szalak, R.; Biała, G. The Evaluation of Pro-Cognitive and Antiamnestic Properties of Berberine and Magnoflorine Isolated from Barberry Species by Centrifugal Partition Chromatography (CPC), in Relation to QSAR Modelling. Int. J. Mol. Sci. 2017, 18, 2511. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.X.; Xu, Z.; Su, H.W.; Hu, J.; Yan, Y.J. Emodin-8-O-β-D-glucoside from Polygonum amplexicaule D. Don var. sinense Forb. promotes proliferation and differentiation of osteoblastic MC3T3-E1 cells. Molecules 2011, 16, 728–737. [Google Scholar] [CrossRef]
- Yao, S.; Li, Y.; Kong, L. Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography. J. Chromatogr. A 2006, 1115, 64–71. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Wang, C.; Du, W.; Sun, H.; Sun, W.; Jin, Y.; Zuo, G.; Tong, S. Orthogonality in the selection of biphasic solvent systems for off-line two-dimensional countercurrent chromatography from Polygonum cuspidatum Sieb. et Zucc. J. Chromatogr. A 2020, 1634, 461666. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liu, Q.; Xie, S.; Zhu, F.; Chen, X. Preparative isolation and purification of 12 main antioxidants from the roots of Polygonum multiflorum Thunb. using high-speed countercurrent chromatography and preparative HPLC guided by 1,1′-diphenyl-2-picrylhydrazyl-HPLC. J. Sep. Sci. 2020, 43, 1415–1422. [Google Scholar] [CrossRef]
- Liu, S. Effects of emodin-8-O-β-D-glucoside on cell apoptosis and expression of Bcl-2/Bax in cervical cancer SKOV3 cells. Zhonghua Yi Xue Za Zhi 2015, 95, 3541–3544. [Google Scholar]
- Xu, L.; Liu, Y.; Chen, Y.; Zhu, R.; Li, S.; Zhang, S.; Zhang, J.; Xie, H.Q.; Zhao, B. Emodin inhibits U87 glioblastoma cells migration by activating aryl hydrocarbon receptor (AhR) signaling pathway. Ecotoxicol. Environ. Saf. 2022, 234, 113357. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, C.W.; Lee, W.S.; Jeong, H.J.; Park, M.J.; Jang, W.I.; Kim, E.H. Emodin coupled with high LET neutron beam-a novel approach to treat on glioblastoma. J. Radiat. Res. 2022, 63, 817–827. [Google Scholar] [CrossRef]
- Arcella, A.; Oliva, M.A.; Staffieri, S.; Sanchez, M.; Madonna, M.; Riozzi, B.; Esposito, V.; Giangaspero, F.; Frati, L. Effects of aloe emodin on U87MG glioblastoma cell growth: In vitro and in vivo study. Environ. Toxicol. 2018, 33, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Staffieri, S.; Russo, V.; Oliva, M.A.; Alborghetti, M.; Russo, M.; Arcella, A. Aloe-Emodin Overcomes Anti-Cancer Drug Resistance to Temozolomide and Prevents Colony Formation and Migration in Primary Human Glioblastoma Cell Lines NULU and ZAR. Molecules 2023, 28, 6024. [Google Scholar] [CrossRef] [PubMed]
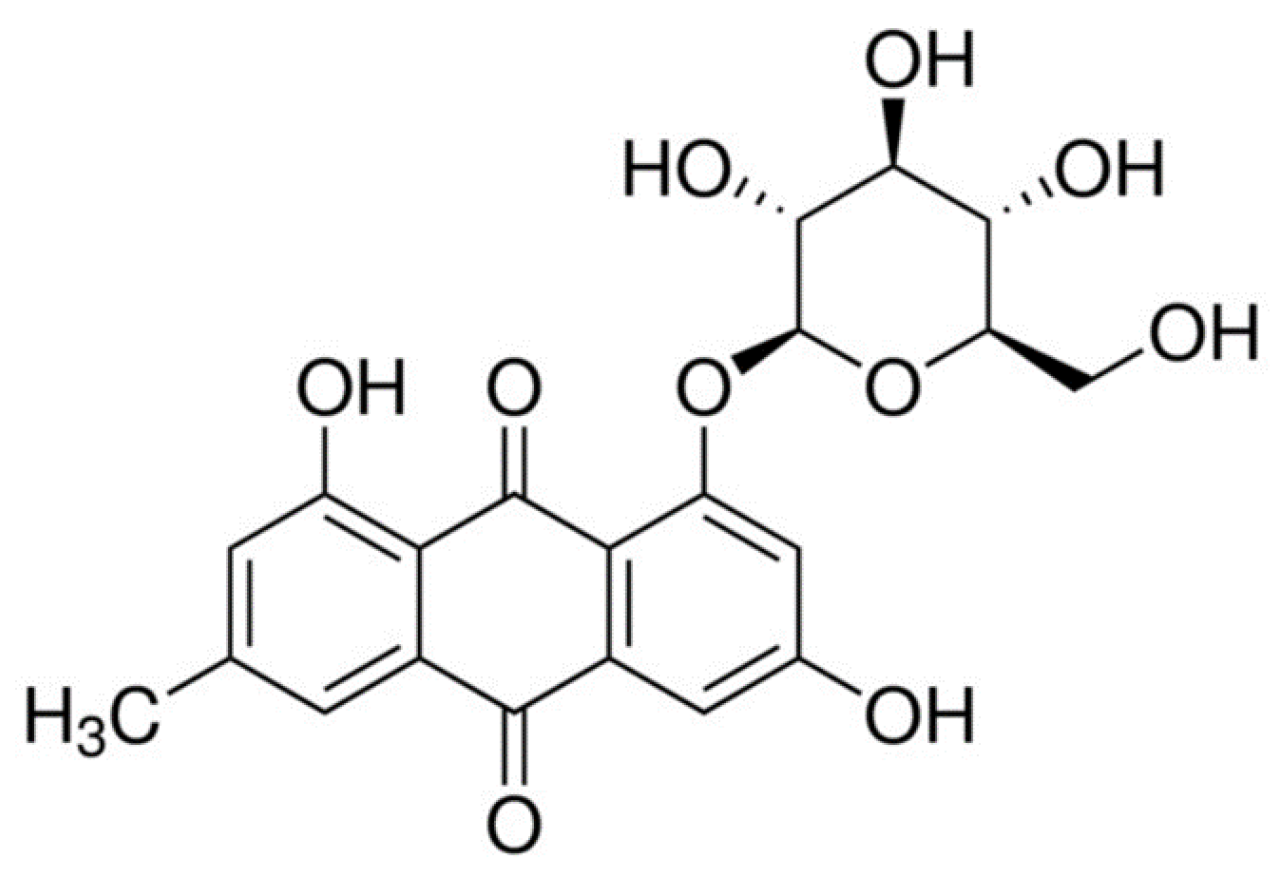

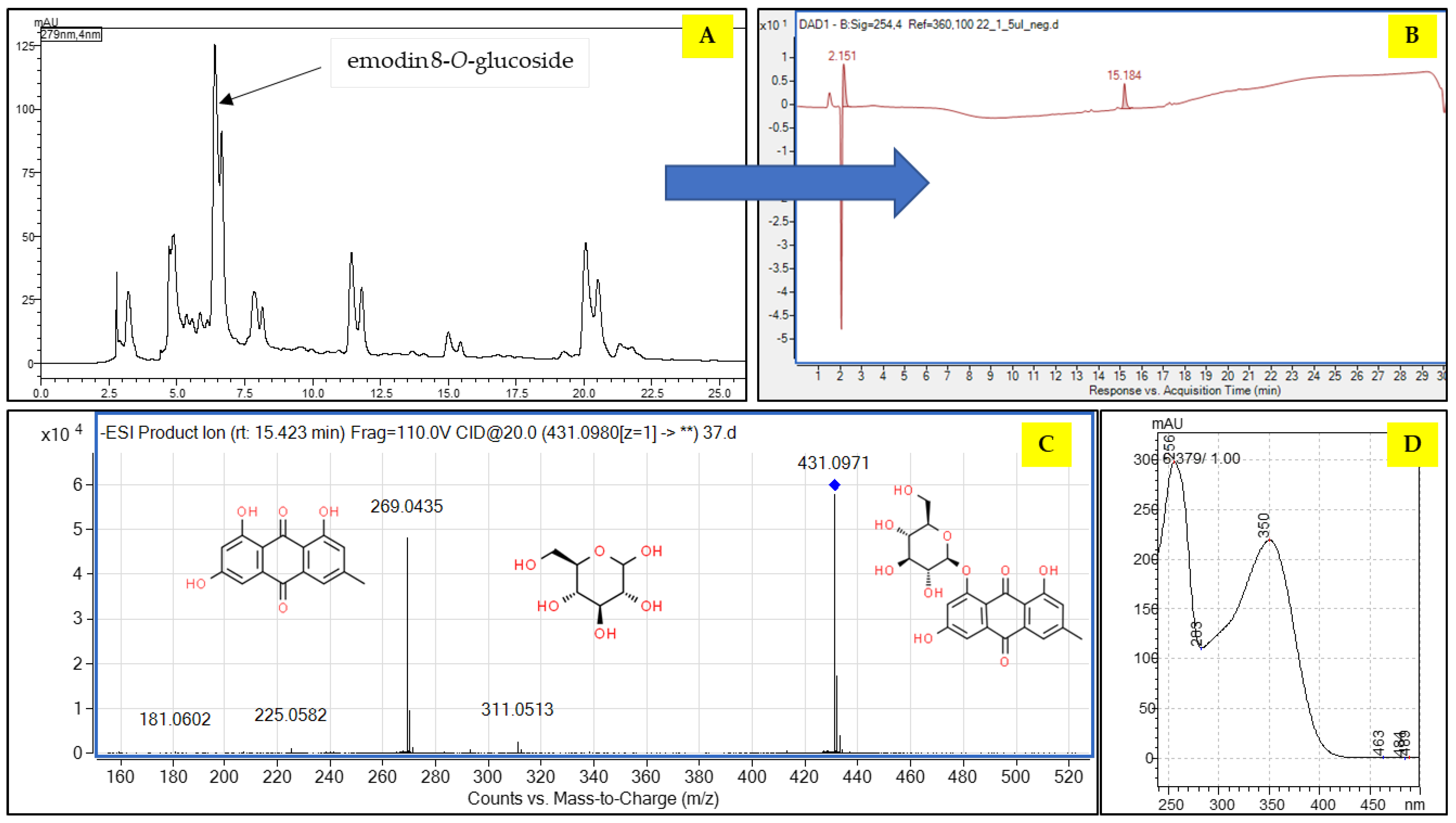

| No | Compound | Formula | Rt (min) | Pos/Neg (−/+) | Theoretical Mass | Experimental Mass | Error | DBE | MS/MS Fragments | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Emodin | C15H10O5 | 24.4 | − | 269.0455 | 269.0446 | 3.51 | 11 | 241, 225 | 9 |
| 2 | Emodin-6-O-glucoside | C21H20O10 | 21.1 | − | 431.0984 | 431.1003 | −4.47 | 12 | 304, 311, 283, 269 | 1.6 |
| 3 | Emodin-8-O-glucoside | C21H20O10 | 21.3 | − | 431.0984 | 431.0994 | −2.38 | 12 | 341, 311, 283, 271 | 1.4 |
| 4 | Physcion | C16H12O5 | 22.4 | − | 283.0612 | 283.0626 | −4.94 | 11 | 112, 248 | 0.11 |
| 5 | Quercetin | C15H10O7 | 22.3 | − | 301.0354 | 301.0368 | −5.38 | 11 | 151, 178, 273, 107 | 8 |
| 6 | Rutin | C27H30O16 | 21.1 | − | 609.1461 | 609.1482 | −4.58 | 13 | - | 0.6 |
| 7 | Luteolin | C15H10O6 | 22.7 | − | 285.0405 | 285.0419 | −5.03 | 11 | 241, 268 | 1.5 |
| 8 | Luteolin 7-O-glucoside | C21H20O11 | 20.591 | − | 447.0933 | 447.0950 | −3.83 | 12 | 429, 357, 327, 285 | 1.6 |
| 9 | Citric acid | C6H8O7 | 3.672 | − | 191.0197 | 191.0205 | −4.03 | 3 | 111, 87 | 8 |
| 10 | Gallic acid | C7H6O5 | 16.174 | − | 169.0140 | 169.0154 | −6.78 | 5 | 151, 125, 83 | 7 |
| 11 | Tartaric acid | C4H6O6 | 18.257 | − | 149.0092 | 149.0096 | −2.92 | 2 | - | 0.6 |
| 12 | Cafraic acid | C13H12O9 | 18.257 | 311.0409 | 311.0426 | −5.59 | 8 | - | 5 | |
| 13 | Bergapten | C12H8O4 | 2.006 | − | 215.0350 | 215.0331 | 11.95 | 9 | 181, 89 | 7 |
| 14 | Malic acid | C4H6O5 | 2.922 | − | 133.0142 | 133.0139 | 2.59 | 2 | 115, 71 | 1.5 |
| 15 | Coumarin | C9H6O2 | 19.085 | + | 147.0441 | 147.0440 | 0.38 | 7 | - | 0.4 |
| 16 | Catechin | C15H14O6 | 20.418 | + | 291.0863 | 291.0868 | −1.67 | 9 | - | 0.3 |
| 17 | Procyanidin B1 | C30H26O12 | 19.918 | + | 579.1497 | 579.1498 | −1.03 | 18 | - | 4.5 |
| 18 | Quinic acid | C7H12O6 | 2.089 | − | 191.0561 | 191.0564 | −1.5 | 2 | - | 0.5 |
| 19 | 3-p-coumaroylquinic acid | C16H18O8 | 19.174 | − | 337.0929 | 337.0933 | −1.21 | 8 | 163, 191 | 3 |
| 20 | Isoquercetin | C21H20O12 | 21.4 | − | 463.0882 | 463.0901 | −4.09 | 12 | 317, 300, 255 | 1.6 |
| 21 | Sacranoside A | C20H32O10 | 19.8 | − | 431.1995 | 431.1950 | −6.3 | 5 | 387, 179 | 7 |
| 22 | Procyanidin B6 | C30H26O12 | 19.9 | − | 577.1351 | 577.1374 | −3.89 | 18 | 426, 289, 194 | 7 |
| 23 | Isoschaftoside | C26H28O14 | 20.6 | − | 563.1406 | 563.1436 | −5.27 | 13 | 521, 483, 303, 263 | 3 |
| No | Solvent System | Ratio | References |
|---|---|---|---|
| 1 | Petroleum ether:ethyl acetate:water | 1:5:5 | [26] |
| 2 | Hexane:ethyl acetate:methanol:water | 3:7:5:5 | [25] |
| 3 | Hexane:ethyl acetate:methanol:water | 9:1:5:5 | modified |
| 4 | Hexane:ethyl acetate:methanol:water | 8:2:6:4 | modified |
| 5 | Hexane:ethyl acetate:methanol:water | 8:2:9:1 | modified |
| 6 | Ethyl acetate:methanol:water | 70:1:70 | [26] |
| 7 | Petroleum ether:ethyl acetate:methanol:water | 4:5:4:5 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okon, E.; Koval, M.; Wawruszak, A.; Slawinska-Brych, A.; Smolinska, K.; Shevera, M.; Stepulak, A.; Kukula-Koch, W. Emodin-8-O-Glucoside—Isolation and the Screening of the Anticancer Potential against the Nervous System Tumors. Molecules 2023, 28, 7366. https://doi.org/10.3390/molecules28217366
Okon E, Koval M, Wawruszak A, Slawinska-Brych A, Smolinska K, Shevera M, Stepulak A, Kukula-Koch W. Emodin-8-O-Glucoside—Isolation and the Screening of the Anticancer Potential against the Nervous System Tumors. Molecules. 2023; 28(21):7366. https://doi.org/10.3390/molecules28217366
Chicago/Turabian StyleOkon, Estera, Maryna Koval, Anna Wawruszak, Adrianna Slawinska-Brych, Katarzyna Smolinska, Myroslav Shevera, Andrzej Stepulak, and Wirginia Kukula-Koch. 2023. "Emodin-8-O-Glucoside—Isolation and the Screening of the Anticancer Potential against the Nervous System Tumors" Molecules 28, no. 21: 7366. https://doi.org/10.3390/molecules28217366
APA StyleOkon, E., Koval, M., Wawruszak, A., Slawinska-Brych, A., Smolinska, K., Shevera, M., Stepulak, A., & Kukula-Koch, W. (2023). Emodin-8-O-Glucoside—Isolation and the Screening of the Anticancer Potential against the Nervous System Tumors. Molecules, 28(21), 7366. https://doi.org/10.3390/molecules28217366







