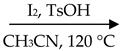Abstract
An iodine-mediated one-pot synthesis of pyrrolo/indolo [1,2-a]quinoxalines and quinazolin-4-one via utilizing epoxides as alkyl precursors under metal-free conditions has been described. Both 1-(2-aminophenyl)-pyrrole and 2-aminobenzamide could be applied to this protocol. A total of 33 desired products were obtained with moderate to good yields. This methodology was suitable for wide-scale preparation and the obtained products could be further modified into promising pharmaceutically active reagents.
1. Introduction
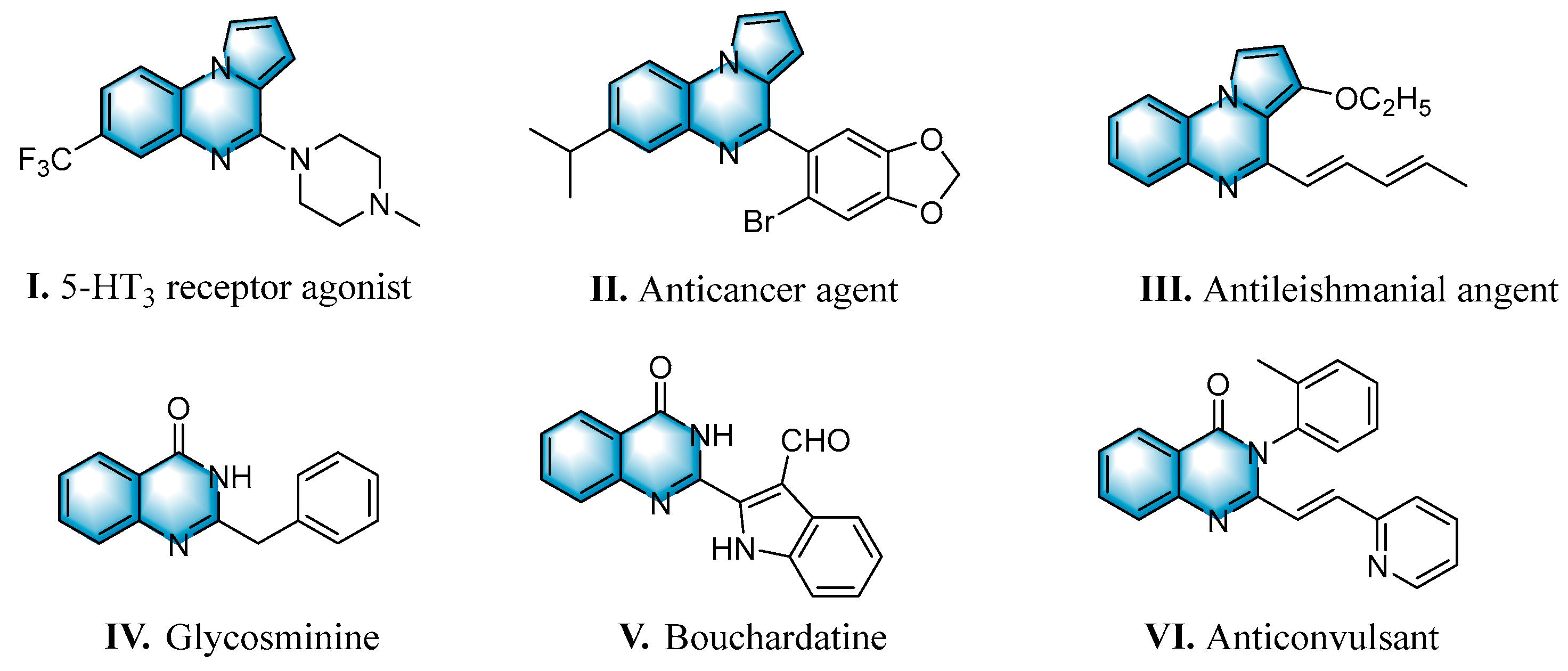
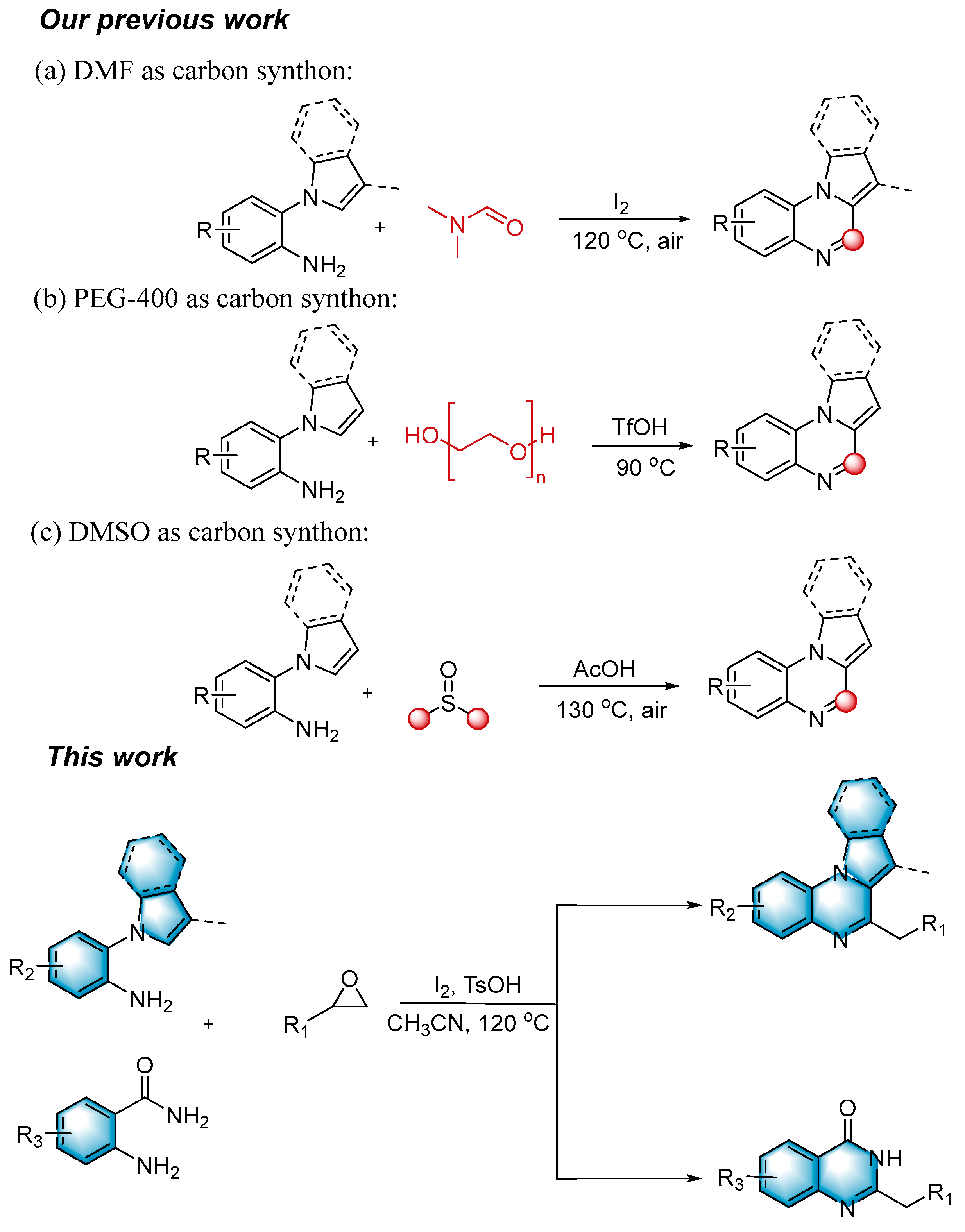
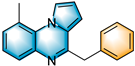
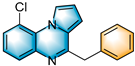
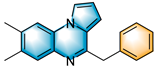
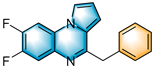
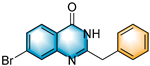
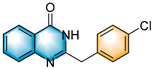
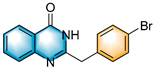
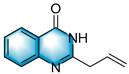
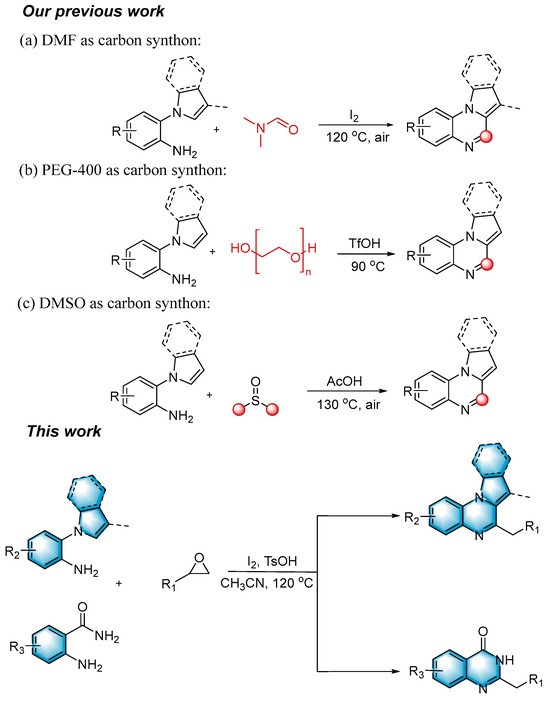
N-heterocycles are inseparable from our life for use as drug molecules, functional materials and dyes [1,2,3]. Pyrrolo [1,2-a]quinoxalines and quinazolin-4-ones are two essential heterocyclic skeletons with outstanding biological properties for antimalarial, anticancer, anti-HIV, antibacterial, etc [4,5,6,7,8,9,10,11,12,13,14]. As shown in Figure 1, compound I exhibits excellent activity towards the 5-HT3 receptor, and compounds II and III have anticancer and antileishmanial properties, respectively. Glycosminine (Figure 1, IV) and alkaloid bouchardatine (Figure 1, V) are present in natural products. Compound VI has anticonvulsant activity. Given their excellent applications, especially in medicinal chemistry, their efficient and green synthesis has been a long-pursued research topic in the field of organic synthetic chemistry. In 1966, Cheeseman and Tuck [15] first synthesized pyrrolo [1,2-a]quinoxalines using 1-(2-aminophenyl)-pyrrole as the starting material with aqueous formic acid under reflux conditions. Since then, a number of groups have made tremendous efforts to construct these compounds [16,17,18,19,20,21,22,23,24,25]. In 2022, Ma [16] reported a Cu(II)-catalyzed synthesis of pyrrolo[1,2-a]quinoxalines using N,N-dimethylethanolamine (DMEA) as a C1 synthon. In addition, the method of Ma’s group was also applicable to the synthesis of quinazolin-4-one and benzo[4,5]imidazoquinazoline. During the preparation of our manuscript, by means of an I2-DMSO synergistic system, Lin [17] reported a method for the synthesis of acyl-substituted pyrrolo[1,2-a]quinolines using epoxides as acyl precursors. As part of our research interest in the green synthesis of nitrogen-containing heterocycles under metal-free conditions, PEG-400 [18], DMF [19] and DMSO [20] as carbon synthons have been reported (Scheme 1).
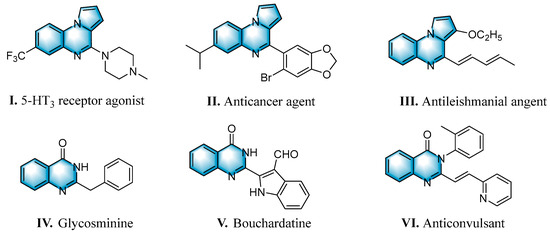
Figure 1.
Drug Molecules Containing Quinoxalines and Quinazolines.
Scheme 1.
Synthesis of Pyrrolo[1,2-a]quinoxalines.
On the other hand, the synthesis of quinazolin-4-ones has also attracted much attention from chemists. In traditional synthesis methods, quinazolinone was obtained by an acid/base facilitated condensation reaction of esters, aldehydes or carboxylic acids with amides by means of some homogeneous catalytic systems and expensive raw materials [26,27,28,29,30]. In 2018, Wang [31] reported a novel synthetic strategy for the synthesis of quinazolinones from olefins, carbon monoxide and amines over a heterogeneous Ru cluster/cerium oxide catalyst under acid/ base-free and oxidant-free conditions with H2O as the only by-product. In 2019, Zheng [32] developed a visible light-mediated intramolecular C-N cross-coupling reaction to synthesize a series of fused N-substituted polycyclic quinazolinone derivatives under mild reaction conditions via long-lived photoactive photoisomer complexes. In 2023, Fan [33] reported a method for the synthesis of 5H-phthalazino[1,2-b]quinazolin-8(6H)-one derivatives via a t-BuOK-catalyzed intramolecular hydrogen amination reaction of quinazolinones. Also in 2023, Zhu [34] reported a cobalt homeostatic catalysis system for the synthesis of quinazolinones from the coupling of enaminones and oxadiazolones.
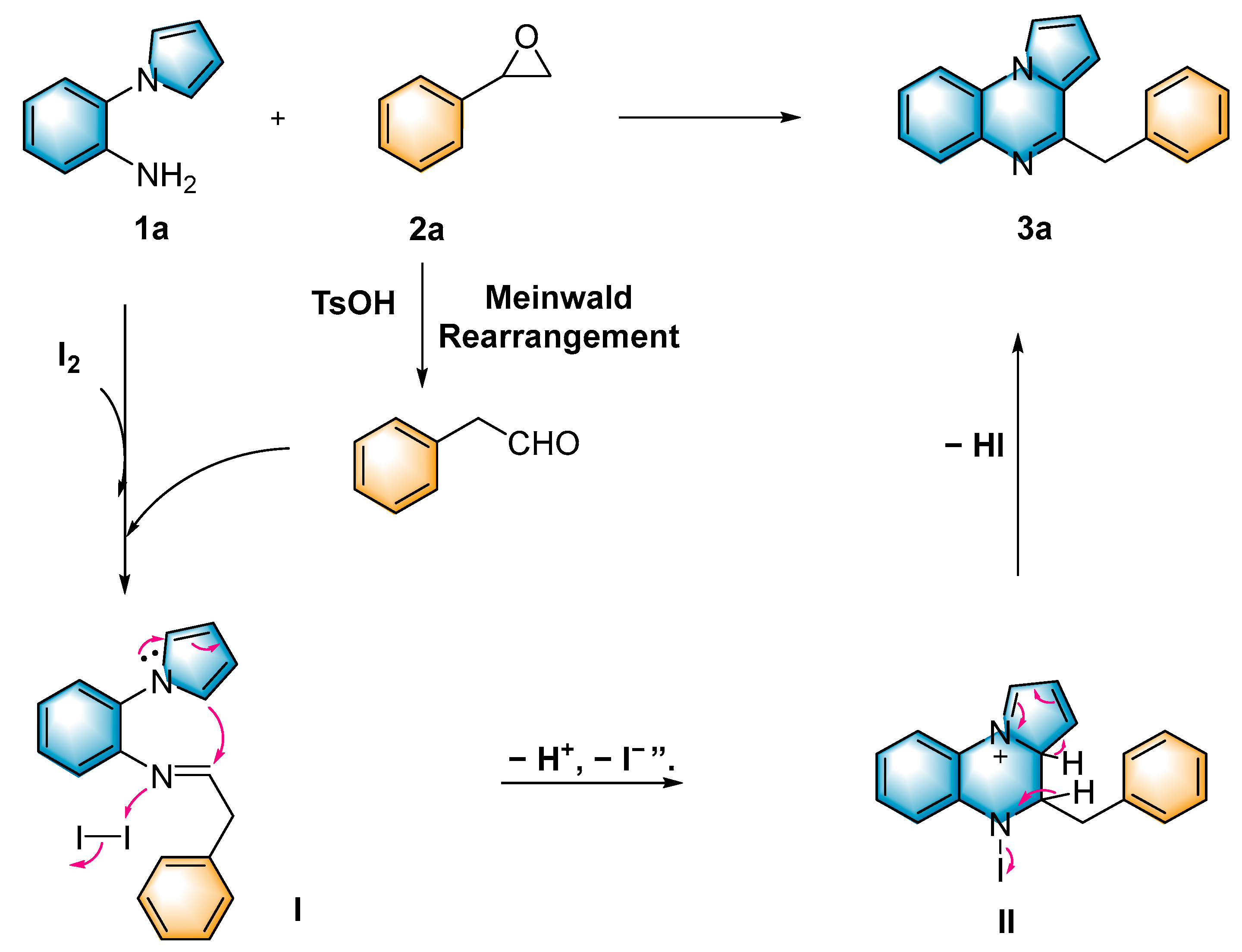
Epoxides are well-known electrophiles that can react with various nucleophiles. They are readily available from olefins or ketones and are usually air-stable and easily stored. The Meinwald rearrangement [35] is an acid-catalyzed rearrangement reaction in which epoxides form aldehydes or ketones via ring-opening followed by a 1,2-shift of the hydride or alkyl group. Aldehydes, especially enolizable aliphatic aldehydes, are susceptible to self-condensation which makes them unstable and hard to store in pure form. Therefore, it is more desirable to utilize epoxides to replace the original aldehydes. In 2023, Moran [36] synthesized a series of functionalized isochromans using epoxides as an alternative to aldehydes. In 2021, Feng [37] reported the first catalytic asymmetric multi-insertion olefin addition reaction triggered by an epoxy-vinyl Meinwald rearrangement using a chiral N,N′-dioxide/ScIII complex catalyst. Encouraged by these discoveries and to further explore the applications of epoxides [38,39,40,41,42], herein, we developed a method for synthesizing pyrrolo[1,2-a]quinoxalines and quinazolin-4-ones via a tandem Meinwald rearrangement and cyclization in one pot.
2. Results and Discussion
2.1. Optimization
We commenced our exploration with 1-(2-aminophenyl)-pyrrole (1a; 0.5 mmol) and styrene oxide (2a; 1.0 mmol) as the model substrates. In the presence of I2 (0.5 mmol) and AcOH (0.5 mmol) in CH3CN (1.0 mL) at 120 °C, the target product 4-benzylpyrrolo[1,2-a]quinoxaline (3a) was obtained in 52% yield. Then, we screened several common Brønsted acids, such as HCOOH, trifluoromethanesulfonic acid (TfOH) and p-toluenesulfonic acid (TsOH) (Table 1, entries 1–5), and 3a had the best yield (76%) when TsOH was used (Table 1, entry 5). In addition, we found that in the absence of iodine or acid, yields showed a significant decline (Table 1, entries 6–7). It can be noted that iodine and acid play a crucial role in this reaction system. Furthermore, when the acid was reduced to 0.5 equivalents, the yield decreased; when the acid equivalent was increased to 1.5 equivalents, the yield did not increase significantly (Table 1, entries 8–9). In addition, common solvents, such as 1,2-dichloroethane (DCE), EtOH, toluene, N-methylpyrrolidone (NMP) and PhCl, were selected, and CH3CN was the best (Table 1, entries 10–14). Lowering the reaction temperature did not improve the reaction yield (Table 1, entries 15–16). For the sake of economy and safety, we did not raise the temperature further. We finally determined that subsequent experiments will be performed with I2 (0.5 mmol) and TsOH (0.5 mmol) in CH3CN at 120 °C for 4 h (Table 1, entry 5).

Table 1.
Optimization a.
2.2. Scope of Substrates
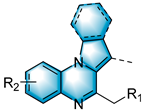
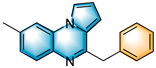
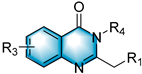
With the optimal reaction conditions in hand, various substituted 1-(2-aminophenyl)-pyrroles were first explored, and the results are shown in Table 2. For monosubstituted 1-(2-aminophenyl)-pyrroles, some bearing electron-donating groups (EDGs), like methyl, methoxy or tert-butyl, work well in this method (3b–3c, 3e–3f and 3k). The method was also compatible with substrates containing electron-withdrawing groups (EWGs), such as fluoro, chloro, bromo and iodine (3d, 3g–3j and 3l). Disubstituted 1-(2-aminophenyl)-pyrroles were also well tolerated under these conditions; for 4,5-dimethyl-2-(1H-pyrrol-1-yl)aniline, 4,5-difluoro-2-(1H-pyrrol-1-yl)aniline and 4,5-dichloro-2-(1H-pyrrol-1-yl)aniline, the target products 3m, 3n and 3o were obtained with the yields of 64%, 76% and 65%, respectively. When we replaced the pyrrole ring with indole, the reaction still proceeded with 35–48% yields (3p–3r). The reaction also proceeds smoothly when alkyl epoxides, such as 1,2-epoxybutane, were used (3s); when 3,4-epoxy-1-butene was used, the product 3t’ was expected to suffer a facile double-bond migration, affording the conjugated structure 3t.

Table 2.
Substrate Scope of 2-(1H-Pyrrolo/indolo-1-yl)anilines a,b.
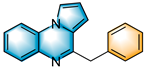
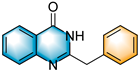
Inspired by the experimental results in Table 2, we then turned our focus to the synthesis of quinazolin-4-ones. We attempted to react 2-aminobenzamide (4a) with styrene oxide (2a) under optimal conditions and successfully obtained 2-benzylquinazolin-4(3H)-one (5a) in 70% yield (Table 3). Whether the R3 group of 2-aminobenzamide was EDG or EWG participated successfully in the reaction with yields ranging from 58% to 74% (5a-5j). Whether the R3 group of the 2-aminobenzamide was an EDG or EWG and could smoothly participate in this reaction, were demonstrated with yields ranging from 58% to 74% (5a–5j). The reaction also performed well when styrene oxide (2a) was attached to EWGs, such as chloro and bromo (5k–5l). When we used 3,4-epoxy-1-butene to react with 4a, the expected product quinazolinone 5m’ suffered an easy double-bond migration to give the conjugated product 5m. Unfortunately, we did not obtain the desired products 5n and 5o when the R4 group was replaced with an alkyl or aryl group.

Table 3.
Substrate Scope of 2-Aminobenzamide a,b.
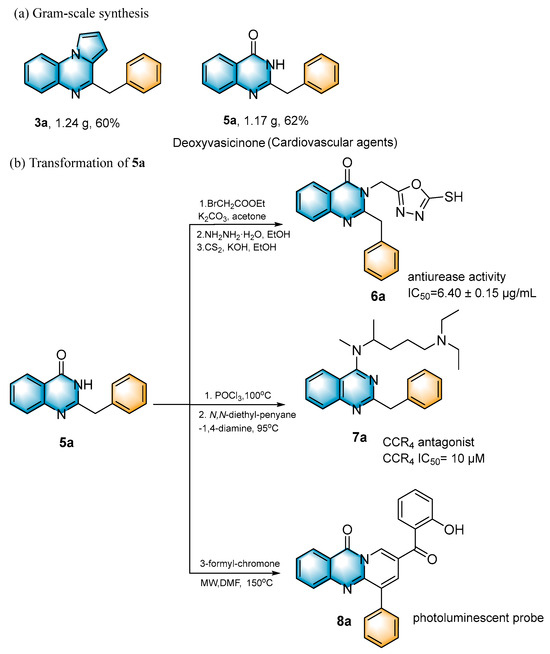
2.3. Large Scale Reaction and Synthetic Applications
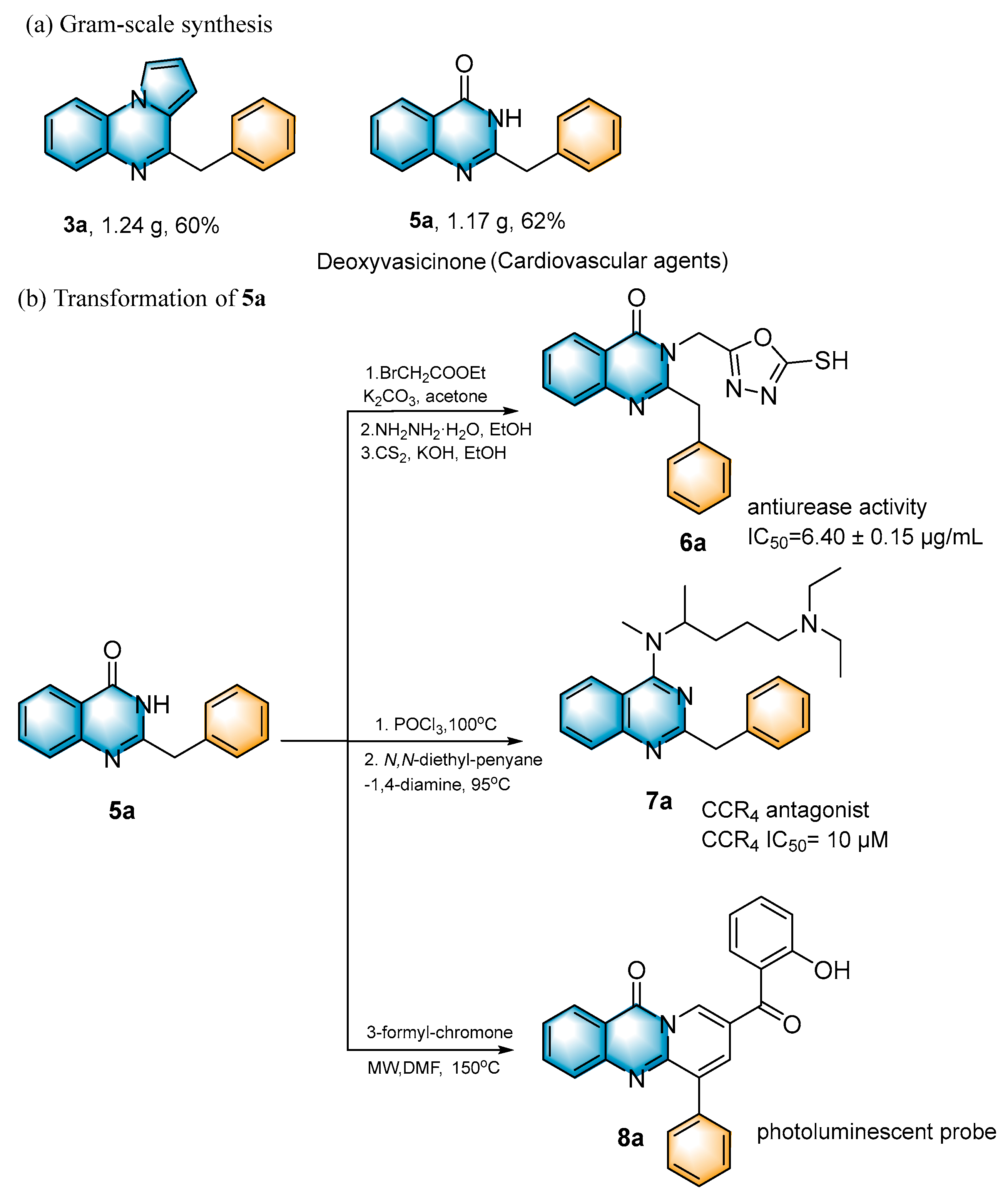
Subsequently, several synthetic applications were performed to demonstrate the practicality of this methodology (Scheme 2). Under standard conditions, the gram-scale synthesis of 3a and 5a was carried out. Pleasantly, the desired product was successfully obtained in 60% and 62% yields, respectively, making the procedure suitable for a broad-scale preparation. Of these, 5a can be used as a cardiovascular agent [14]. In addition, the 2-benzylquinazolin-4(3H)-one (5a) obtained in this protocol could be further modified into an active antiurease reagent [43], molecular antagonists of CCR4 [44] and a photoluminescent probe [45]. The photoluminescent probe could be used for the label-free, highly selective and sensitive detection of Fe3+ and Ag+ metal ions.
Scheme 2.
Large-scale reaction and synthetic applications.
2.4. Mechanism Investigation
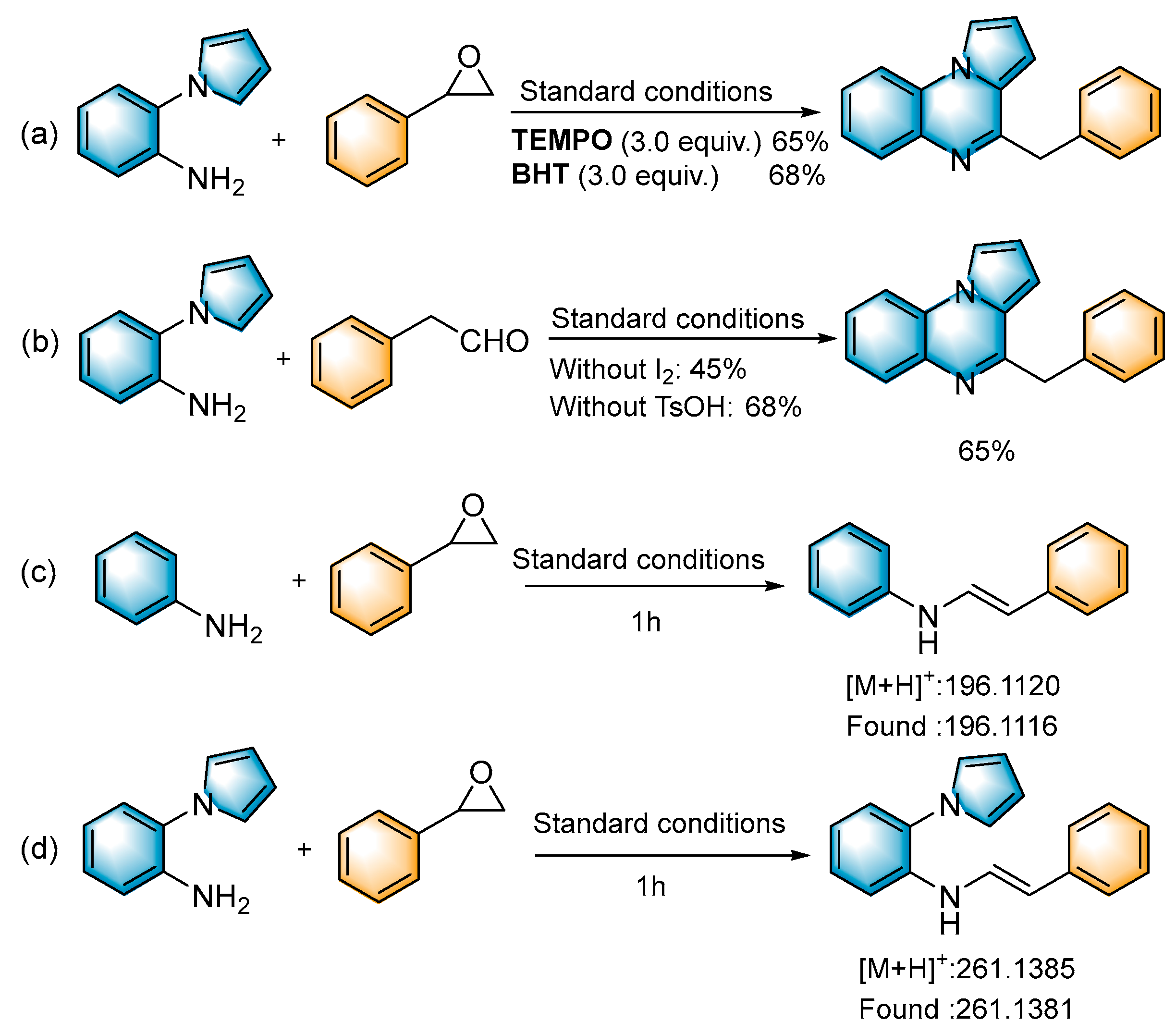
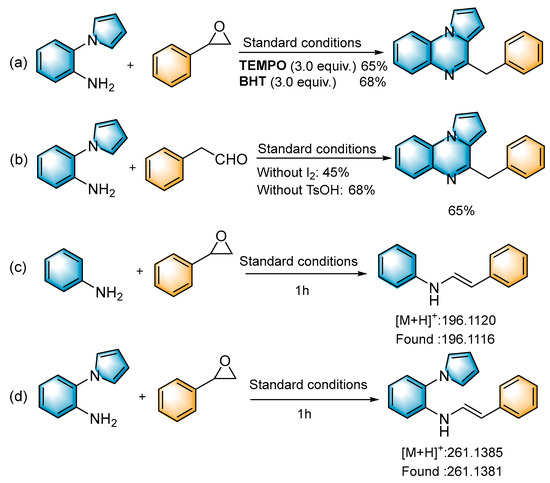
To shed light on the mechanism of the reaction, several control experiments were performed (Scheme 3). When the radical scavengers 2,2,6,6-tetramethylpiperidinyl-1-oxide (TEMPO, 3.0 equiv.) and 2,6-di-tert-butyl-4-methylphenol (BHT, 3.0 equiv.) were added to the reaction system under standard conditions, the desired product was obtained in 65% and 68% yields (Scheme 3a), respectively. This indicated that the conversion was a non-radical process. When the reaction was carried out without the addition of I2, the yield exhibited a significant decrease (27%). When the reaction system was performed without the addition of TsOH, the target product was obtained with a 50% yield; we presumed that it is the I2, as a Lewis acid, that plays a role in the conversion process (Table 1, entries 6–7). To investigate the reaction mechanism in more depth, we used 2-phenylacetaldehyde to react with 1a under standard conditions to obtain the target product in 65% yield (Scheme 3b). This indicated that 2-phenylacetaldehyde may be a key intermediate in the reaction. In the absence of I2, the yield decreased significantly, indicating that iodine plays a key role in the subsequent cyclization reaction. Under standard conditions, aniline and 1-(2-aminophenyl)-pyrrole were reacted with styrene oxide (2a), respectively, and the intermediate imide was detected with HRMS (Scheme 3c,d).
Scheme 3.
Control experiments (a–d).
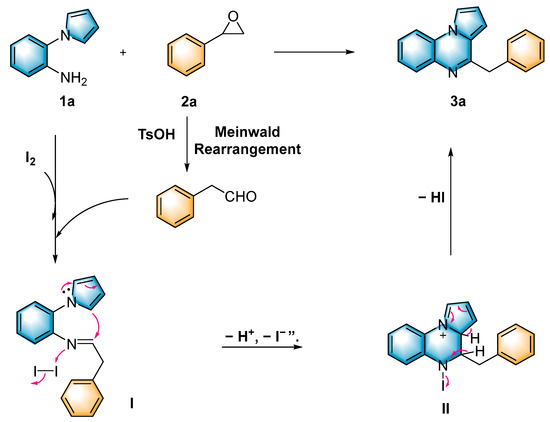
Based on the aforementioned control experiments and related literature studies, a plausible mechanism of this reaction is described in Scheme 4. Initially, in the presence of TsOH, styrene oxide (2a) underwent a Meinwald rearrangement to afford 2-phenylacetaldehyde. The 2-phenylacetaldehyde reacted with 1-(2-aminophenyl)-pyrrole (1a) accompanied by the elimination of H2O to generate the intermediate imine I. Afterwards, intramolecular cyclization was accomplished with the assistance of molecular iodine to afford the dihydropyrrolo[1,2-a]quinoxaline II, which was ultimately aromatized to afford the target product 3a.
Scheme 4.
Proposed Mechanism.
3. Materials and Methods
3.1. General Information
2-(1H-pyrrol-1-yl)anilines/2-(1H-indolo-1-yl)anilines and 2-aminobenzamides were obtained based on procedures reported in the literature [46,47]. All other reagents were market available and used with no further purification. We monitored the reactions using thin layer chromatography (TLC). Reactions requiring heat were performed in an oil bath. 1H NMR (400 MHz or 500 MHz) and 13C NMR (100 MHz or 125 MHz) spectra were recorded on a Bruker spectrometer, with CDCl3 or DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal standard (Supplementary Materials). HRMS spectra (ESI) were acquired on a Bruker impact II spectrograph in positive-ion mode with an ESI ion source.
3.2. General Experimental Procedures for 2-(1H-Pyrrol-1-yl) Anilines (1)
The substituted 2-nitroaniline (2.7626 g, 20 mmol, 1 equiv.) and 2, 5-dimethoxytetrahydrofuran (2.9075 g, 22 mmol, 1.1 equiv.) were mixed in HOAc (30 mL) and stirred vigorously under reflux conditions for 2−3 h. To neutralize the reaction mixture, Na2CO3 aqueous solution was added and extracted three times with EtOAc. The organic layer was then dried with anhydrous Na2SO4 and the residue was obtained after vacuum evaporation. Iron powder (4.4680 g, 80 mmol, 5 equiv.) and NH4Cl (1.0698 g, 20 mmol, 1 equiv.) were added to the residue in water (40 mL) and refluxed for 4–9 h. When the reaction was complete, the mixture was extracted three times with EtOAc. The organic layer was then dried with anhydrous Na2SO4, and the residue was obtained after vacuum evaporation. The residue was subsequently purified by silica gel chromatography to yield the desirable compounds.
3.3. General Experimental Procedures for 2-(1H-Indolo-1-yl)anilines (1)
The substituted fluoro-2-nitrobenzene (2.8220 g, 20 mmol, 1 equiv.), indole (2.3430 g, 20 mmol, 1 equiv.) and NaOH (0.8000 g, 20 mmol, 1 equiv.) were mixed in DMSO (30 mL) and stirred at room temperature for 4 h. The reaction mixture was extracted with EtOAc (3 × 30 mL). The organic layer was then dried with anhydrous Na2SO4 and a solid was obtained after vacuum evaporation. Iron powder (5.6850 g, 100 mmol, 5 equiv.) and NH4Cl (1.0700 g, 20 mmol, 1 equiv.) were added to the solvent and refluxed for 5−9 h. After cooling, the reaction mixture was extracted three times with EtOAc and brine solution and subsequently dried with anhydrous Na2SO4. After filtration, a vacuum was used to remove the solvent. The resulting solid was then purified by silica gel column chromatography to obtain the desired compound.
3.4. General Experimental Procedures for Compounds 3a–3t and 5a–5m
Various substituted amines (0.5 mmol, 1 equiv.), epoxides (1.0 mmol, 2 equiv.), iodine (0.1269 g, 0.5 mmol, 1 equiv.) and TsOH (0.0861 g, 0.5 mmol, 1 equiv.) were added to CH3CN (1mL) in a sealed tube. The reaction mixture was stirred vigorously at 120 °C for 4 h. After completion of the reaction, monitored by TLC, the reaction mixture was neutralized with Na2S2O3 and extracted three times with EtOAc and H2O, followed by drying with anhydrous Na2SO4. The product was purified by silica gel column chromatography to give the desired compounds 3a–3t and 5a–5m.
3.5. Characterization Data
- 4-benzylpyrrolo[1,2-a]quinoxaline (3a), purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3a as a light yellow solid (98.2 mg, 76% yield); 1H NMR (400 MHz, CDCl3): δ 7.86–7.84 (m, 1 H), 7.65 (dd, J = 2.9, 1.4 Hz, 1 H), 7.60–7.57 (m, 1 H), 7.31–7.24 (m, 4 H), 7.14 (t, J = 8.0 Hz, 2 H), 7.10–7.03 (m, 1 H), 6.69 (dd, J = 4.0, 1.2 Hz, 1 H), 6.62–6.62 (m, 1 H), 4,23 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 154.1, 136.9, 134.7, 128.5, 127.8, 127.8, 127.4, 126.1, 125.5, 124.7, 124.0, 113.2, 112.6, 112.5, 106.1, 41.5. HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H15N2+: 259.1229; found: 259.1233.
- 4-benzyl-8-methylpyrrolo[1,2-a]quinoxaline (3b), purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3b as a yellow solid (84.4 mg, 62% yield); 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 8.2 Hz, 1 H), 7.72 (dd, J = 2.7, 1.3 Hz, 1 H), 7.50 (s, 1 H), 7.32 (d, J = 7.4 Hz, 2 H), 7.20–7.10 (m, 4 H), 6.71 (dd, J = 4.0, 1.3 Hz, 1 H), 6.67 (dd, J = 4.0, 2.7 Hz, 1 H), 4.26 (s, 2 H), 2.43 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 153.2, 137.1, 136.6, 132.8, 128.3, 127.8, 127.4, 126.0, 125.5, 125.3, 124.9, 112.8, 112.6, 112.5, 105.7, 41.5, 20.7. HRMS (ESI-TOF): m/z [M + H]+ calcd for C19H17N2+: 273.1385; found: 273.1381.
- 4-benzyl-8-methoxypyrrolo[1,2-a]quinoxaline (3c), purification on a silica gel (petroleum ether/ethyl acetate = 8:1) afforded compound 3c as a yellow solid (85.1 mg, 59% yield); 1H NMR (400 MHz, CDCl3): δ 7.77 (d, J = 8.9 Hz, 1 H), 7.57 (dd, J = 2.8, 1.3 Hz, 1 H), 7.29 (d, J = 6.8 Hz, 2 H), 7.14 (t, J = 7.4 Hz, 2 H), 7.08–7.03 (m, 2 H), 6.88 (dd, J = 8.9, 2.7 Hz, 1 H), 6.66–6.61(m, 2 H), 4.21 (s, 2 H), 3.75 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 157.7, 151.5, 137.2, 129.8, 129.2, 127.8, 127.4, 126.9, 125.4, 124.7, 112.7, 112.6, 111.5, 105.5, 96.4, 54.6, 41.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C19H17ON2+: 289.1335; found: 289.1339.
- 4-benzyl-8-fluoropyrrolo[1,2-a]quinoxaline (3d), purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3d as a yellow solid (102.3 mg, 74% yield); 1H NMR (400 MHz, CDCl3): δ 7.63 (dd, J = 2.8, 1.3 Hz, 1 H), 7.56–7.50 (m, 2 H), 7.31 (d, J = 7.2 Hz, 2 H), 7.16 (t, J = 7.5 Hz, 2 H), 7.11–7.00 (m, 2 H), 6.74 (d, J = 4.0 Hz, 1 H), 6.64 (dd, J = 4.1, 2.7 Hz, 1 H), 4.22 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 158.7 (d, JC-F = 243.4 Hz), 155.4, 136.7, 136.0 (d, JC-F = 11.4 Hz), 127.65 (d, JC-F = 34.6 Hz), 125.6, 124.5, 122.86 (d, JC-F = 2.3 Hz), 113.9 (d, JC-F = 22.2 Hz), 113.7 (d, JC-F = 21.1 Hz), 113.6, 113.5, 113.4, 112.7, 106.4, 41.4. 19F NMR (377 MHz, CDCl3): δ −116.7 (s). HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H14FN2+: 277.1135; found: 277.1132.
- 4-benzyl-7-methylpyrrolo[1,2-a]quinoxaline (3e), purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3e as a yellow solid (84.4 mg, 62% yield); 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 1.5 Hz, 2 H), 7.52 (d, J = 8.3 Hz, 1 H), 7.31 (d, J = 7.2 Hz, 2 H), 7.18–7.12 (m, 3 H), 7.08 (t, J = 7.3 Hz, 1 H), 6.70 (dd, J = 4.0, 1.3 Hz, 1 H), 6.63 (dd, J = 4.0, 2.7 Hz, 1 H), 4.24 (s, 2 H), 2.36 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 154.1, 137.0, 134.8, 133.8, 128.5, 127.8, 127.4, 127.2, 125.5, 124.7, 124.1, 112.9, 112.3, 112.2, 105.7, 41.5, 20.0. HRMS (ESI-TOF): m/z [M + H]+ calcd for C19H17N2+: 273.1385; found: 273.1384.
- 4-benzyl-7-(tert-butyl)pyrrolo[1,2-a]quinoxaline (3f), purification on a silica gel (petroleum ether/ethyl acetate = 20:1) afforded compound 3f as a yellow solid (114.8 mg, 73% yield); 1H NMR (400 MHz, CDCl3): δ 7.90 (s, 1 H), 7.68 (dd, J = 2.8, 1.3 Hz, 1 H), 7.59 (d, J = 8.6 Hz, 1 H), 7.40 (dd, J = 8.6, 2.3 Hz, 1 H), 7.30 (d, J = 6.8 Hz, 2 H), 7.14 (t, J = 7.5 Hz, 2 H), 7.08–7.04 (m, 1 H), 6.69 (d, J = 4.0 Hz, 1 H), 6.63 (dd, J = 4.0, 2.7 Hz, 1 H), 4.25 (s, 2 H), 1.30 (s, 9 H). 13C NMR (100 MHz, CDCl3): δ 154.0, 147.3, 137.0, 134.5, 127.7, 127.4, 125.5, 125.0, 124.7, 124.0, 123.8, 112.9, 112.4, 112.1, 105.8, 41.6, 33.7, 30.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C22H23N2+: 315.1855; found: 315.1859.
- 4-benzyl-7-fluoropyrrolo[1,2-a]quinoxaline (3g), purification on a silica gel (petroleum ether/ethyl acetate = 20:1) afforded compound 3g as a yellow solid (98.1 mg, 71% yield); 1H NMR (400 MHz, CDCl3): δ 7.67 (dd, J = 2.8, 1.3 Hz, 1 H), 7.59 (dd, J = 9.0, 5.0 Hz, 1 H), 7.53 (dd, J = 9.5, 2.9 Hz, 1 H), 7.32 (d, J = 7.1 Hz, 2 H), 7.18 (t, J = 7.4 Hz, 2 H), 7.12–7.04 (m, 2 H), 6.76 (dd, J = 4.0, 1.3 Hz, 1 H), 6.66 (dd, J = 4.1, 2.7 Hz, 1 H), 4.23 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 158.7 (d, JC-F= 243.5 Hz), 155.4, 136.7, 136.1 (d, JC-F = 11.6 Hz), 127.8, 127.5, 125.6, 124.5, 122.9 (d, JC-F= 2.2 Hz), 114.0 (d, JC-F = 22.0 Hz), 113.8 (d, JC-F = 24.0 Hz), 113.6 (d, JC-F= 9.0 Hz), 113.4, 112.7, 106.4, 41.4. 19F NMR (377 MHz, CDCl3): δ −116.8 (s). HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H14FN2+: 277.1135; found: 277.1332.
- 4-benzyl-7-chloropyrrolo[1,2-a]quinoxaline (3h), purification on a silica gel (petroleum ether/ethyl acetate = 20:1) afforded compound 3h as a yellow solid (92.2 mg, 63% yield); 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 2.4 Hz, 1 H), 7.74 (d, J = 1.7 Hz, 1 H), 7.63 (d, J = 8.7 Hz, 1 H), 7.33 (d, J = 8.6 Hz, 3 H), 7.22–7.18 (m, 2 H), 7.14–7.11 (m, 1 H), 6.81 (d, J = 4.5 Hz, 1 H), 6.73–6.72 (m, 1 H), 4.27 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 155.4, 136.6, 135.7, 129.1, 128.0, 127.8, 127.5, 126.0, 125.6, 124.8, 124.6, 113.6, 113.5, 112.9, 106.5, 41.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H14ClN2+: 293.0839; found: 293.0837.
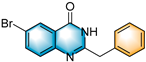
- 4-benzyl-7-bromopyrrolo[1,2-a]quinoxaline (3i), purification on a silica gel (petroleum ether/ethyl acetate = 20:1) afforded compound 3i as a yellow solid (69.1 mg, 41% yield); 1H NMR (400 MHz, CDCl3): δ 7.98 (d, J = 2.3 Hz, 1 H), 7.63 (d, J = 1.5 Hz, 1 H), 7.44 (d, J = 8.8 Hz, 1 H), 7.37 (dd, J = 8.8, 2.3 Hz, 1 H), 7.31 (d, J = 7.6 Hz, 2 H), 7.18 (t, J = 7.5 Hz, 2 H), 7.10 (t, J = 7.3 Hz, 1 H), 6.75 (d, J = 4.1 Hz, 1 H), 6.67–6.65 (m, 1 H), 4.23 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 155.3, 136.6, 136.0, 131.1, 128.8, 127.8, 127.5, 125.7, 125.2, 124.6, 116.5, 113.9, 113.5, 113.0, 106.6, 41.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H14BrN2+: 337.0334; found: 337.0333.
- 4-benzyl-7-iodopyrrolo[1,2-a]quinoxaline (3j), purification on a silica gel (petroleum ether/ethyl acetate = 20:1) afforded compound 3i as a yellow solid (76.8 mg, 40% yield); 1H NMR (400 MHz, CDCl3): δ 8.22 (d, J = 2.0 Hz, 1 H), 7.69 (dd, J = 2.8, 1.4 Hz, 1 H), 7.59 (dd, J = 8.6, 2.0 Hz, 1 H), 7.38 (d, J = 8.6 Hz, 1 H), 7.32 (d, J = 7.5 Hz, 2 H), 7.22–7.15 (m, 2 H), 7.11 (t, J = 7.3 Hz, 1 H), 6.78 (dd, J = 4.0, 1.4 Hz, 1 H), 6.70 (dd, J = 4.1, 2.7 Hz, 1 H), 4.24 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 155.2, 137.3, 136.6, 136.2, 134.5, 127.8, 127.5, 125.9, 125.7, 124.6, 114.2, 113.5, 113.0, 106.7, 87.1, 41.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H14IN2+: 385.0195; found: 385.0196.
- 4-benzyl-9-methylpyrrolo[1,2-a]quinoxaline (3k), purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3k as a yellow solid (87.1 mg, 64% yield); 1H NMR (400 MHz, CDCl3): δ 8.03 (dd, J = 2.9, 1.4 Hz, 1 H), 7.72 (dd, J = 7.9, 1.8 Hz, 1 H), 7.28 (d, J = 6.8 Hz, 2 H), 7.16–7.10 (m, 3 H), 7.07–7.02 (m, 2 H), 6.72 (dd, J = 4.1, 1.3 Hz, 1 H), 6.59 (dd, J = 4.2, 2.8 Hz, 1 H), 4.21 (s, 2 H), 2.68 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 153.7, 137.0, 136.4, 129.6, 127.7, 127.4, 127.1, 126.5, 126.0, 125.4, 124.2, 123.4, 118.8, 111.8, 105.4, 41.3, 22.7. HRMS (ESI-TOF): m/z [M + H]+ calcd for C19H17N2+: 273.1385; found: 273.1390.
- 4-Benzyl-9-chloropyrrolo[1,2-a]quinoxaline (3l). Purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3l as a yellow solid (95.2 mg, 65% yield); 1H NMR (400 MHz, CDCl3): δ 8.90 (dd, J = 2.9, 1.3 Hz, 1 H), 7.72 (dd, J = 8.0, 1.5 Hz, 1 H), 7.27 (dd, J = 8.0, 1.5 Hz, 3 H), 7.15–7.09 (m, 3 H), 7.07–7.03 (m, 1 H), 6.76 (dd, J = 4.2, 1.3 Hz, 1 H), 6.60 (dd, J = 4.2, 2.9 Hz, 1 H), 4.18(s, 2 H). 13C NMR (100 MHz, CDCl3): δ 154.6, 137.7, 136.7, 128.5, 127.8, 127.7, 127.4, 125.8, 125.6, 124.4, 123.5, 119.8, 119.8, 112.0, 106.4, 41.2. HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H14ClN2+: 293.0839; found: 293.0844.
- 4-benzyl-7,8-dimethylpyrrolo[1,2-a]quinoxaline (3m), purification on a silica gel (petroleum ether/ethyl acetate = 10:1) afforded compound 3m as a yellow solid (91.6 mg, 64% yield); 1H NMR (400 MHz, CDCl3): δ 7.64 (s, 1 H), 7.62 (s, 1 H), 7.41 (d, J = 5.3 Hz, 1 H), 7.30 (d, J = 8.1 Hz, 2 H), 7.15 (t, J = 7.4 Hz, 2 H), 7.07 (t, J = 7.3 Hz, 1 H), 6.67 (dd, J = 4.0, 1.4 Hz, 1 H), 6.62–6.60 (m, 1 H), 4.23 (s, 2 H), 2.29 (s, 3 H), 2.25 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 153.1, 137.2, 135.5, 133.0, 132.8, 128.8, 127.8, 127.4, 125.4, 124.8, 124.2, 113.0, 112.6, 112.2, 105.4, 41.5, 19.1, 18.5. HRMS (ESI-TOF): m/z [M + H]+ calcd for C20H19N2+: 287.1542; found: 287.1540.
- 4-benzyl-7,8-difluoropyrrolo[1,2-a]quinoxaline (3n), purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3n as a yellow solid (108.8 mg, 76% yield); 1H NMR (400 MHz, CDCl3): δ 7.68 (dd, J = 11.0, 8.1 Hz, 1 H), 7.62 (dd, J = 2.7, 1.3 Hz, 1 H), 7.48 (dd, J = 10.5, 7.3 Hz, 1 H), 7.32 (d, J = 6.9 Hz, 2 H), 7.22–7.17 (m, 2 H), 7.14–7.11 (m, 1 H), 6.80 (dd, J = 4.1, 1.3 Hz, 1 H), 6.73 (dd, J = 4.0, 2.8 Hz, 1 H), 4.25 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 154.8 (d, JC-F = 2.9 Hz), 148.3 (dd, JC-F = 248.7, 14.4 Hz), 146.9 (dd, JC-F = 245.2, 13.7 Hz), 136.6, 129.9, 127.8, 127.5, 125.7, 124.4, 122.6 (d, JC-F = 8.7 Hz), 116.2 (d, JC-F = 19.6 Hz), 113.5, 113.2, 106.6, 101.1 (d, JC-F = 22.2 Hz), 41.3. 19F NMR (377 MHz, CDCl3): δ -135.1 (s), -140.2 (s). HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H13F2N2+: 295.1041; found: 295.1039.
- 4-benzyl-7,8-dichloropyrrolo[1,2-a]quinoxaline (3o), purification on a silica gel (petroleum ether/ethyl acetate = 25:1) afforded compound 3o as a yellow solid (106.3 mg, 65% yield); 1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1 H), 7.75 (s, 1 H), 7.64 (dd, J = 2.8, 1.3 Hz, 1 H), 7.31 (d, J = 6.9 Hz, 2 H), 7.22–7.11 (m, 3 H), 6.80 (dd, J = 4.0, 1.3 Hz, 1 H), 6.71 (dd, J = 4.0, 2.8 Hz, 1 H), 4.23 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 155.6, 136.4, 134.3, 129.6, 129.6, 127.8, 127.6, 127.5, 125.7, 125.4, 124.5, 114.1, 113.8, 113.4, 107.1, 41.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C18H13Cl2N2+: 327.0450; found: 327.0455.
- 6-benzylindolo[1,2-a]quinoxaline (3p), purification on a silica gel (petroleum ether/ethyl acetate = 25:1) afforded compound 3p as a yellow solid (74.1 mg, 48% yield); 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 15.8, 8.6 Hz, 2 H), 7.89 (dd, J = 7.9, 1.8 Hz, 1 H), 7.71 (d, J = 8.0 Hz, 1 H), 7.42–7.23 (m, 6 H), 7.17 (t, J = 7.8 Hz, 2 H), 7.09 (t, J = 8.1 Hz, 1 H), 6.95 (s, 1 H), 4.30 (s, 2 H). 13C NMR (100 MHz, CDCl3): δ 155.6, 136.6, 134.7, 131.7, 129.2, 128.9, 128.1, 127.9, 127.7, 127.5, 127.0, 125.6, 123.1, 122.9, 121.6, 121.4, 113.5, 113.4, 99.5, 41.6. HRMS (ESI-TOF): m/z [M + H]+ calcd for C22H17N2+: 309.1385; found: 309.1382.
- 6-qenzyl-3-methylindolo[1,2-a]quinoxaline (3q), purification on a silica gel (petroleum ether/ethyl acetate = 20:1) afforded compound 3q as a yellow solid (61.3 mg, 38% yield); 1H NMR (400 MHz, CDCl3): δ 8.31 (d, J = 8.6 Hz, 1 H), 8.14 (s, 1 H), 7.79 (dd, J = 16.9, 8.0 Hz, 2 H), 7.42–7.27 (m, 3 H), 7.29 (t, J = 7.4 Hz, 1 H), 7.23–7.06 (m, 4 H), 6.98 (s, 1 H), 4.33(s, 2 H), 2.51(s, 3 H). 13C NMR (100 MHz, CDCl3): δ 154.6, 137.5, 136.7, 132.7, 131.7, 129.1, 128.7, 128.3, 128.0, 127.7, 127.5, 125.6, 124.0, 122.9, 121.6, 121.5, 113.9, 113.6, 99.4, 41.6, 21.1. HRMS (ESI-TOF): m/z [M + H]+ calcd for C23H19N2+: 323.1542; found: 323.1546.
- 6-Benzyl-7-methylindolo[1,2-a]quinoxaline (3r). Purification on a silica gel (petroleum ether/ethyl acetate = 15:1) afforded compound 3r as a yellow solid (56.4 mg, 35% yield); 1H NMR (400 MHz, CDCl3): δ 8.44 (t, J = 7.9 Hz, 2 H), 7.99 (s, 1 H), 7.88 (d, J = 8.1 Hz, 1 H), 7.56 (q, J = 7.1 Hz, 2 H), 7.41 (dt, J = 14.1, 7.1 Hz, 2 H), 7.27–7.25 (m, 4 H), 7.20 (q, J = 4.3 Hz, 1 H), 4.64 (s, 2 H), 2.63 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ 156.8, 137.9, 132.1, 130.6, 130.0, 129.5, 128.9, 128.6, 128.5, 128.4, 128.2, 126.5, 124.8, 124.0, 123.8, 122.0, 120.7, 114.5, 114.4, 29.7, 11.0. HRMS (ESI-TOF): m/z [M + H]+ calcd for C23H19N2+: 323.1542; found: 323.1543.
- 4-propylpyrrolo[1,2-a]quinoxaline (3s), purification on a silica gel (petroleum ether/ethyl acetate = 10:1) afforded compound 3s as a yellow solid (42.1 mg, 40% yield); 1H NMR (500 MHz, CDCl3): δ 7.84 (dd, J = 7.9, 1.5 Hz, 1 H), 7.81–7.79 (m, 1 H), 7.73 (d, J = 8.0 Hz, 1 H), 7.35 (dtd, J = 21.0, 7.4, 1.4 Hz, 2 H), 6.82 (dd, J = 3.9, 1.2 Hz, 1 H), 6.75 (dd, J = 3.8, 2.8 Hz, 1 H), 2.91 (dd, J = 8.5, 7.1 Hz, 2 H), 1.86 (dq, J = 15.0, 7.4 Hz, 2 H), 1.00 (t, J = 7.4 Hz, 3 H). 13C NMR (125 MHz, CDCl3): δ 156.4, 135.0, 128.4, 126.2, 125.8, 125.1, 124.0, 113.0, 112.6, 112.4, 105.2, 36.8, 20.9, 13.3. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H15N2+: 211.1229; found: 211.1233.
- (E)-4-(prop-1-en-1-yl)pyrrolo[1,2-a]quinoxaline (3t), purification on a silica gel (petroleum ether/ethyl acetate = 10:1) afforded compound 3t as a yellow solid (52.1 mg, 40% yield); 1H NMR (500 MHz, CDCl3): δ 7.90–7.82 (m, 2 H), 7.77–7.72 (dd, J = 8.0, 1.0 Hz, 1 H), 7.42–7.29 (m, 2 H), 7.18–7.09 (qd, J = 13.5, 7.0 Hz, 1 H), 6.93–6.90 (dd, J = 4.0, 1.0 Hz, 1 H), 6.80 (dd, J = 4.0, 1.5 Hz, 1 H), 6.78 (dd, J = 7.0, 2.5 Hz, 1 H), 1.98 (dd, J = 6.8, 1.7 Hz, 3 H). 13C NMR (125 MHz, CDCl3): δ 149.2, 135.1, 134.4, 128.6, 126.2, 125.9, 125.7, 124.6, 124.2, 113.3, 112.6, 112.5, 105.0, 17.8. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H14N2+: 209.1072; found: 209.1071.
- 2-benzylquinazolin-4(3H)-one (5a), purification on a silica gel (petroleum ether/ethyl acetate = 2:1) afforded compound 5a as a white solid (82.7 mg, 70% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.43 (s, 1 H), 8.09 (dd, J = 7.9, 1.6 Hz, 1 H), 7.78 (ddd, J = 8.4, 7.0, 1.6 Hz, 1 H), 7.62 (d, J = 8.5 Hz, 1 H), 7.50–7.44 (m, 1 H), 7.39 (d, J = 7.9 Hz, 2 H), 7.33 (t, J = 7.6 Hz, 2 H), 7.28–7.22 (m, 1 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 167.1, 161.2, 154.1, 141.8, 139.6, 134.1, 133.7, 132.2, 132.0, 131.4, 130.9, 126.0, 46.0. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H13ON2+: 237.1002; found: 237.1005.
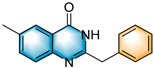
- 2-benzyl-6-methylquinazolin-4(3H)-one (5b), purification on a silica gel (petroleum ether/ethyl acetate = 5:1) afforded compound 5b as a white solid (92.6 mg, 74% yield). 1H NMR (400 MHz, DMSO-d6): δ 12.32 (s, 1 H), 7.87 (s, 1 H), 7.59 (dd, J = 8.3, 2.2 Hz, 1 H), 7.51 (d, J = 8.2 Hz, 1 H), 7.38 (d, J = 6.8 Hz, 2 H), 7.32 (t, J = 7.5 Hz, 2 H), 7.24 (t, J = 7.2 Hz, 1 H), 3.92 (s, 2 H), 2.42 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 162.3, 155.5, 147.4, 137.1, 136.3, 136.1, 129.3, 129.0, 127.3, 127.2, 125.5, 121.0, 41.2, 21.2. HRMS (ESI-TOF): m/z [M + H]+ calcd for C16H15ON2+: 251.1178; found: 251.1177.
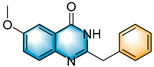
- 2-benzyl-6-methoxyquinazolin-4(3H)-one (5c), purification on a silica gel (petroleum ether/ethyl acetate = 1:1) afforded compound 5c as a white solid (77.3 mg, 58% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.38 (s, 1 H), 7.57 (d, J = 8.8 Hz, 1 H), 7.48 (d, J = 2.9 Hz, 1 H), 7.41–7.36 (m, 3 H), 7.32 (d, J = 15.0 Hz, 2 H), 7.24 (t, J = 7.2 Hz, 1 H), 3.92 (s, 2 H), 3.85 (s, 3 H). 13C NMR (100 MHz, DMSO-d6): δ 162.2, 157.8, 154.1, 143.9, 137.2, 129.3, 129.1, 129.0, 127.2, 124.3, 122.0, 106.2, 56.0, 41.1. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H15ON2+: 267.1127; found: 267.1123.
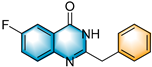
- 2-benzyl-6-fluoroquinazolin-4(3H)-one (5d), purification on a silica gel (petroleum ether/ethyl acetate = 5:1) afforded compound 5d as a white solid (90.3 mg, 71% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.60 (s, 1 H), 7.76 (dd, J = 8.6, 2.7 Hz, 1 H), 7.71–7.65 (m, 2 H), 7.39 (d, J = 6.9 Hz, 2 H), 7.33 (t, J = 7.6 Hz, 2 H), 7.25 (t, J = 7.3 Hz, 1 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 161.8 (d, JC-F = 3.3 Hz), 160.2 (d, JC-F = 244.9 Hz), 156.0 (d, JC-F = 2.2 Hz), 146.2 (d, JC-F = 1.8 Hz), 136.9, 130.2 (d, JC-F = 8.0 Hz), 129.4, 129.0, 127.3, 123.3 (d, JC-F = 24.0 Hz), 122.4 (d, JC-F = 8.4 Hz), 110.8 (d, JC-F = 23.3 Hz), 41.1. 19F NMR (377 MHz, DMSO-d6): δ −114.1 (s). HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OFN2+: 255.0927; found: 255.0928.
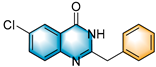
- 2-benzyl-6-chloroquinazolin-4(3H)-one (5e), purification on a silica gel (petroleum ether/ethyl acetate = 5:1) afforded compound 5e as a white solid (101.55 mg, 75% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.61 (s, 1 H), 8.01 (d, J = 2.5 Hz, 1 H), 7.80 (dd, J = 8.8, 2.6 Hz, 1 H), 7.64 (d, J = 8.7 Hz, 1 H), 7.38 (d, J = 6.8 Hz, 2 H), 7.33 (t, J = 7.5 Hz, 2 H), 7.25 (t, J = 7.2 Hz, 1 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 161.4, 157.1, 148.1, 136.8, 135.0, 130.9, 129.7, 129.4, 129.0, 127.3, 125.2, 122.5, 41.2. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OClN2+:271.0632; found: 271.0635.
- 2-benzyl-6-bromoquinazolin-4(3H)-one (5f), purification on a silica gel (petroleum ether/ethyl acetate = 5:1) afforded compound 5f as a white solid (93.0 mg, 59% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.65 (s, 1 H), 8.15 (d, J = 2.4 Hz, 1 H), 7.92 (dd, J = 8.7, 2.4 Hz, 1 H), 7.57 (d, J = 8.8 Hz, 1 H), 7.38 (d, J = 6.9 Hz, 2 H), 7.33 (t, J = 7.5 Hz, 2 H), 7.25 (t, J = 7.2 Hz, 1 H), 3.94 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 161.2, 157.2, 148.4, 137.7, 136.8, 129.8, 129.4, 129.0, 128.3, 127.3, 122.9, 119.0, 41.2. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OBrN2+: 315.0127; found: 315.0124.
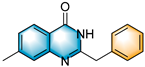
- 2-benzyl-7-methylquinazolin-4(3H)-one (5g), purification on a silica gel (petroleum ether/ethyl acetate = 2:1) afforded compound 5g as a white solid (75.2 mg, 60% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.31 (s, 1 H), 7.97 (d, J = 8.1 Hz, 1 H), 7.47–7.37 (m, 3 H), 7.35–7.20 (m, 4 H), 3.93 (s, 2 H), 2.43 (s, 3 H). 13C NMR (100 MHz, DMSO-d6): δ 162.2, 156.5, 149.5, 145.3, 137.1, 129.3, 128.9, 128.0, 127.2, 127.1, 126.0, 118.8, 41.2, 21.8. HRMS (ESI-TOF): m/z [M + H]+ calcd for C16H15ON2+: 251.1178; found: 251.1175.
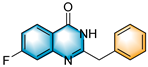
- 2-benzyl-7-fluoroquinazolin-4(3H)-one (5h), purification on a silica gel (petroleum ether/ethyl acetate = 2:1) afforded compound 5h as a white solid (85.2 mg, 67% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.54 (s, 1 H), 8.15 (dd, J = 8.9, 6.4 Hz, 1 H), 7.42–7.37 (m, 3 H), 7.36–7.30 (m, 3 H), 7.25 (t, J = 7.3 Hz, 1 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ = 166.2 (d, J C-F = 250.7 Hz), 161.6, 158.0, 151.6 (d, J C-F = 13.2 Hz), 136.8, 129.4, 129.2, 129.0, 127.3, 118.3 (d, JC-F = 1.8 Hz), 115.2 (d, JC-F = 23.6 Hz), 112.5 (d, JC-F = 21.8 Hz), 41.2. 19F NMR (377 MHz, DMSO-d6): δ −104.5 (s). HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OFN2+: 255.0927; found: 255.0930.
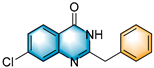
- 2-benzyl-7-chloroquinazolin-4(3H)-one (5i), purification on a silica gel (petroleum ether/ethyl acetate = 2:1) afforded compound 5i as a white solid (88.1 mg, 65% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.58 (s, 1 H), 8.07 (d, J = 8.4 Hz, 1 H), 7.66 (d, J = 2.1 Hz, 1 H), 7.50 (dd, J = 8.6, 2.1 Hz, 1 H), 7.39 (d, J = 7.0 Hz, 2 H), 7.33 (t, J = 7.5 Hz, 2 H), 7.25 (t, J = 7.2 Hz, 1 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 161.8, 158.1, 150.5, 139.5, 136.8, 129.4, 129.0, 128.3, 127.3, 127.0, 126.6, 120.1, 41.2. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OClN2+: 271.0632; found: 271.0630.
- 2-benzyl-7-bromoquinazolin-4(3H)-one (5j), purification on a silica gel (petroleum ether/ethyl acetate = 3:1) afforded compound 5j as a white solid (100.9 mg, 64% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.58 (s, 1 H), 7.99 (d, J = 8.4 Hz, 1 H), 7.81 (d, J = 2.1 Hz, 1 H), 7.62 (dd, J = 8.4, 2.0 Hz, 1 H), 7.39 (d, J = 6.8 Hz, 2 H), 7.33 (t, J = 7.5 Hz, 2 H), 7.25 (t, J = 7.2 Hz, 1 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 161.9, 158.1, 150.6, 136.8, 129.7, 129.7, 129.4, 129.0, 128.5, 128.3, 127.3, 120.4, 41.2. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OBrN2+: 315.0127; found: 315.0131.
- 2-(4-chlorobenzyl)quinazolin-4(3H)-one (5k), purification on a silica gel (petroleum ether/ethyl acetate = 2:1) afforded compound 5k as a white solid (89.4 mg, 66% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.43 (s, 1 H), 8.09 (dd, J = 7.9, 1.6 Hz, 1 H), 7.78 (ddd, J = 8.6, 7.1, 1.6 Hz, 1 H), 7.60 (dd, J = 8.3, 1.1 Hz, 1 H), 7.50–7.45 (m, 1 H), 7.44–7.37 (m, 4 H), 3.95 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 162.3, 156.1, 149.3, 136.0, 134.9, 132.0, 131.3, 128.9, 127.4, 126.8, 126.2, 121.3, 40.4. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OClN2+: 271.0632; found: 271.0633.
- 2-(4-bromobenzyl)quinazolin-4(3H)-one (5l), purification on a silica gel (petroleum ether/ethyl acetate = 3:1) afforded compound 5l as a white solid (99.3 mg, 63% yield); 1H NMR (400 MHz, DMSO-d6): δ 12.43 (s, 1 H), 8.09 (d, J = 9.6 Hz, 1 H), 7.82–7.73 (m, 1 H), 7.60 (d, J = 7.4 Hz, 1 H), 7.53 (d, J = 8.4 Hz, 2 H), 7.48 (t, J = 7.5 Hz, 1 H), 7.35 (d, J = 8.5 Hz, 2 H), 3.93 (s, 2 H). 13C NMR (100 MHz, DMSO-d6): δ 162.3, 156.0, 149.3, 136.4, 134.9, 131.8, 131.7, 127.4, 126.8, 126.2, 121.3, 120.5, 40.5. HRMS (ESI-TOF): m/z [M + H]+ calcd for C15H12OBrN2+: 315.0127; found: 315.0125.
- (E)-2-(prop-1-en-1-yl)quinazolin-4(3H)-one (5m). Purification on a silica gel (petroleum ether/ethyl acetate = 1:1) afforded compound 5m as a white solid (44.7 mg, 48% yield); 1H NMR (500 MHz, DMSO-d6): δ 12.17 (s, 1 H), 8.14–8.01 (m, 1 H), 7.82–7.72 (m, 1 H), 7.60 (d, J = 8.1 Hz, 1 H), 7.44 (t, J = 7.5 Hz, 1 H), 7.21–7.04 (m, 1 H), 6.27 (dd, J = 15.7, 1.7 Hz, 1 H), 1.92 (dd, J = 6.9, 1.5 Hz, 3 H). 13C NMR (125 MHz, DMSO-d6): δ 162.3, 151.6, 149.5, 138.7, 134.9, 127.5, 126.4, 126.2, 125.1, 121.4, 18.7. HRMS (ESI-TOF): m/z [M + H]+ calcd for C11H11ON2+: 187.0865; found: 187.0867.
4. Conclusions
To conclude, we disclosed an iodine-mediated one-pot procedure for the synthesis of pyrrolo/indolo[1,2-a]quinoxalines and quinazolin-4-ones under metal-free conditions. This methodology tandems the Meinwald rearrangement and annulation process. A series of N-heterocycles were obtained with medium to good yields. The protocol has a broad substrate scope, has a simple work-up and was suitable for wide-scale preparation. Furthermore, the obtained products could be further modified to afford promising pharmaceutical reagents. This study injects new vitality into the methodology for the synthesis of N-heterocycles. Related research is still in progress in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28217391/s1, Figure S1: 1H NMR spectrum of compound 3a in CDCl3 (400 MHz); Figure S2: 13C {1H} NMR spectrum of compound 3a in CDCl3 (100 MHz); Figure S3: 1H NMR spectrum of compound 3b in CDCl3 (400 MHz); Figure S4: 13C {1 H} NMR spectrum of compound 3b in CDCl3 (100 MHz); Figure S5: 1H NMR spectrum of compound 3c in CDCl3 (400 MHz); Figure S6: 13C {1 H} NMR spectrum of compound 3c in CDCl3 (100 MHz); Figure S7: 1H NMR spectrum of compound 3d in CDCl3 (400 MHz); Figure S8: 13C {1 H} NMR spectrum of compound 3d in CDCl3 (100 MHz); Figure S9: 19F {1 H} NMR spectrum of compound 3d in CDCl3 (377 MHz); Figure S10: 1H NMR spectrum of compound 3e in CDCl3 (400 MHz); Figure S11: 13C {1 H} NMR spectrum of compound 3e in CDCl3 (100 MHz); Figure S12: 1H NMR spectrum of compound 3f in CDCl3 (400 MHz); Figure S13: 13C {1 H} NMR spectrum of compound 3f in CDCl3 (100 MHz); Figure S14: 1H NMR spectrum of compound 3g in CDCl3 (400 MHz); Figure S15: 13C {1 H} NMR spectrum of compound 3g in CDCl3 (100 MHz); Figure S16: 19F {1H} NMR spectrum of compound 3g in CDCl3 (377 MHz); Figure S17: 1H NMR spectrum of compound 3h in CDCl3 (400 MHz); Figure S18: 13C {1 H} NMR spectrum of compound 3h in CDCl3 (100 MHz); Figure S19: 1H NMR spectrum of compound 3i in CDCl3 (400 MHz); Figure S20: 13C {1 H} NMR spectrum of compound 3i in CDCl3 (100 MHz); Figure S21: 1H NMR spectrum of compound 3j in CDCl3 (400 MHz); Figure S22: 13C {1 H} NMR spectrum of compound 3j in CDCl3 (100 MHz); Figure S23: 1H NMR spectrum of compound 3k in CDCl3 (400 MHz); Figure S24: 13C {1 H} NMR spectrum of compound 3k in CDCl3 (100 MHz); Figure S25: 1H NMR spectrum of compound 3l in CDCl3 (400 MHz); Figure S26: 13C {1 H} NMR spectrum of compound 3l in CDCl3 (100 MHz); Figure S27: 1H NMR spectrum of compound 3m in CDCl3 (400 MHz); Figure S28: 13C {1 H} NMR spectrum of compound 3m in CDCl3 (100 MHz); Figure S29: 1H NMR spectrum of compound 3n in CDCl3 (400 MHz); Figure S30: 13C {1 H} NMR spectrum of compound 3n in CDCl3 (100 MHz); Figure S31: 19F {1 H} NMR spectrum of compound 3n in CDCl3 (377 MHz); Figure S32: 1H NMR spectrum of compound 3o in CDCl3 (400 MHz); Figure S33: 13C {1 H} NMR spectrum of compound 3o in CDCl3 (100 MHz); Figure S34: 1H NMR spectrum of compound 3p in CDCl3 (400 MHz); Figure S35: 13C {1 H} NMR spectrum of compound 3p in CDCl3 (100 MHz); Figure S36: 1H NMR spectrum of compound 3q in CDCl3 (400 MHz); Figure S37: 13C {1 H} NMR spectrum of compound 3q in CDCl3 (100 MHz); Figure S38: 1H NMR spectrum of compound 3r in CDCl3 (400 MHz); Figure S39: 13C {1 H} NMR spectrum of compound 3r in CDCl3 (100 MHz); Figure S40: 1H NMR spectrum of compound 3s in CDCl3 (500 MHz); Figure S41: 13C {1 H} NMR spectrum of compound 3s in CDCl3 (125 MHz); Figure S42: 1H NMR spectrum of compound 3t in CDCl3 (500 MHz); Figure S43: 13C {1 H} NMR spectrum of compound 3t in CDCl3 (125 MHz); Figure S44: 1H NMR spectrum of compound 5a in DMSO-d6 (400 MHz); Figure S45: 13C {1 H} NMR spectrum of compound 5a in DMSO-d6 (100 MHz); Figure S46: 1H NMR spectrum of compound 5b in DMSO-d6 (400 MHz); Figure S47: 13C {1 H} NMR spectrum of compound 5b in DMSO-d6 (100 MHz); Figure S48: 1H NMR spectrum of compound 5c in DMSO-d6 (400 MHz); Figure S49: 13C {1 H} NMR spectrum of compound 5c in DMSO-d6 (100 MHz); Figure S50: 1H NMR spectrum of compound 5d in DMSO-d6 (400 MHz); Figure S51: 13C {1 H} NMR spectrum of compound 5d in DMSO-d6 (100 MHz); Figure S52: 19F {1 H} NMR spectrum of compound 5d in DMSO-d6 (377 MHz); Figure S53: 1H NMR spectrum of compound 5e in DMSO-d6 (400 MHz); Figure S54: 13C {1 H} NMR spectrum of compound 5e in DMSO-d6 (100 MHz); Figure S55: 1H NMR spectrum of compound 5f in DMSO-d6 (400 MHz); Figure S56: 13C {1 H} NMR spectrum of compound 5f in DMSO-d6 (100 MHz); Figure S57: 1H NMR spectrum of compound 5g in DMSO-d6 (400 MHz); Figure S58: 13C {1 H} NMR spectrum of compound 5g in DMSO-d6 (100 MHz); Figure S59: 1H NMR spectrum of compound 5h in DMSO-d6 (400 MHz); Figure S60: 13C {1 H} NMR spectrum of compound 5h in DMSO-d6 (100 MHz); Figure S61: 19F {1 H} NMR spectrum of compound 5h in DMSO-d6 (377 MHz); Figure S62: 1H NMR spectrum of compound 5i in DMSO-d6 (400 MHz); Figure S63: 13C {1 H} NMR spectrum of compound 5i in DMSO-d6 (100 MHz); Figure S64: 1H NMR spectrum of compound 5j in DMSO-d6 (400 MHz); Figure S65: 13C {1 H} NMR spectrum of compound 5j in DMSO-d6 (100 MHz); Figure S66: 1H NMR spectrum of compound 5k in DMSO-d6 (400 MHz); Figure S67: 13C {1 H} NMR spectrum of compound 5k in DMSO-d6 (100 MHz); Figure S68: 1H NMR spectrum of compound 5l in DMSO-d6 (400 MHz); Figure S69: 13C {1 H} NMR spectrum of compound 5l in DMSO-d6 (100 MHz); Figure S70: 1H NMR spectrum of compound 5m in DMSO-d6 (500 MHz); Figure S71: 13C {1 H} NMR spectrum of compound 5m in DMSO-d6 (125 MHz); Figure S72. HRMS of intermediate.
Author Contributions
Methodology: S.L. and X.L.; validation: X.L., C.D. and L.L.; investigation: S.L. and X.L.; writing—original draft preparation: X.L.; writing—review and editing: B.Y. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
Shandong Key Research Program (No. 2019JZZY021015) and Zhejiang Xin’an Chemical Group Company Basic Research Project (No. YJG202108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The supporting data are available in the Supplementary Materials.
Acknowledgments
We are grateful for the financial support from the Shandong Key Research Program (No. 2019JZZY021015) and Zhejiang Xin’an Chemical Group Company Basic Research Project (No. YJG202108). We are also grateful for the Analytical Center for Structural Constituent and the Physical Property of Core Facilities Sharing Platform, Shandong University for their technology and service support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Li, R.; Li, B.; Zhang, H.; Ju, C.; Qin, Y.; Xue, X.; Zhao, D. A ring expansion strategy towards diverse azaheterocycles. Nat. Chem. 2021, 13, 1006–1016. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Sun, Z.; Dai, Z.; Tang, Z.; Ma, J.; Ma, C. Direct Olefination of Fluorinated Quinoxalines via Cross-Dehydrogenative Coupling Reactions: A New Near-Infrared Probe for Mitochondria. Adv. Synth. Catal. 2017, 359, 2259–2268. [Google Scholar] [CrossRef]
- Jonet, A.; Guillon, J.; Mullié, C.; Cohen, A.; Sonnet, P. Synthesis and Antimalarial Activity of New Enantiopure Aminoalcohol pyrrolo [1,2-a]quinoxaline. Med. Chem. 2018, 14, 293–303. [Google Scholar] [CrossRef]
- Liu, K.; Li, D.; Zheng, W.; Shi, M.; Chen, Y.; Tang, M.; Yang, T.; Zhao, M.; Deng, D.; Zhang, C.; et al. Discovery, Optimization, and Evaluation of Quinazolinone Derivatives with Novel Linkers as Orally Efficacious Phosphoinositide-3-Kinase Delta Inhibitors for Treatment of Inflammatory Diseases. J. Med. Chem. 2021, 64, 8951–8970. [Google Scholar] [CrossRef]
- Safakish, M.; Hajimahdi, Z.; Aghasadeghi, M.R.; Vahabpour, R.; Zarghi, A. Design, Synthesis, Molecular Modeling and Anti-HIV Assay of Novel Quinazolinone Incorporated Coumarin Derivatives. Curr. HIV Res. 2020, 18, 41–51. [Google Scholar]
- Li, Z.; Zhao, L.; Bian, Y.; Li, Y.; Qu, J.; Song, F. The Antibacterial Activity of Quinazoline and Quinazolinone Hybrids. Curr. Top. Med. Chem. 2022, 22, 1035–1044. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Yao, X.; Li, Y.; Tan, J.; Wang, L.; Qiao, W.; Geng, Y.; Liu, Y.; Wang, Q. Design, synthesis and antiviral activity of novel quinazolinones. Eur. J. Med. Chem. 2012, 53, 275–282. [Google Scholar] [CrossRef]
- Sakr, A.; Rezq, S.; Ibrahim, S.M.; Soliman, E.; Baraka, M.M.; Romero, D.G.; Kothayer, H. Design and synthesis of novel quinazolinones conjugated ibuprofen, indoleacetamide, or thioacetohydrazide as selective COX-2 inhibitors: Anti-inflammatory, analgesic and anticancer activities. J. Enzym. Inhib. Med. Chem. 2021, 36, 1810–1828. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Chen, J.; Wu, X.F. Recent Advances in 4(3H)-quinazolinone Syntheses. RSC Adv. 2014, 4, 12065–12077. [Google Scholar] [CrossRef]
- El-Subbagh, H.I.; Sabry, M.A. 2-Substituted-mercapto-quinazolin-4(3H)-ones as DHFR Inhibitors. Mini-Rev. Med. Chem. 2021, 21, 2249–2260. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B.; Wang, Y.; Wang, X.; Gou, S. Discovery of phthalazino [1,2-b]-quinazolinone derivatives as multitarget HDAC inhibitors for the treatment of hepatocellular carcinoma via activating the p53 signal pathway. Eur. J. Med. Chem. 2022, 229, 114058. [Google Scholar] [CrossRef]
- Chatterjee, A.; Majumdar, S.G. Alkaloids of Glycosmis pentaphylla (Retz.) DC. Part I. J. Am. Chem. Soc. 1954, 76, 2459–2463. [Google Scholar] [CrossRef]
- Cao, L.; Huo, H.; Zeng, H.; Yu, Y.; Lu, D.; Gong, Y. One-Pot Synthesis of Quinazolin-4(3H)-ones through Anodic Oxidation and the Related Mechanistic Studies. Adv. Synth. Catal. 2018, 360, 4764–4773. [Google Scholar] [CrossRef]
- Cheeseman, G.W.H.; Tuck, B. The Synthesis of pyrrolo[l,2-a]quinoxalines from N-(2-acylaminophenyl)-pyrroles. J. Chem. Soc. C 1966, 852–855. [Google Scholar] [CrossRef]
- Geng, M.; Huang, M.; Kuang, J.; Fang, W.; Miao, M.; Ma, Y. Application of N,N-Dimethylethanolamine as a One-Carbon Synthon for the Synthesis of Pyrrolo [1,2-a]quinoxalines, Quinazolin-4-ones, and Benzo [4,5]imidazoquinazolines via [5 + 1] Annulation. J. Org. Chem. 2022, 87, 14753–14762. [Google Scholar] [CrossRef]
- Jayaram, A.; Govindan, K.; Kannan, V.R.; Seenivasan, V.T.; Chen, N.Q.; Lin, W.Y. Iodine-Promoted Oxidative Cyclization of Acylated and Alkylated Derivatives from Epoxides toward the Synthesis of Aza Heterocycles. J. Org. Chem. 2023, 88, 1749–1761. [Google Scholar] [CrossRef]
- Ding, C.; Li, S.; Feng, K.; Ma, C. PEG-400 as a carbon synthon: Highly selective synthesis of quinolines and methylquinolines under metal-free conditions. Green Chem. 2021, 23, 5542–5548. [Google Scholar] [CrossRef]
- Li, S.; Ren, J.; Ding, C.; Wang, Y.; Ma, C. N,N-Dimethylformamide as Carbon Synthons for the Synthesis of N-Heterocycles: Pyrrolo/Indolo [1,2-a]quinoxalines and Quinazolin-4-ones. J. Org. Chem. 2021, 86, 16848–16857. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Z.; Li, D.; Gong, J.; Han, X.; Liu, X.; Ma, C. Dimethyl Sulfoxide Involved One-Pot Synthesis of Quinoxaline Derivatives. J. Org. Chem. 2017, 82, 3491–3499. [Google Scholar] [CrossRef]
- Liu, H.; Mai, X.; Xian, J.; Liu, S.; Zhang, X.; Li, B.; Chen, X.; Li, Y.; Xie, F. Construction of Spirocyclic Pyrrolo [1,2-a]quinoxalines via Palladium-Catalyzed Hydrogenative Coupling of Phenols and Nitroarenes. J. Org. Chem. 2022, 87, 16449–16457. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Ma, Q.; Yin, J.; Liang, C.; Tian, L.; Ma, Y. RhIII-Catalyzed formal [5 + 1] cyclization of 2-pyrrolyl/indolylanilines using vinylene carbonate as a C1 synthon. Org. Chem. Front. 2021, 8, 1764–1769. [Google Scholar] [CrossRef]
- Nan, J.; Chen, P.; Gong, X.; Hu, Y.; Ma, Q.; Wang, B.; Ma, Y. Metal-Free C-H [5 + 1] Carbonylation of 2-Alkenyl/Pyrrolylanilines Using Dioxazolones as Carbonylating Reagents. Org. Lett. 2021, 23, 3761–3766. [Google Scholar] [CrossRef]
- He, Z.; Bae, M.; Wu, J.; Jamison, T. Synthesis of Highly Functionalized Polycyclic Quinoxaline Derivatives Using Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2014, 53, 14451–14455. [Google Scholar] [CrossRef]
- Liu, S.; Liang, J.; Zhang, P.; Li, Z.; Jiao, L.Y.; Jia, W.; Ma, Y.; Szostak, M. Ruthenium-catalyzed divergent deaminative and denitrative C–N cleavages: Facile access to quinoxalines. Org. Chem. Front. 2023, 10, 22–29. [Google Scholar] [CrossRef]
- Liu, X.; Fu, H.; Jiang, Y.; Zhao, Y. A Simple and Efficient Approach to Quinazolinones under Mild Copper-Catalyzed Conditions. Angew. Chem. Int. Ed. 2009, 48, 348–351. [Google Scholar] [CrossRef]
- Huang, D.; Li, X.; Xu, F.; Li, L.; Lin, X. Highly Enantioselective Synthesis of Dihydroquinazolinones Catalyzed by SPINOL-Phosphoric Acids. ACS Catal. 2013, 3, 2244–2247. [Google Scholar] [CrossRef]
- Li, H.; He, L.; Neumann, H.; Beller, M.; Wu, X. Cascade synthesis of quinazolinones from 2-aminobenzonitriles and aryl bromides via palladium-catalyzed carbonylation reaction. Green Chem. 2014, 16, 1336–1343. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Chen, X.; Li, Q.; Zhou, Y.; Yin, S. Metal- and Oxidant-Free Synthesis of Quinazolinones from β-Ketoesters with o-Aminobenzamides via Phosphorous Acid Catalyzed Cyclocondensation and Selective C−C Bond Cleavage. J. Org. Chem. 2015, 80, 9392–9400. [Google Scholar] [CrossRef]
- Sharma, R.; Vishwakarma, R.A.; Bharate, S.B. Ligand-Free Copper-Manganese Spinel Oxide-Catalyzed Tandem One-Pot C-H Amidation and N-Arylation of Benzylamines: A Facile Access to 2-Arylquinazolin-4(3H)-ones. Adv. Synth. Catal. 2016, 358, 3027–3033. [Google Scholar] [CrossRef]
- An, J.; Wang, Y.; Zhang, Z.; Zhao, Z.; Zhang, J.; Wang, F. The Synthesis of Quinazolinones from Olefins, CO, and Amines over a Heterogeneous Ru-clusters/Ceria Catalyst. Angew. Chem. Int. Ed. 2018, 57, 12308–12312. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Lu, C.; Chen, Z.; Jin, S.; Xie, L.; Meng, Z.; Su, Z.; Zheng, K. Light-Driven Intramolecular C-N Cross-Coupling via a Long-Lived Photoactive Photoisomer Complex. Angew. Chem. Int. Ed. 2019, 58, 14666–14672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Guo, S.; Fan, J.; Fan, X. t-BuOK-Catalyzed Regio-and Stereoselective Intramolecular Hydroamination Reaction Leading to Phthalazinoquinazolinone Derivatives. J. Org. Chem. 2023, 88, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Fan, S.; Wu, X.; Fang, L.; Zhu, J. Cobalt Homeostatic Catalysis for Coupling of Enaminones and Oxadiazolones to Quinazolinones. J. Org. Chem. 2023, 88, 1945–1962. [Google Scholar] [CrossRef]
- Meinwald, J.; Labana, S.S.; Chadha, M.S. Peracid Reactions. III. The Oxidation of Bicyclo [2.2.1]heptadiene. J. Am. Chem. Soc. 1963, 85, 582–585. [Google Scholar] [CrossRef]
- Muller, C.; Horky, F.; Vayer, M.; Golushko, A.; Leboeuf, D.; Moran, J. Synthesis of functionalised isochromans: Epoxides as aldehyde surrogates in hexafluoroisopropanol. Chem. Sci. 2023, 14, 2983. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; He, J.; Dong, S.; Lin, L.; Feng, X. Asymmetric Catalytic Vinylogous Addition Reactions Initiated by Meinwald Rearrangement of Vinyl Epoxides. Angew. Chem. Int. Ed. 2021, 60, 14521–14527. [Google Scholar] [CrossRef]
- Mehedi, M.S.A.; Tepe, J.J. Sc(OTf)3-Mediated One-Pot Synthesis of 2,3-Disubstituted Quinolines from Anilines and Epoxides. J. Org. Chem. 2020, 85, 6741–6746. [Google Scholar] [CrossRef]
- Mehedi, M.S.A.; George, D.E.; Tepe, J.J. Sc(OTf)3-Mediated One-Pot Synthesisof 3,4-Disubstituted 1H-Pyrazolesand 3,5-Disubstituted Pyridinesfrom Hydrazine or Ammonia with Epoxides. J. Org. Chem. 2022, 87, 16820–16828. [Google Scholar] [CrossRef]
- Rao, C.J.; Sudheer, M.; Battula, V.R. Triflic-Acid-Catalyzed Tandem Epoxide Rearrangement and Annulation with Alkynes: An Efficient Approach for Regioselective Synthesis of Naphthalenes. ChemistrySelect 2022, 7, e20220047. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.T. Ytterbium(III) triflate catalyzed domino reaction of arylamines and styrene oxides: Synthesis of 2-benzyl-3-arylquinoline derivatives. Tetrahedron Lett. 2021, 70, 152981. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.T. An environmentally benign regioselective synthesis of 2-benzyl-4-arylquinoline derivatives using aryl amines, styrene oxides and aryl acetylenes. Org. Biomol. Chem. 2021, 19, 8772–8782. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, G. Synthesis and urease inhibition studies of some new quinazolinones. J. Heterocycl. Chem. 2021, 58, 1164–1170. [Google Scholar] [CrossRef]
- Purandare, A.V.; Gao, A.; Wan, H.; Somerville, J.; Burke, C.; Seachord, C.; Vaccaro, W.; Wityak, J.; Poss, M.A. Identification of chemokine receptor CCR4 antagonist. Bioorg. Med. Chem. Lett. 2005, 15, 2669–2672. [Google Scholar] [CrossRef]
- Yun, E.S.; Akhtar, M.S.; Mohandoss, S.; Lee, Y.R. Microwave-assisted annulation for the construction of pyrido-fused heterocycles and their application as photoluminescent chemosensors. Org. Biomol. Chem. 2022, 20, 3397–3407. [Google Scholar] [CrossRef]
- Bao, Y.; Yan, Y.; Xu, K.; Su, J.; Zha, Z.; Wang, Z. Copper-catalyzed radical methylation/C−H amination/oxidation cascade for the synthesis of quinazolinones. J. Org. Chem. 2015, 80, 4736–4742. [Google Scholar] [CrossRef]
- Dai, C.; Meschini, F.; Narayanam, J.M.R.; Stephenson, C.R.J. Friedel−Crafts Amidoalkylation via Thermolysis and Oxidative Photocatalysis. J. Org. Chem. 2012, 77, 4425–4431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).