Abstract
Urate transporter 1 (URAT1) is a clinically validated target for the treatment of hyperuricemia and gout. Due to the absence of protein structures, the molecular design of new URAT1 inhibitors generally resorts to ligand-based approaches. Two series of biphenyl carboxylic acids were designed based on the structures of URAT1 inhibitors Epaminurad and Telmisartan via a strategy of pharmacophore fusion. Fifty-one novel compounds were synthesized and most of them showed obvious inhibition against human URAT1. A1 and B21 were identified as the most potent URAT1 inhibitors in series A and B, respectively. They exhibited IC50 values of 0.93 μM and 0.17 μM, which were comparable or superior to the clinical uricosuric drug benzbromarone. The results confirmed the effectiveness of ligand-based approaches in identifying novel and potent URAT1 inhibitors.
1. Introduction
High serum uric acid concentration is an important risk factor for the development of gout. Chronically elevated uric acid levels, which is termed hyperuricemia, can lead to deposition of monosodium urate crystals in articular and non-articular structures, and hyperuricemia is the major cause of gout flares [1,2]. Besides anti-inflammatory treatment during gout attacks, effective control of the uric acid level is a key strategy for the prevention and treatment of gout. Urate lowering therapies (ULTs) aim to reverse hyperuricemia, which thereby dissolve monosodium urate crystals and prevent gout attacks in the long term.
Insufficient urate excretion is the primary reason for high serum urate (SU) levels, and urate excretion is predominantly regulated by urate transporters in the kidneys, specifically URAT1 (SLC22A12) [3,4,5,6], GLUT9 (SLC2A9) [5,7], and ABCG2 [8]. Therefore, uricosuric agents represented by URAT1 inhibitors are principal components of currently available small-molecule ULTs [9,10,11].
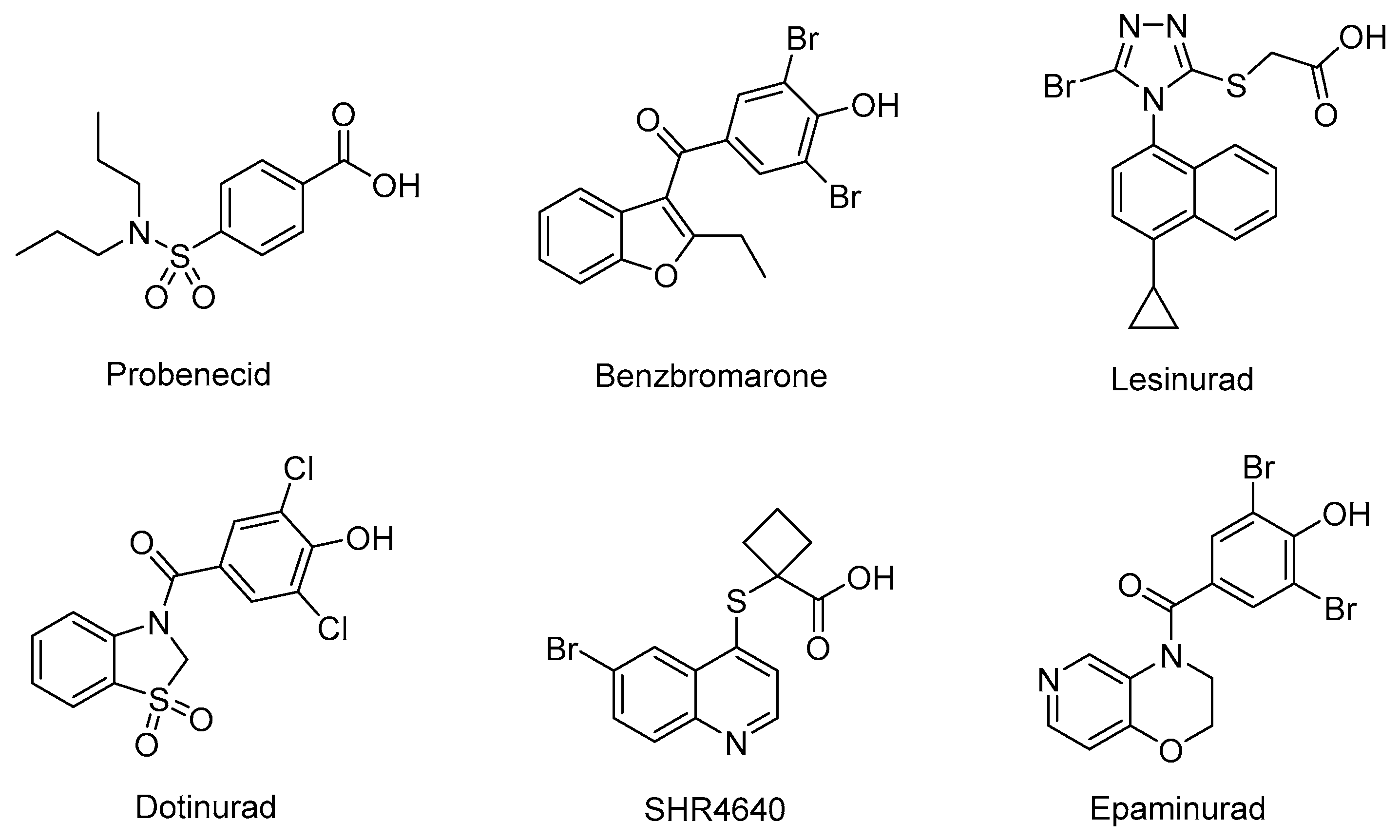
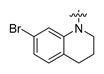
URAT1 inhibitors can inhibit urate reabsorption, promote urate excretion, and thus lower the serum urate level. Hence, URAT1 inhibitors have drawn extensive attention in recent years as an effective urate-lowering therapy [11,12]. Currently, four URAT1 inhibitors, namely Probenecid, Benzbromarone, Lesinurad, and Dotinurad (Figure 1), are used in clinic for the management of hyperuricemia. Probenecid exerts its effects by inhibiting both URAT1 and GLUT9. Despite its poor efficacy, Probenecid is still an option for patients who cannot tolerate allopurinol or fail to achieve target SU in monotherapy with xanthine oxidase (XO) inhibitors [9]. Benzbromarone is a highly active URAT1 inhibitor with an IC50 at submicromolar level. Although it is much more effective than Probenecid, occurrence of liver toxicity has been occasionally reported. Therefore, liver function monitoring is required when prescribing this drug [9,13,14]. Lesinurad is a relatively weak URAT1 inhibitor with an IC50 at micromolar level. Due to its poor efficacy, Lesinurad is usually used in clinic in combination with XO inhibitors to achieve reasonable urate lowering effects [15,16]. Dotinurad is a selective urate reabsorption inhibitor (SURI). It inhibits URAT1 selectively, yet only shows minimal effects on ABCG2, OAT1, and OAT3. Dotinurad was approved in Japan for the treatment of hyperuricemia and gout in 2020 [17,18,19]. The clinic application of the marketed URAT1 inhibitors is more or less limited due to poor efficacy and potential toxicity associated with these drugs. Thus, safe and effective management of hyperuricemia is still an unmet clinic need. Most URAT1 inhibitors currently in clinical investigations are structurally derived from the marketed drugs, which might inherently share similar pharmacological and safety profiles with the previous drugs. Therefore, it is imperative to discover new chemical prototypes of URAT1 inhibitors [20,21].
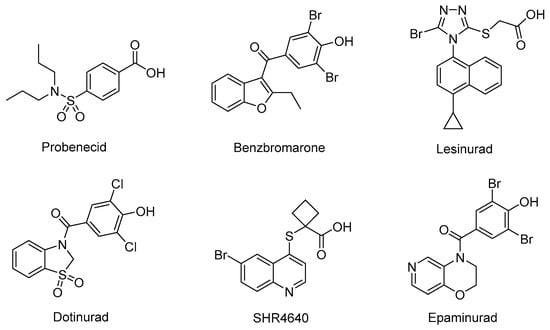
Figure 1.
The structures of representative URAT1 Inhibitors.
Due to the transmembrane nature of URAT1, its structure has not been resolved, and the design of new URAT1 inhibitors mainly relies on ligand-based approaches [22,23,24,25]. Among the diverse URAT1 inhibitors reported, SHR4640 and Epaminurad (Figure 1) are highlighted as representative inhibitors progressed to clinical investigations. SHR4640 is a potent URAT1 inhibitor exhibiting good pharmacokinetic properties [26]. In a Phase II clinical trial in China, SHR4640 exhibited obvious urate lowering effects and good tolerability with a regimen of 5 mg or 10 mg once daily by oral administration for five weeks. SHR4640 is currently undergoing a phase III clinical trial [27,28]. Epaminurad (UR-1102) is a highly selective URAT1 inhibitor derived from benzbromarone, yet it has selectivity and safety profiles superior to benzbromarone [13,29,30].
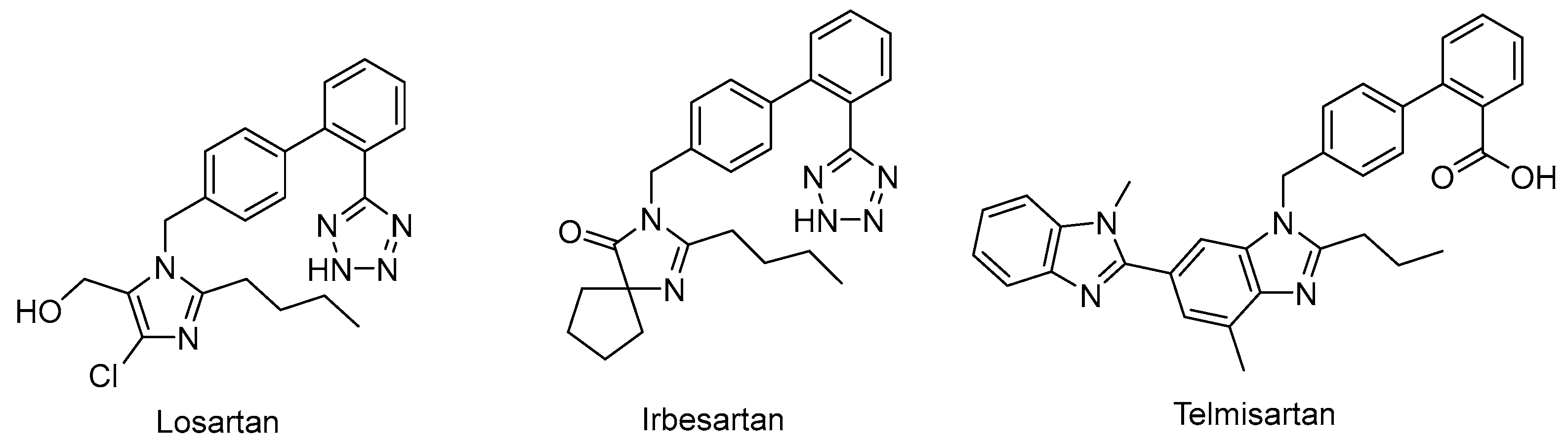
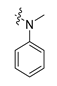
Drug repurposing is not only a promising field to identify new therapeutic uses for existing drugs, but also an effective approach to recognize potential leads in drug discovery [31]. For example, the angiotensin II–receptor blockers (ARBs) Losartan, Irbesartan, and Telmisartan (Figure 2) are therapeutic drugs to treat hypertension. However, urate-lowering effects were observed during their clinical application, and they were later recognized as weak URAT1 inhibitors [32], which provided a new chemical prototype for URAT1 inhibitors.
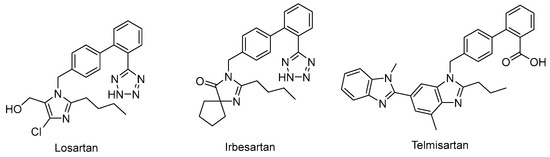
Figure 2.
Representative angiotensin II receptor blockers with URAT1 inhibitory activity.
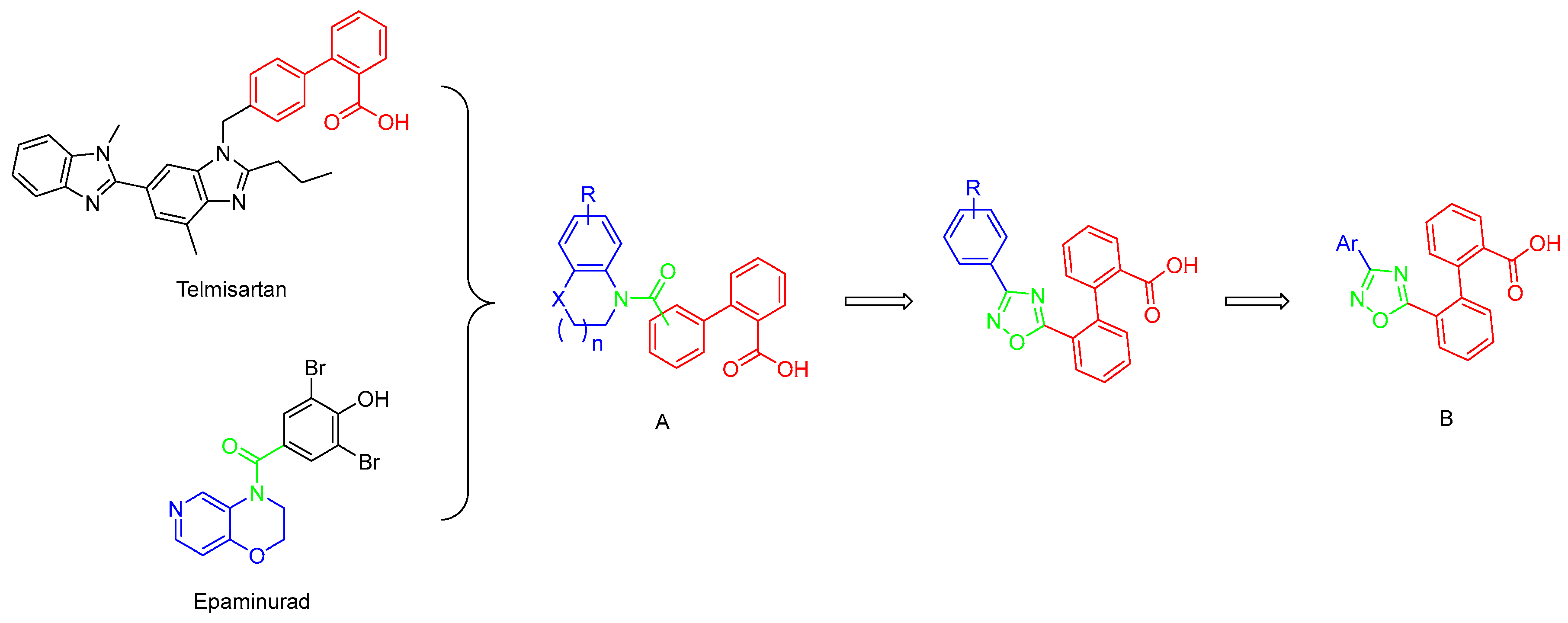
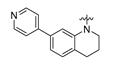
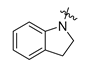
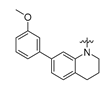
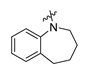
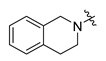
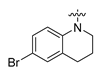
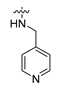
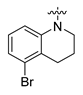
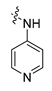
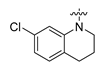
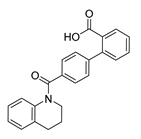
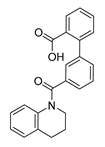
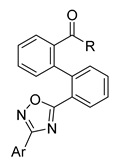
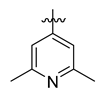
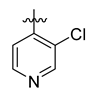
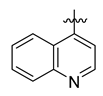
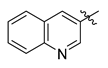
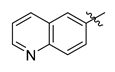
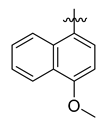
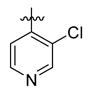
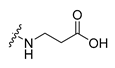
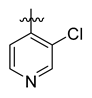
Careful examination of the structures of representative URAT1 inhibitors revealed the pharmacophore of URAT1 inhibitors, which includes anionic and hydrophobic components. To identify novel URAT1 inhibitors with better therapeutic profiles, we exploited a pharmacophore fusion strategy based on the structures of Telmisartan and Epaminurad (Figure 3). It was reported that the 3,5-dibromo-4-hydroxyphenyl fragment might be responsible for metabolic activation and resultant hepatic toxicity of Benzbromarone derivatives [14]. The biphenyl carboxylic acid fragment from Telmisartan was thus taken as the anionic component, while fragments similar to the 3,4-dihydro-2H-pyrido [4,3-b][1,4]oxazine part in Epaminurad were introduced as the hydrophobic component. The two parts are fused together through amide bonds to provide target compounds of series A (Figure 3), which were designed to reduce toxicity (compared to Epaminurad) and improve chemical accessibility (compared to Telmisartan) simultaneously. The amide bond in series A compounds was replaced with 1,2,4-oxadiazol via a bioisosteric strategy, and a variety of aromatic moieties were incorporated as the hydrophobic part to obtain target compounds of series B (Figure 3). These two series of compounds were both featured by the presence of the biphenyl carboxylic acid moiety, and we conducted preliminary structure–activity relationship (SAR) exploration on these compound classes to discover new chemical prototypes as potent URAT1 inhibitors.
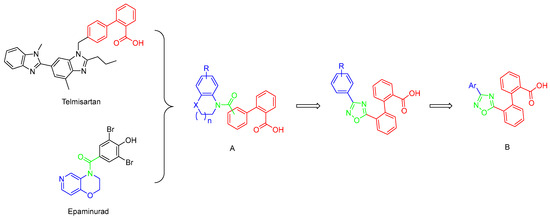
Figure 3.
Novel biphenyl carboxylic acid-based URAT1 inhibitors designed by pharmacophore fusion. The anionic (presented in red) and hydrophobic (presented in blue) components were derived from Telmisartan and Epaminurad, respectively. The linker between the two parts was presented in green. A and B represent the general formula for compounds in Series A and B, respectively.
2. Results and Discussion
2.1. Chemistry
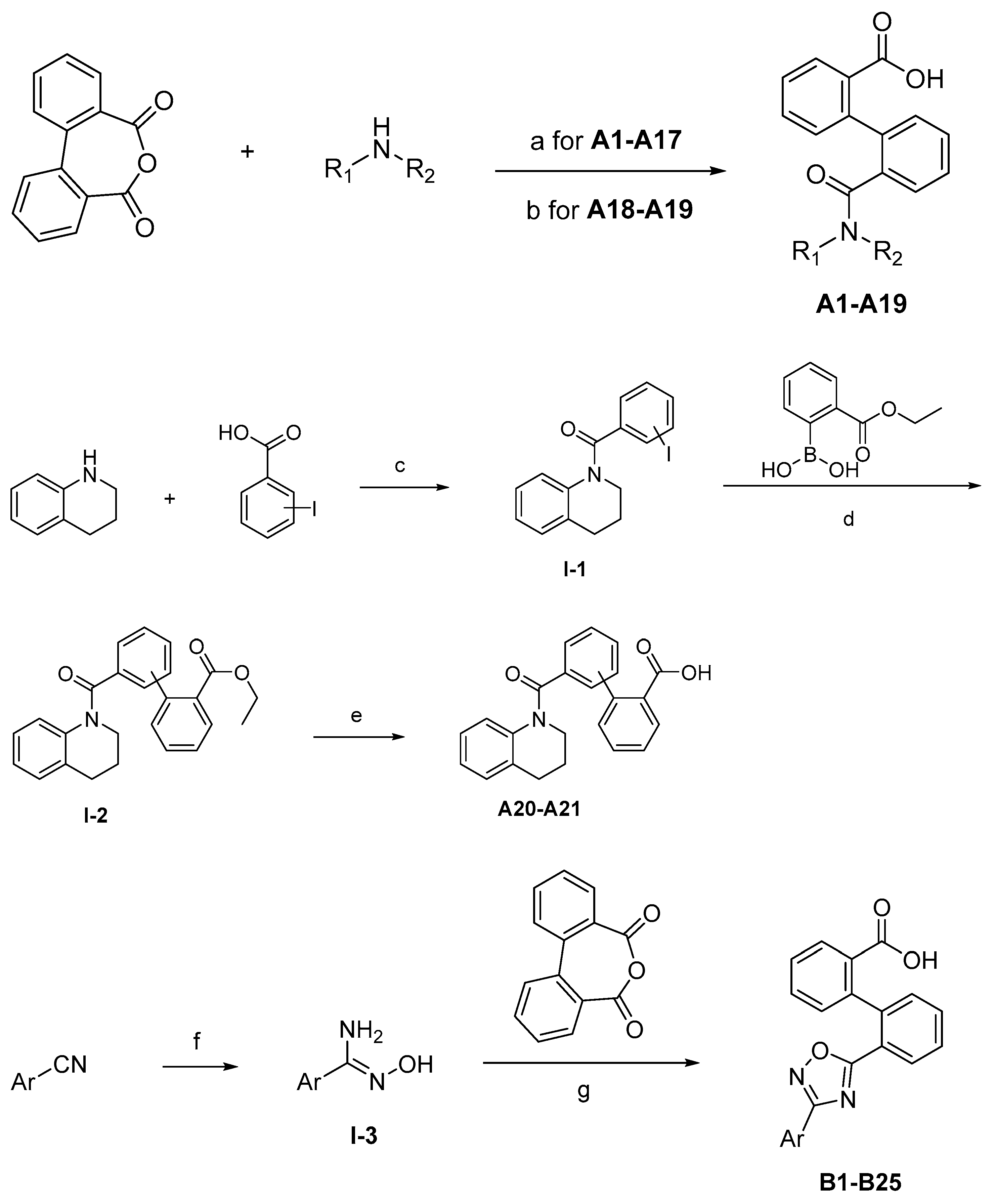
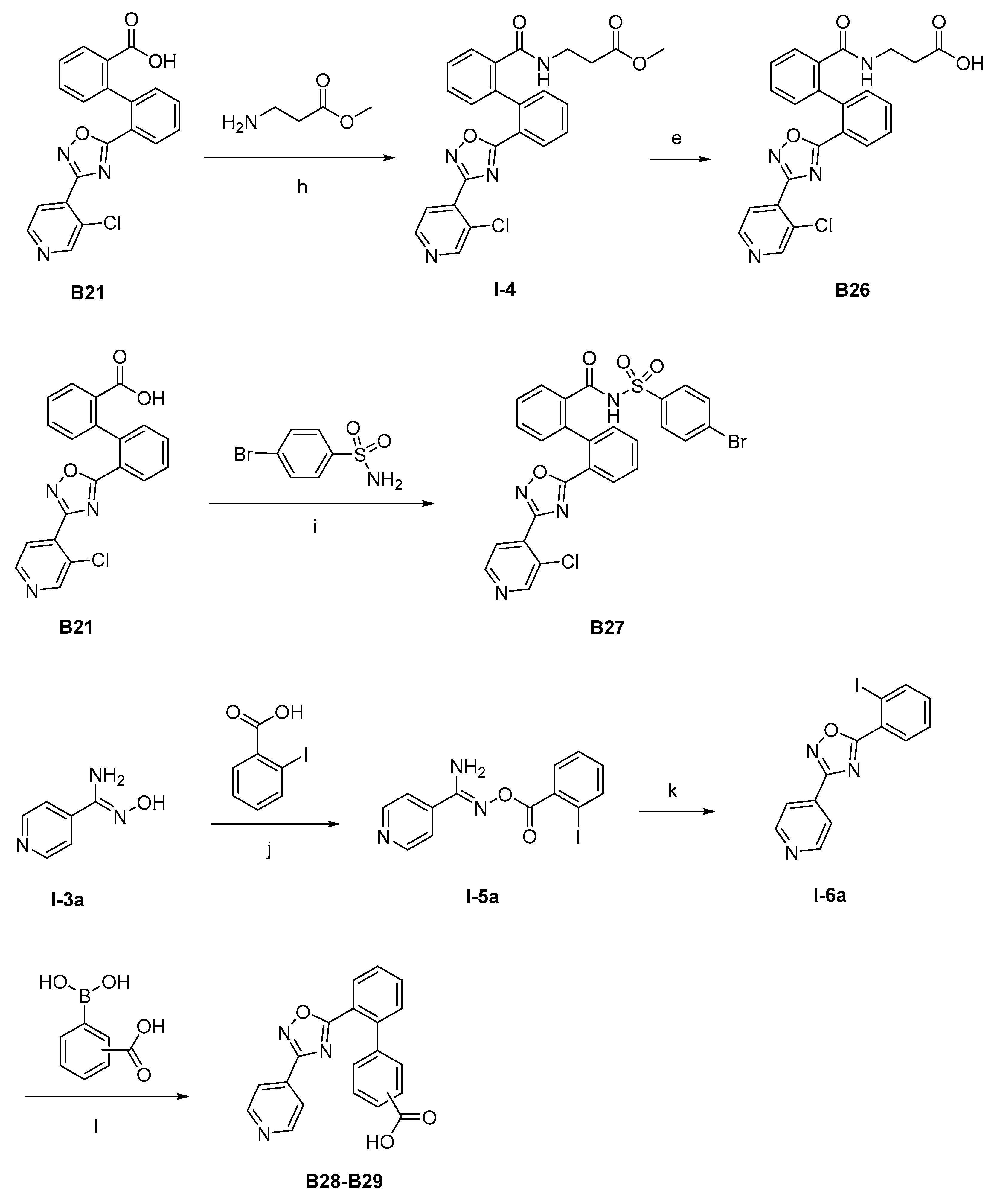
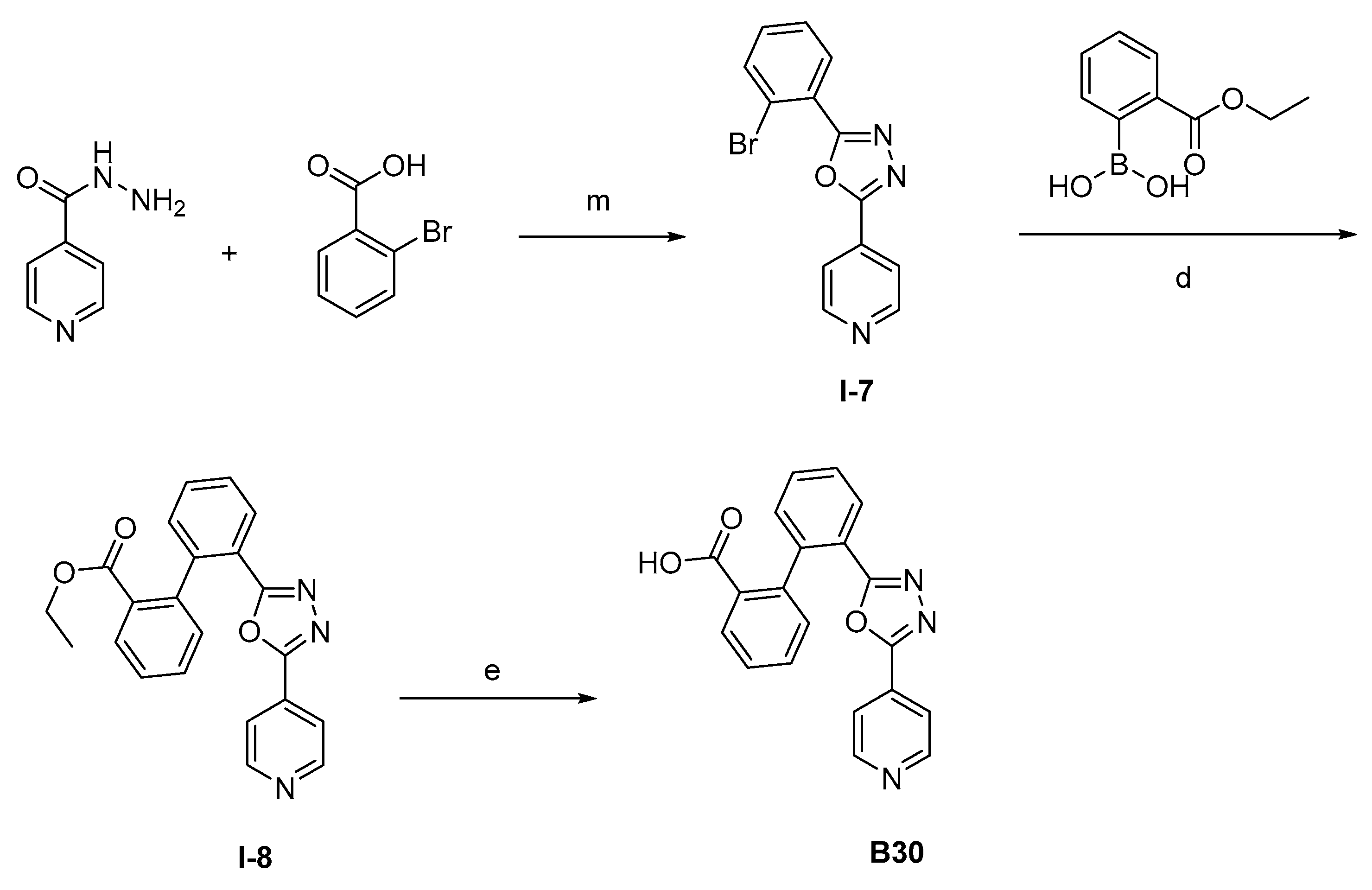
The synthesis of the target compounds were demonstrated in Scheme 1. Briefly, diphenic anhydride underwent amide condensation with substituted amines to form compounds A1–A19. Intermediate I-1 was obtained through condensation of iodine substituted benzoic acid with 1,2,3,4-tetrahydroquinoline. I-1 underwent a coupling reaction with carboxylic ester substituted phenylboronic acid to give intermediate I-2, which is then hydrolyzed to provide compounds A20–A21. Cyanides reacted with hydroxylamine hydrochloride to afford intermediate I-3, which reacted with diphenic anhydride to furnish the oxadiazole ring and provide compounds B1–B25. Compound B21 was subjected to successive amidation and hydrolysis to obtain compound B26. Compound B27 was also obtained from B21 by reacting with 4-bromobenzenesulfonamide. Intermediate I-3a condensed with iodine substituted benzoic acid to get intermediate I-5a, which was then cyclized to give the oxadiazole intermediate I-6a. I-6a underwent Suzuki reaction with substituted phenylboronic acid to produce compounds B28 and B29. Intermediate I-7 furnished the 1,3,4-oxadiazole ring through the hydrazine and carboxylic acid condensation. I-7 then underwent Suzuki reaction with substituted phenylboronic acid to form intermediate I-8, and subsequently hydrolyzed to obtain compound B30.
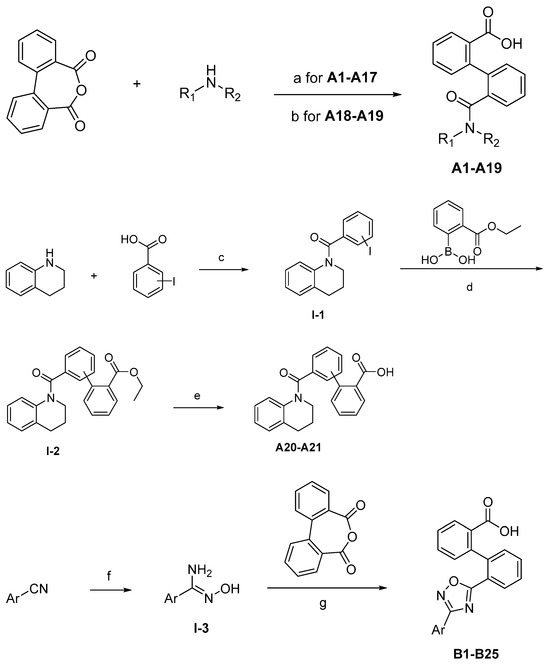
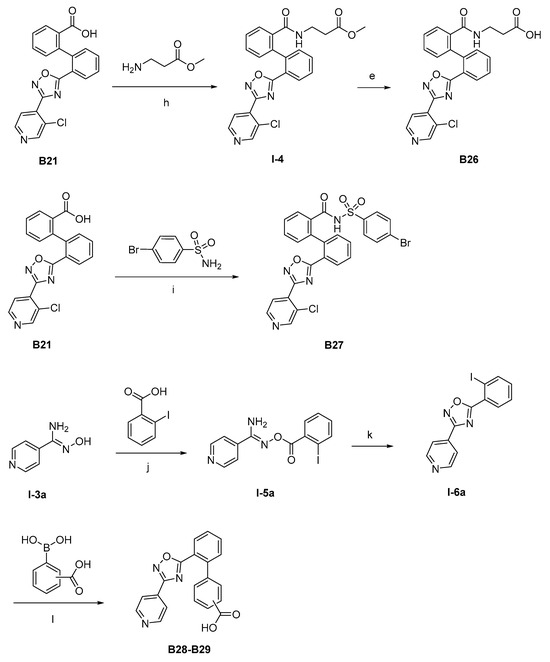
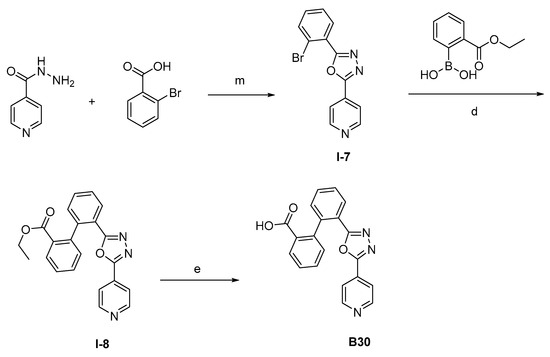
Scheme 1.
Synthetic routes of target compounds A1–A21, B1–B30. Reagents and conditions: (a) Toluene, 120 °C; (b) Triethylamine, DMAP, CH2Cl2, r.t., then 1N aq. HCl; (c) HATU, Triethylamine, Acetonitrile, r.t.; (d) K2CO3, Pd(PPh3)4, Toluene:95%Ethanol (1:1), 110 °C, N2; (e) LiOH.H2O, Ethanol:H2O (4:1), r.t., then 1N aq. HCl; (f) NH2OH.HCl, Na2CO3, Ethanol:H2O (4:1), 60 °C; (g) DMSO, r.t., then NaOH, r.t., then 1N aq. HCl; (h) Triethylamine, HATU, DMF, 60 °C; (i) EDCI, DMAP, CH2Cl2, r.t.; (j) Triethylamine, HATU, CH2Cl2, r.t.; (k) Cs2CO3, DMSO, r.t.; (l) K2CO3, Pd(PPh3)4, Toluene:95%Ethanol (1:1), 110 °C, N2, then 1N aq. HCl; (m) POCl3, 110 °C. DMAP: 4-dimethylaminopyridine; HATU: 2-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate; EDCI: 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride.
2.2. In Vitro Evaluation and Structure–Activity Relationships
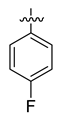
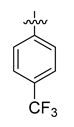
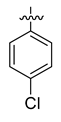
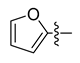
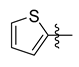
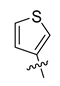
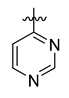
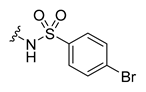
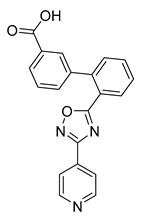
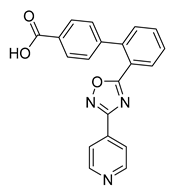
All the target compounds were evaluated for their URAT1 inhibitory activities in HEK293-URAT1 cells using 14C-labeled uric acid as the substrate. The activity data are listed in Table 1 and Table 2. Currently available data revealed some SAR clues. The SAR exploration on series A compounds mainly focused on the hydrophobic component. Regioisomers A1, A20 and A21 were first investigated to determine the regioselectivity for URAT1 inhibition, and the ortho-substituted pattern (A1) emerged to be optimal. With the ortho-substituted biphenyl carboxylic acid fixed, a variety of hydrophobic components were introduced as R. This molecular area seemed sensitive to steric hindrance. Moieties with sizes similar to tetrahydroquinoline in A1 are prone to maintain certain URAT1 inhibitory activities (A2–A11), though the activity was more or less decreased. However, moieties with sizes much larger (A12, A14) or smaller (A16–A19) than tetrahydroquinoline generally led to significantly decreased inhibitory activity. A13 and A15 were exceptions. The introduction of an electron-deficient pyridyl retained the URAT1 inhibitory activity (A13), while tetrahydroisoquinoline as R caused significant loss of activity (A15). These results implied the involvement of factors other than steric hindrance in affecting the inhibitory activity. For series B compounds, the ortho-substituted biphenyl carboxylic acid was initially kept, and various aromatic components were incorporated (B1–B25). Substituted phenyl (B1–B7), furanyl (B8), thienyl (B9 and B10), pyrimidyl (B11), and pyridyl (B12–B14) were initially attempted, and only compound B14 with 4-pyridyl showed significant inhibitory activity. Different substituents were introduced onto the 4-pyridyl (B15–B21). Except dimethyl substitution (B20), all the other substitution patterns led to increased inhibitory activities. In contrast, quinolyl (B22–B24) or methoxyl substituted naphthyl (B25) resulted in decreased URAT1 inhibition. Extending the carboxyl acid with β-alanine (B26) caused dramatic loss of activity, yet amidation of the carboxyl acid with 4-bromobenzenesulfonamide (B27) retained considerable inhibition. Regioisomers B28–B30 were also explored, and ortho-substituted biphenyl carboxylic acid and 1,2,4-oxadiazole were preferred.

Table 1.
Structures and URAT1 inhibitory activities of compounds A1–A21.

Table 2.
Structures and URAT1 inhibitory activities of compounds B1–B30.
3. Materials and Methods
3.1. Chemistry
High-resolution mass spectra (HR-MS) were measured with an Agilent 6530 Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) LC/MS system equipped with electrospray ionization (ESI). 1H NMR and 13C NMR spectra were recorded on a Quantum-I 400 or AVANCE NEO 700 spectrometer with TMS as the internal standard. All reagents and solvents were commercially obtained from local suppliers and were used directly without further purification.
General preparation of compounds A1–A17 .The diphenic anhydride (1.0 eq) and substituted amines (1.0 eq) were mixed in 20 mL toluene. The mixture was stirred at 110 °C for 12 h, and the solvent was evaporated in a vacuum. The residue was purified by column chromatography to give A1–A17.
Compound A1: Yield 61%; white solid; mp: 147–149 °C; 1H NMR (400 MHz, DMSO-d6) δ7.84–7.77 (m, 1H), 7.50–7.37 (m, 5H), 7.21–7.15 (m, 1H), 7.14–7.09 (m, 1H), 7.03–6.96 (m, 2H), 6.92 (ddd, J = 14.6, 7.1, 1.8 Hz, 2H), 3.45 (t, J = 6.0 Hz, 2H), 2.43 (t, J = 6.9 Hz, 2H), 1.59 (p, J = 6.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6) δ168.07, 167.40, 139.05, 137.64, 137.03, 135.38, 130.89, 130.08, 129.13, 128.06, 126.97, 126.73, 124.36, 123.47, 25.22, 22.05; HR-ESI-MS: m/z = 358.1455 [M + H]+, calculated for C23H20NO3: 358.1438.
Compound A2: Yield 63%; white solid; mp: 174–176 °C; 1H NMR (400 MHz, DMSO-d6) δ12.36 (s, 1H), 7.87–7.74 (m, 1H), 7.55–7.37 (m, 6H), 7.24 (dd, J = 6.3, 2.3 Hz, 1H), 7.08 (s, 1H), 6.95 (td, J = 7.8, 1.6 Hz, 1H), 6.81–6.62 (m, 2H), 3.83 (s, 2H), 3.70–3.42 (m, 2H); HR-ESI-MS: m/z = 360.1252 [M + H]+, calculated for C22H18NO4: 360.1230.
Compound A3: Yield 51%; white solid; mp: 193–195 °C; 1H NMR (400 MHz, DMSO-d6) δ12.22 (s, 1H), 8.01–7.78 (m, 1H), 7.55–7.36 (m, 5H), 7.20 (d, J = 7.3 Hz, 1H), 7.09–7.02 (m, 2H), 7.00–6.94 (m, 1H), 6.92–6.77 (m, 2H), 3.71 (s, 2H), 2.93 (t, J = 5.5 Hz, 2H); HR-ESI-MS: m/z = 376.1001 [M + H]+, calculated for C22H18NO3S: 376.1002.
Compound A4: Yield 66%; whsite solid; mp: 195–196 °C; 1H NMR (400 MHz, DMSO-d6) δ12.72 (s, 1H), 7.93 (d, J = 8.0 Hz, 1H), 7.77 (dd, J = 7.7, 1.4 Hz, 1H), 7.57–7.43 (m, 4H), 7.37 (dt, J = 8.9, 7.2 Hz, 2H), 7.28 (d, J = 6.9 Hz, 1H), 7.21–7.08 (m, 2H), 6.99 (d, J = 7.7 Hz, 1H), 3.79 (s, 2H), 2.87 (s, 2H); HR-ESI-MS: m/z = 344.1277 [M + H]+, calculated for C22H18NO3: 344.1281.
Compound A5: Yield 64%; white solid; mp: 215–217 °C; 1H NMR (400 MHz, DMSO-d6) δ7.86 (d, J = 7.6 Hz, 1H), 7.63–6.93 (m, 10H), 6.75–6.59 (m, 1H), 3.25 (s, 2H), 2.56 (s, 1H), 1.57 (m, 4H); HR-ESI-MS: m/z = 372.1594 [M + H]+, calculated for C24H22NO3: 372.1594.
Compound A6: Yield 66%; white solid; mp: 231–233 °C; 1H NMR (400 MHz, DMSO-d6) δ12.29 (s, 1H), 7.86–7.77 (m, 1H), 7.52–7.36 (m, 6H), 7.25–7.20 (m, 1H), 7.12 (dd, J = 8.2, 2.0 Hz, 1H), 7.05–6.99 (m, 1H), 6.96 (d, J = 8.2 Hz, 1H), 3.43 (t, J = 6.0 Hz, 2H), 2.39 (d, J = 6.9 Hz, 2H), 1.56 (p, J = 6.5 Hz, 2H); HR-ESI-MS: m/z = 436.0553 [M + H]+, calculated for C23H19BrNO3: 436.0543.
Compound A7: Yield 65%; white solid; mp: 261–262 °C; 1H NMR (400 MHz, DMSO-d6) δ12.27 (s, 1H), 7.81 (dd, J = 6.7, 2.3 Hz, 1H), 7.51–7.37 (m, 5H), 7.20 (q, J = 2.8 Hz, 2H), 7.13–7.01 (m, 3H), 3.43 (t, J = 6.0 Hz, 2H), 2.44 (d, J = 6.9 Hz, 2H), 1.58 (p, J = 6.5 Hz, 2H); HR-ESI-MS: m/z = 436.0544 [M + H]+, calculated for C23H19BrNO3: 436.0543.
Compound A8: Yield 61%; white solid; mp: 201–202 °C; 1H NMR (400 MHz, DMSO-d6) δ12.26 (s, 1H), 7.82 (dt, J = 6.7, 2.1 Hz, 1H), 7.52–7.37 (m, 5H), 7.27 (d, J = 7.9 Hz, 1H), 7.17 (dt, J = 6.5, 2.0 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.88 (tt, J = 9.9, 8.1, 3.5 Hz, 2H), 3.45 (t, J = 5.8 Hz, 2H), 2.40 (t, J = 7.2 Hz, 2H), 1.59 (t, J = 6.5 Hz, 2H); HR-ESI-MS: m/z = 436.0552 [M + H]+, calculated for C23H19BrNO3: 436.0543.
Compound A9: Yield 65%; white solid; mp: 233–234 °C; 1H NMR (400 MHz, DMSO-d6) δ12.27 (s, 1H), 7.83 (dt, J = 7.4, 1.7 Hz, 1H), 7.53–7.38 (m, 5H), 7.31–7.19 (m, 2H), 7.08–6.93 (m, 3H), 3.43 (t, J = 6.1 Hz, 2H), 2.43 (t, J = 6.9 Hz, 2H), 1.57 (t, J = 6.4 Hz, 2H); HR-ESI-MS: m/z = 392.1064 [M + H]+, calculated for C23H19ClNO3: 392.1048.
Compound A10: Yield 65%; white solid; mp: 198–200 °C; 1H NMR (400 MHz, DMSO-d6) δ12.27 (s, 1H), 7.87–7.74 (m, 1H), 7.48–7.35 (m, 5H), 7.18 (dq, J = 4.2, 2.4 Hz, 1H), 7.05 (s, 2H), 6.58 (d, J = 2.9 Hz, 1H), 6.52 (dd, J = 8.9, 2.9 Hz, 1H), 3.69 (s, 3H), 3.41 (t, J = 6.1 Hz, 2H), 2.41 (d, J = 8.5 Hz, 2H), 1.57 (p, J = 6.4 Hz, 2H); HR-ESI-MS: m/z = 388.1536 [M + H]+, calculated for C24H22NO4: 388.1543.
Compound A11: Yield 67%; white solid; mp: 220–222 °C; 1H NMR (400 MHz, DMSO-d6) δ7.89–7.72 (m, 1H), 7.41 (dq, J = 5.3, 2.5, 1.9 Hz, 5H), 7.26–7.15 (m, 1H), 7.01 (s, 2H), 6.81 (d, J = 2.0 Hz, 1H), 6.73 (dd, J = 8.3, 2.1 Hz, 1H), 3.42 (t, J = 6.1 Hz, 2H), 2.40 (t, J = 6.9 Hz, 2H), 2.19 (s, 3H), 1.57 (p, J = 6.5 Hz, 2H); HR-ESI-MS: m/z = 372.1588 [M + H]+, calculated for C24H22NO3: 372.1594.
Compound A12: Yield 55%; white solid; mp: 245–246 °C; 1H NMR (400 MHz, DMSO-d6) δ12.07 (s, 1H), 7.82 (dd, J = 7.0, 2.2 Hz, 1H), 7.59–7.12 (m, 12H), 7.05 (d, J = 7.9 Hz, 1H), 6.85 (d, J = 6.9 Hz, 1H), 3.50 (t, J = 6.0 Hz, 2H), 2.40 (d, J = 6.8 Hz, 2H), 1.59 (p, J = 6.4 Hz, 2H); HR-ESI-MS: m/z = 452.1649 [M + H]+, calculated for C29H23FNO3: 452.1656.
Compound A13: Yield 55%; white solid; mp: 266–268 °C; 1H NMR (400 MHz, DMSO-d6) δ12.22 (s, 1H), 8.62–8.40 (m, 2H), 7.96–7.76 (m, 1H), 7.58 (dd, J = 7.0, 2.0 Hz, 1H), 7.49 (dtd, J = 14.1, 7.4, 1.7 Hz, 2H), 7.44–7.31 (m, 4H), 7.28 (d, J = 5.1 Hz, 2H), 7.19 (dd, J = 7.0, 1.9 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 6.85 (s, 1H), 3.49 (d, J = 6.1 Hz, 2H), 2.40 (s, 2H), 1.58 (p, J = 6.4 Hz, 2H); HR-ESI-MS: m/z = 435.1696 [M + H]+, calculated for C28H23N2O3: 435.1703.
Compound A14: Yield 54%; white solid; mp: 177–179 °C; 1H NMR (400 MHz, DMSO-d6) δ12.19 (s, 1H), 7.81 (dd, J = 7.4, 1.7 Hz, 1H), 7.60–7.53 (m, 1H), 7.51–7.44 (m, 2H), 7.43–7.34 (m, 2H), 7.28 (t, J = 7.9 Hz, 1H), 7.20 (ddd, J = 13.1, 7.5, 2.2 Hz, 3H), 7.04 (d, J = 7.9 Hz, 1H), 6.93–6.75 (m, 4H), 3.80 (s, 3H), 3.50 (s, 2H), 2.38 (s, 2H), 1.58 (t, J = 6.4 Hz, 2H); HR-ESI-MS: m/z = 464.1849 [M + H]+, calculated for C30H26NO4: 464.1856.
Compound A15: Yield 62%; white solid; mp: 171–173 °C; 1H NMR (400 MHz, DMSO-d6) δ12.74 (s, 1H), 7.76 (dd, J = 7.7, 1.5 Hz, 1H), 7.50–6.48 (m, 11H), 4.29 (s, 2H), 3.33 (s, 2H), 2.73–2.17 (m, 2H); HR-ESI-MS: m/z = 358.1448 [M + H]+, calculated for C23H20NO3: 358.1438.
Compound A16: Yield 53%; white solid; mp: 169–172 °C; 1H NMR (400 MHz, DMSO-d6) δ12.64 (s, 1H), 7.85 (d, J = 7.7 Hz, 1H), 7.69–6.66 (m, 12H), 3.04 (s, 3H); HR-ESI-MS: m/z = 332.1284 [M + H]+, calculated for C21H18NO3: 332.1281.
Compound A17: Yield 58%; white solid; mp: 118–120 °C; 1H NMR (400 MHz, DMSO-d6) δ12.74 (s, 1H), 8.42 (t, J = 6.1 Hz, 1H), 8.39–8.31 (m, 2H), 7.81 (dd, J = 7.5, 1.7 Hz, 1H), 7.59–7.41 (m, 5H), 7.16 (td, J = 6.4, 2.4 Hz, 2H), 6.90–6.83 (m, 2H), 4.25 (d, J = 5.9 Hz, 2H); HR-ESI-MS: m/z = 333.1246 [M + H]+, calculated for C20H17N2O3: 333.1234.
General preparation of compounds A18 and A19 . The diphenic anhydride (1.0 eq), aromatic amines (1.0 eq), triethylamine (3.0 eq), and 4-dimethylaminopyridine (0.2 eq) were mixed in 20 mL dichloromethane. The mixture was stirred at room temperature for 10 h, and the solvent was evaporated in a vacuum. The reaction mixture was poured into water (50 mL), 1 M HCl solution was added dropwise to separated water phase to adjust the pH to 6 and the aqueous residue was extracted with dichloromethane. The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to A18 and A19.
Compound A18: Yield 55%; white solid; mp: 190–191 °C; 1H NMR (400 MHz, DMSO-d6) δ12.63 (s, 1H), 8.33–8.25 (m, 2H), 7.68–7.56 (m, 2H), 7.52–7.43 (m, 2H), 7.39–7.35 (m, 2H), 7.27 (tt, J = 7.4, 5.7 Hz, 2H), 7.16–7.05 (m, 1H), 6.99–6.91 (m, 1H); HR-ESI-MS: m/z = 319.1092 [M + H]+, calculated for C19H14N2O3: 319.1077.
Compound A19: Yield 53%; white solid; mp: 176–178 °C; 1H NMR (400 MHz, DMSO-d6) δ8.68 (d, J = 5.0 Hz, 1H), 7.88 (dd, J = 12.7, 8.5 Hz, 2H), 7.82 (d, J = 5.0 Hz, 1H), 7.70–7.61 (m, 2H), 7.52–7.45 (m, 3H), 7.39 (t, J = 7.7 Hz, 1H), 7.24 (dd, J = 6.2, 2.8 Hz, 2H), 7.10 (dd, J = 5.9, 3.0 Hz, 1H), 7.05 (d, J = 4.0 Hz, 1H); HR-ESI-MS: m/z = 369.1253 [M + H]+, calculated for C23H17N2O3: 369.1234.
General preparation of intermediate I-1. Iodine substituted benzoic acid (1.2 eq) and 1,2,3,4-tetrahydroquinoline (1.0 eq) were dissolved in 20 mL acetonitrile, then HATU (1.5 eq) and triethylamine (2.0 eq) were added. The mixture was stirred at room temperature for 10 h, and the solvent was evaporated in a vacuum. The residue was purified by column chromatography to give intermediate I-1.
General preparation of intermediate I-2. The intermediate I-1 (1.0 eq), substituted phenylboronic acids (1.0 eq), potassium carbonate (3.0 eq), and tetrakis(triphenylphosphine)palladium (0.2 eq) were added into 20 mL of a mixed solvent of toluene: 95%ethanol (1:1). The mixture was stirred at 110 °C under N2 for 12 h. The solvent was then evaporated in a vacuum and the residue was purified by column chromatography to get intermediate I-2.
General preparation of compounds A20 and A21. The intermediate I-2 (1.0 eq) and lithium hydroxide monohydrate (2.0 eq) were dissolved in 20 mL of a mixed solvent of ethanol: H2O (4:1). The mixture was stirred at room temperature for 8 h, and the solvent was evaporated in a vacuum. The reaction mixture was poured into water (50 mL). 1 M HCl solution was added dropwise to adjust the pH to 2. The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to A20 and A21.
Compound A20: Yield 59%; white solid; mp: 165–167 °C; 1H NMR (400 MHz, DMSO-d6) δ7.74 (dd, J = 7.7, 1.4 Hz, 1H), 7.66–7.53 (m, 2H), 7.47 (td, J = 7.5, 1.3 Hz, 1H), 7.42–7.34 (m, 3H), 7.33–7.28 (m, 2H), 7.23–7.15 (m, 1H), 7.01 (td, J = 7.3, 1.6 Hz, 1H), 6.93 (dd, J = 14.9, 7.8 Hz, 2H), 3.77 (t, J = 6.6 Hz, 2H), 2.83 (t, J = 6.6 Hz, 2H), 1.96 (p, J = 6.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6) δ168.71, 168.60, 142.00, 139.68, 138.20, 134.56, 130.49, 130.35, 129.79, 128.67, 128.12, 127.94, 127.49(2C), 127.27(2C), 127.04, 124.83, 124.32, 123.68, 44.09, 25.62, 22.96; HR-ESI-MS: m/z = 358.1445 [M + H]+, calculated for C23H20NO3: 358.1438.
Compound A21: Yield 62%; white solid; mp: 100–102 °C; 1H NMR (400 MHz, DMSO-d6) δ12.85 (s, 1H), 7.74 (dd, J = 7.7, 1.4 Hz, 1H), 7.53 (td, J = 7.5, 1.5 Hz, 1H), 7.45 (td, J = 7.6, 1.3 Hz, 1H), 7.39–7.34 (m, 2H), 7.33–7.25 (m, 2H), 7.18 (ddd, J = 9.5, 7.5, 1.5 Hz, 2H), 7.02 (td, J = 7.4, 1.4 Hz, 1H), 6.94 (td, J = 7.7, 7.2, 1.6 Hz, 1H), 6.86 (d, J = 8.2 Hz, 1H), 3.77 (t, J = 6.6 Hz, 2H), 2.81 (t, J = 6.6 Hz, 2H), 1.95 (p, J = 6.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6) δ168.78, 168.51, 140.16, 139.60, 138.24, 135.55, 130.69, 130.24, 129.71, 129.29, 128.67, 127.87, 127.36 (d, J = 3.3 Hz), 126.96, 126.17, 124.84, 124.50, 123.68, 43.96, 25.62, 22.99; HR-ESI-MS: m/z = 358.1443 [M + H]+, calculated for C23H20NO3: 358.1438
General preparation of intermediate I-3. The aromatic cyanides (1.0 eq), hydroxylamine hydrochloride (1.5 eq), and sodium carbonate (2.0 eq) were added into 20 mL of a mixed solvent of ethanol: H2O (4:1). The mixture was stirred at 60 °C for 6 h. The solvent was then evaporated in a vacuum and the residue was purified by column chromatography to provide intermediate I-3.
General preparation of compounds B1–B25. The diphenic anhydride (1.0 eq) and intermediate I-3 (1.0 eq) were mixed in 20 mL dimethyl sulfoxide. The mixture was stirred at room temperature for 2 h, then sodium hydroxide (2.0 eq) was added to the reaction solution, the mixture was stirred at room temperature for 1 h. The reaction mixture was poured into water (50 mL), 1 M HCl solution was added dropwise to separated water phase to adjust the pH to 5, and the solid precipitates were filtered. The residue was purified by column chromatography to afford B1–B25.
Compound B1: Yield 55%; white solid; mp: 150–153 °C; 1H NMR (400 MHz, DMSO-d6) δ12.54 (s, 1H), 8.18 (d, J = 7.8 Hz, 1H), 7.96 (d, J = 7.7 Hz, 1H), 7.94–7.86 (m, 2H), 7.76–7.67 (m, 1H), 7.68–7.59 (m, 2H), 7.55 (t, J = 7.6 Hz, 1H), 7.38 (t, J = 8.9 Hz, 3H), 7.28 (d, J = 7.5 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 175.95, 167.61, 166.67, 163.92 (d, J = 249.1 Hz), 142.53, 141.07, 132.28, 131.58, 130.77 (2C), 130.71, 129.69 (2C), 129.44, 129.35, 129.25, 127.85 (d, J = 13.9 Hz, 2C), 122.66 (d, J = 2.4 Hz), 122.33, 116.39 (d, J = 22.2 Hz, 2C); HR-MS(ESI) m/z = 361.0983 [M + H]+, calculated for C21H14FN2O3: 361.0983.
Compound B2: Yield 59%; white solid; mp: 173–175 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 8.20 (d, J = 7.3 Hz, 1H), 8.06 (d, J = 8.1 Hz, 2H), 7.97 (d, J = 7.5 Hz, 1H), 7.90 (d, J = 8.3 Hz, 2H), 7.72 (t, J = 7.5 Hz, 1H), 7.63 (t, J = 7.5 Hz, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.40 (d, J = 7.4 Hz, 1H), 7.29 (d, J = 7.1 Hz, 1H); 13C NMR (101MHz, DMSO-d6) δ 176.37, 167.64, 166.54, 142.59, 141.00, 132.43, 131.61, 131.39 (d, J = 32.1 Hz), 130.77, 130.75, 129.94, 129.73, 129.34, 127.90 (d, J = 15.6 Hz, 2C), 127.72 (3C), 126.21 (d, J = 3.5 Hz, 2C), 123.80 (d, J = 272.6 Hz), 122.22; HR-ESI-MS: m/z = 411.0946 [M + H]+, calculated for C22H14F3N2O3: 411.0951.
Compound B3: Yield 59%; white solid; mp: 154–156 °C; 1H NMR (400 MHz, DMSO-d6) δ12.55 (s, 1H), 8.18 (d, J = 7.8 Hz, 1H), 7.96 (d, J = 7.7 Hz, 1H), 7.86 (d, J = 8.7 Hz, 2H), 7.72 (t, J = 7.3 Hz, 1H), 7.66–7.59 (m, 4H), 7.55 (t, J = 7.4 Hz, 1H), 7.39 (d, J = 7.6 Hz, 1H), 7.28 (d, J = 7.5 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 176.09, 167.61, 166.68, 142.53, 141.03, 136.30, 132.32, 131.59, 130.77, 130.72 (2C), 129.71, 129.39 (2C), 129.29, 128.65 (2C), 127.94, 127.79, 124.94, 122.30; HR-ESI-MS: m/z = 377.0684 [M + H]+, calculated for C21H14ClN2O3: 377.0687.
Compound B4: Yield 51%; white solid; mp: 148–150 °C; 1H NMR (400 MHz, DMSO-d6) δ12.54 (s, 1H), 8.18 (d, J = 7.7 Hz, 1H), 7.95 (d, J = 7.6 Hz, 1H), 7.79 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H), 7.70 (d, J = 7.5 Hz, 1H), 7.62 (t, J = 7.2 Hz, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.39 (d, J = 7.5 Hz, 1H), 7.28 (d, J = 7.4 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ176.10, 167.61, 166.79, 142.53, 141.02, 132.31 (3C), 131.59, 130.77, 130.72, 129.71, 129.30, 128.81 (3C), 127.94, 127.79, 125.28, 125.16, 122.29; HR-ESI-MS: m/z = 421.0187 [M + H]+, calculated for C21H14BrN2O3: 421.0182.
Compound B5: Yield 51%; white solid; mp: 154–157 °C; 1H NMR (400 MHz, DMSO-d6) δ12.53 (s, 1H), 8.17 (dd, J = 1.2, 7.8 Hz, 1H), 7.97 (dd, J = 1.3, 7.7 Hz, 1H), 7.83–7.75 (m, 2H), 7.70 (td, J = 1.3, 7.6 Hz, 1H), 7.65–7.58 (m, 2H), 7.55 (td, J = 1.3, 7.6 Hz, 1H), 7.38 (dd, J = 1.1, 7.6 Hz, 1H), 7.27 (dd, J = 1.2, 7.5 Hz, 1H), 7.10–7.02 (m, 2H), 3.81(s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 175.50, 167.61, 167.18, 161.68, 142.52, 141.21, 132.10, 131.55, 130.76 (2C), 130.66, 129.71, 129.18, 128.57 (2C), 127.87, 127.73, 122.49, 118.38, 114.58 (2C), 55.38; HR-ESI-MS: m/z = 373.1179 [M + H]+, calculated for C22H17N2O4: 373.1183.
Compound B6: Yield 60%; white solid; mp: 151–153 °C; 1H NMR (400 MHz, DMSO-d6) δ12.52 (s, 1H), 8.18 (d, J = 7.7 Hz, 1H), 7.96 (d, J = 7.6 Hz, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.71 (t, J = 7.5 Hz, 1H), 7.67–7.59 (m, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.38 (d, J = 7.6 Hz, 1H), 7.33 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 7.5 Hz, 1H), 2.36 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 175.69, 167.60, 167.43, 142.52, 141.48, 141.17, 132.15, 131.56, 130.77, 130.74, 130.67, 129.71 (3C), 129.21, 127.88, 127.75, 126.83 (2C), 123.33, 122.47, 21.06; HR-ESI-MS: m/z = 357.1229 [M + H]+, calculated for C22H17N2O3: 357.1234.
Compound B7: Yield 55%; white solid; mp: 119–121 °C; 1H NMR (400 MHz, DMSO-d6) δ12.55 (s, 1H), 8.19 (d, J = 7.8 Hz, 1H), 8.00–7.93 (m, 2H), 7.86 (d, J = 7.8 Hz, 1H), 7.81–7.75 (m, 1H), 7.72 (td, J = 1.2, 7.6 Hz, 1H), 7.67–7.59 (m, 2H), 7.56 (td, J = 1.1, 7.6 Hz, 1H), 7.49 (t, J = 7.9 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 7.5 Hz, 1H); 13C NMR(101 MHz, DMSO-d6) δ 176.05, 167.61, 166.31, 142.61, 141.06, 134.30, 132.40, 131.60, 131.47, 130.78 (2C), 130.69, 129.65, 129.48, 129.25, 128.23, 127.90, 127.81, 125.75, 122.27, 122.17; HR-ESI-MS: m/z = 421.0177 [M + H]+, calculated for C21H14BrN2O3: 421.0182.
Compound B8: Yield 58%; white solid; mp: 171–175 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 8.15 (d, J = 7.7 Hz, 1H), 7.94 (d, J = 9.2 Hz, 2H), 7.75–7.67 (m, 1H), 7.65 –7.58 (m, 2H), 7.58–7.50 (m, 1H), 7.38 (d, J = 7.5 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.06 (d, J = 3.4 Hz, 1H), 6.77–6.67 (m, 1H); 13C NMR (101 MHz, DMSO-d6) δ175.83, 167.60, 160.49, 146.43, 142.48, 141.39, 140.93, 132.33, 131.59, 130.78, 130.72, 130.67, 129.76, 129.38, 127.96, 127.79, 122.25, 114.34, 112.22; HR-ESI-MS: m/z = 333.0869 [M + H]+, calculated for C19H13N2O4: 333.0870.
Compound B9: Yield 58%; white solid; mp: 140–142 °C; 1H NMR (400 MHz, DMSO-d6) δ12.54 (s, 1H), 8.16 (d, J = 7.5 Hz, 1H), 7.96 (d, J = 7.7 Hz, 1H), 7.84 (d, J = 4.9 Hz, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.67–7.58 (m, 3H), 7.54 (t, J = 7.4 Hz, 1H), 7.38 (d, J = 7.5 Hz, 1H), 7.28 (d, J = 7.4 Hz, 1H), 7.23 (t, J = 4.4 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ175.77, 167.59, 163.64, 142.56, 141.02, 132.32, 131.58, 130.76 (2C), 130.69, 130.67, 129.79, 129.75, 129.29, 128.45, 127.93, 127.78, 127.34, 122.21; HR-ESI-MS: m/z = 349.0635 [M + H]+, calculated for C19H13N2O3S: 349.0641.
Compound B10: Yield 50%; white solid; mp: 146–148 °C; 1H NMR (400 MHz, DMSO-d6) δ12.53 (s, 1H), 8.16 (d, J = 7.7 Hz, 1H), 8.10 (d, J = 2.8 Hz, 1H), 7.95 (d, J = 7.5 Hz, 1H), 7.74 (dd, J = 3.0, 5.0 Hz, 1H), 7.70 (t, J = 7.6 Hz, 1H), 7.62 (t, J = 7.4 Hz, 2H), 7.54 (t, J =7.6 Hz, 1H), 7.45 (d, J = 5.0 Hz, 1H), 7.38 (d, J = 7.5 Hz, 1H), 7.27 (d, J = 7.3 Hz, 1H);13C NMR (101 MHz, DMSO-d6) δ 175.61, 167.61, 164.11, 142.51, 141.09, 132.18, 131.55, 130.77 (2C), 130.67, 129.72, 129.25, 128.63, 128.37, 127.92, 127.74, 127.45, 125.55, 122.41; HR-ESI-MS: m/z = 349.0636 [M + H]+, calculated for C19H13N2O3S: 349.0641.
Compound B11: Yield 58%; white solid; mp: 167–169 °C; 1H NMR (400 MHz, DMSO-d6) δ12.59 (s, 1H), 9.38 (d, J = 1.4 Hz, 1H), 9.07 (d, J = 5.1 Hz, 1H), 8.21 (dd, J = 7.8, 1.4 Hz, 1H), 7.96 (dd, J = 7.7, 1.5 Hz, 1H), 7.90 (dd, J = 5.1, 1.4 Hz, 1H), 7.74 (td, J = 7.6, 1.4 Hz, 1H), 7.64 (tdd, J = 7.5, 2.5, 1.5 Hz, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.40 (dd, J = 7.7, 1.3 Hz, 1H), 7.30 (dd, J = 7.6, 1.4 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ177.47, 168.14, 166.91, 159.90, 159.60, 153.03, 142.99, 141.29, 133.09, 132.16, 131.28, 131.16, 130.24, 129.98, 128.56, 128.39, 122.60, 120.13; HR-ESI-MS: m/z = 345.0996 [M + H]+, calculated for C19H13N4O3: 345.0982.
Compound B12: Yield 50%; white solid; mp: 190–193 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 8.76–8.70 (m, 1H), 8.20 (d, J = 7.8 Hz, 1H), 8.03–7.93 (m, 2H), 7.86 (dd, J = 0.8, 8.0 Hz, 1H), 7.72 (t, J = 7.5 Hz, 1H), 7.63 (t, J = 7.4 Hz, 2H), 7.60–7.52 (m, 2H), 7.39 (d, J = 7.6 Hz, 1H), 7.30 (d, J = 7.5 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ176.24, 167.64, 167.53, 150.27 (2C), 145.69, 142.47, 141.03, 137.62, 132.28, 131.61, 130.80, 130.74 (2C), 129.76, 129.37, 127.96, 127.81, 126.07, 123.18, 122.45; HR-ESI-MS: m/z = 344.1026 [M + H]+, cal calculated cd for C20H14N3O3: 344.1030.
Compound B13: Yield 52%; white solid; mp: 210–212 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 9.00(s, 1H), 8.75 (s, 1H), 8.20 (d, J = 7.5 Hz, 2H), 7.97 (d, J = 7.7 Hz, 1H), 7.72 (t, J = 7.5Hz, 1H), 7.63 (t, J = 7.3 Hz, 2H), 7.60–7.52 (m, 2H), 7.40 (d, J = 7.5 Hz, 1H), 7.29 (d, J = 7.4 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ175.58, 167.04, 165.16, 151.74, 147.08, 142.02, 140.42, 133.78, 131.86, 131.03, 130.19, 130.18, 130.14, 129.09, 128.70, 127.37, 127.24, 123.69, 121.74, 121.53; HR-ESI-MS: m/z = 344.1028 [M + H]+, calculated for C20H14N3O3: 344.1030.
Compound B14: Yield 65%; white solid; mp: 188–191 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 8.80 –8.75 (m, 2H), 8.20 (dd, J = 1.3, 7.8 Hz, 1H), 7.96 (dd, J = 1.4, 7.7 Hz, 1H), 7.81–7.76(m, 2H), 7.73 (td, J = 1.4, 7.6 Hz, 1H), 7.68–7.61 (m, 2H), 7.56 (td, J = 1.3, 7.6 Hz, 1H), 7.41 (dd, J = 1.2, 7.6 Hz, 1H), 7.30 (dd, J = 1.2, 7.5 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ176.00, 167.03, 165.58, 150.25, 141.99, 140.31, 132.72, 131.93, 131.04, 130.19, 130.16, 130.14, 129.11, 128.76, 127.41, 127.26, 121.50, 120.18; HR-ESI-MS: m/z = 344.1027 [M + H]+, calculated for C20H14N3O3: 344.1030.
Compound B15: Yield 39%; white solid; mp: 165–168 °C; 1H NMR (400 MHz, DMSO-d6) δ12.57 (s, 1H), 8.59 (d, J = 5.0 Hz, 1H), 8.21 (d, J = 7.7 Hz, 1H), 7.96 (d, J = 7.7 Hz, 1H), 7.93 (s, 1H), 7.84 (d, J = 5.1 Hz, 1H), 7.74 (t, J = 7.5 Hz, 1H), 7.64 (t, J = 7.4 Hz, 2H), 7.56 (t, J = 7.1 Hz, 1H), 7.41 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 7.5 Hz, 1H); 13C NMR (101 MHz, DMSO-d6) δ 176.72, 167.63, 165.05, 151.80 (2C), 142.67, 142.21, 140.88, 136.32, 132.68, 131.65, 130.79 (2C), 129.65, 129.37, 127.98, 127.88, 124.98, 121.88, 120.36; HR-ESI-MS: m/z = 422.0133 [M + H]+, calculated for C20H13BrN3O3: 422.0135.
Compound B16: Yield 61%; white solid; mp: 145–147 °C; 1H NMR (400 MHz, DMSO-d6) δ12.57 (s, 1H), 8.69–8.59 (m, 1H), 8.22 (dd, J = 7.8, 1.3 Hz, 1H), 7.96 (dd, J = 7.8, 1.4 Hz, 1H), 7.84–7.79 (m, 2H), 7.74 (td, J = 7.6, 1.4 Hz, 1H), 7.64 (tt, J = 7.5, 1.4 Hz, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.41 (dd, J = 7.7, 1.2 Hz, 1H), 7.30 (dd, J = 7.6, 1.3 Hz, 1H).; 13C NMR (176 MHz, DMSO-d6) δ 176.16, 167.04, 164.61, 150.80, 150.71, 142.07, 140.27, 136.13, 132.09, 131.07, 130.19, 130.18, 129.07, 128.79, 127.41, 127.29, 121.28, 120.75, 119.57; HR-ESI-MS: m/z = 378.0712 [M + H]+, calculated for C20H13ClN3O3: 378.0640.
Compound B17: Yield 61%; white solid; mp: 98–100 °C; 1H NMR (400 MHz, DMSO-d6) δ12.57 (s, 1H), 8.46 (d, J = 5.1 Hz, 1H), 8.21 (dd, J = 7.7, 1.3 Hz, 1H), 7.96 (dd, J = 7.6, 1.4 Hz, 1H), 7.78–7.70 (m, 2H), 7.68–7.60 (m, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.51 (s, 1H), 7.41 (dd, J = 7.6, 1.4 Hz, 1H), 7.30 (dd, J = 7.6, 1.3 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ 176.18, 167.04, 164.75 (d, J = 3.9 Hz), 162.89 (d, J = 236.0 Hz), 148.80, 148.72, 142.06, 140.25, 138.39 (d, J = 8.7 Hz), 132.08, 131.06, 130.19, 129.08, 128.79, 127.43, 127.29, 121.31, 118.71 (d, J = 4.2 Hz), 106.65, 106.42; HR-ESI-MS: m/z =362.0945 [M + H]+, calculated for C20H13FN3O3: 362.0935.
Compound B18: Yield 61%; white solid; mp: 228–230 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 8.63 (dd, J = 5.2, 0.9 Hz, 1H), 8.20 (dd, J = 7.8, 1.4 Hz, 1H), 7.96 (dd, J = 7.8, 1.5 Hz, 1H), 7.73 (td, J = 7.6, 1.4 Hz, 1H), 7.68–7.60 (m, 3H), 7.60–7.52 (m, 2H), 7.40 (dd, J = 7.6, 1.3 Hz, 1H), 7.29 (dd, J = 7.5, 1.4 Hz, 1H), 2.55 (s, 3H); 13C NMR (176 MHz, DMSO-d6) δ175.90, 167.07, 165.69, 158.77, 149.57, 142.03, 140.33, 132.95, 131.89, 130.99, 130.26, 130.17, 130.13, 129.11, 128.73, 127.36, 127.23, 121.55, 119.49, 117.31, 23.45; HR-ESI-MS: m/z = 358.1198 [M + H]+, calculated for C21H16N3O3: 358.1186.
Compound B19: Yield 57%; white solid; mp: 154–156 °C; 1H NMR (400 MHz, DMSO-d6) δ12.54 (s, 1H), 8.34 (d, J = 5.3 Hz, 1H), 8.19 (dd, J = 7.8, 1.4 Hz, 1H), 7.96 (dd, J = 7.7, 1.5 Hz, 1H), 7.73 (td, J = 7.6, 1.4 Hz, 1H), 7.63 (td, J = 7.5, 1.5 Hz, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.42–7.37 (m, 2H), 7.29 (dd, J = 7.6, 1.3 Hz, 1H), 7.12 (t, J = 1.1 Hz, 1H), 3.90 (s, 3H); 13C NMR (176 MHz, DMSO-d6) δ 175.81, 167.03, 165.42, 163.61, 147.84 (d, J = 2.4 Hz), 142.05, 140.37, 135.70, 131.93, 131.03, 130.19, 130.18, 130.15, 129.07, 128.69, 127.38, 127.25, 121.42, 113.42, 107.36, 53.01; HR-ESI-MS: m/z = 374.1152 [M + H]+, calculated for C21H16N3O4: 374.1135.
Compound B20: Yield 57%; white solid; mp: 214–216 °C; 1H NMR (400 MHz, DMSO-d6) δ12.55 (s, 1H), 8.19 (dd, J = 7.8, 1.4 Hz, 1H), 7.96 (dd, J = 7.7, 1.4 Hz, 1H), 7.73 (td, J = 7.6, 1.4 Hz, 1H), 7.63 (tdd, J = 7.6, 2.7, 1.4 Hz, 2H), 7.56 (td, J = 7.6, 1.4 Hz, 1H), 7.45 (s, 2H), 7.40 (dd, J = 7.7, 1.3 Hz, 1H), 7.29 (dd, J = 7.5, 1.3 Hz, 1H), 2.49 (s, 6H); 13C NMR (176 MHz, DMSO-d6) δ176.88, 168.19, 166.81, 159.16, 142.96, 141.41, 134.47, 133.05, 132.23, 131.23, 131.10, 130.15, 129.77, 128.49, 128.37, 122.56, 117.81, 24.24(2C); HR-ESI-MS: m/z = 372.1361 [M + H]+, calculated for C22H18N3O3: 372.1343.
Compound B21: Yield 59%; white solid; mp: 233–235 °C; 1H NMR (400 MHz, DMSO-d6) δ12.57 (s, 1H), 8.85 (d, J = 0.6 Hz, 1H), 8.70 (d, J = 5.0 Hz, 1H), 8.20 (dd, J = 7.9, 1.3 Hz, 1H), 7.96 (dd, J = 7.8, 1.4 Hz, 1H), 7.77 (dd, J = 5.0, 0.6 Hz, 1H), 7.74 (td, J = 7.6, 1.5 Hz, 1H), 7.64 (tdd, J = 7.5, 3.8, 1.4 Hz, 2H), 7.55 (td, J = 7.6, 1.4 Hz, 1H), 7.40 (dd, J = 7.7, 1.3 Hz, 1H), 7.30 (dd, J = 7.6, 1.3 Hz, 1H).; 13C NMR (176 MHz, DMSO-d6) δ175.33, 166.99, 164.27, 150.18, 148.02, 142.05, 140.35, 131.98, 131.69, 131.08, 130.20, 130.18, 130.01, 129.23, 128.79, 128.41, 127.38, 127.28, 123.89, 121.36; HR-ESI-MS: m/z = 378.0698 [M + H]+, calculated for C20H13ClN3O3: 378.0640.
Compound B22: Yield 58%; white solid; mp: 258–259 °C; 1H NMR (400 MHz, DMSO-d6) δ12.56 (s, 1H), 9.06 (d, J = 4.4 Hz, 1H), 8.42 (dd, J = 8.6, 1.4 Hz, 1H), 8.25 (dd, J = 7.8, 1.4 Hz, 1H), 8.14 (dd, J = 8.5, 1.3 Hz, 1H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 7.96 (d, J = 4.4 Hz, 1H), 7.87 (ddd, J = 8.4, 6.8, 1.4 Hz, 1H), 7.76 (td, J = 7.5, 1.4 Hz, 1H), 7.72–7.64 (m, 3H), 7.61 (td, J = 7.5, 1.4 Hz, 1H), 7.43 (dd, J = 7.7, 1.3 Hz, 1H), 7.36 (dd, J = 7.5, 1.4 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ176.23, 168.19, 167.29, 150.82, 148.72, 143.09, 141.47, 133.05, 132.26, 131.33, 131.30, 131.15, 131.12, 130.65, 130.32, 130.18, 129.82, 128.61, 128.51, 128.44, 126.14, 124.37, 122.57, 122.28; HR-ESI-MS: m/z = 394.1208 [M + H]+, calculated for C24H16N3O3: 394.1186.
Compound B23: Yield 52%; white solid; mp: 236–238 °C; 1H NMR (400 MHz, DMSO-d6) δ12.57 (s, 1H), 9.27 (d, J = 2.1 Hz, 1H), 8.90 (dd, J = 2.2, 0.8 Hz, 1H), 8.26 (dd, J = 7.8, 1.4 Hz, 1H), 8.17 (dd, J = 8.3, 1.5 Hz, 1H), 8.11 (dd, J = 8.4, 1.1 Hz, 1H), 8.00 (dd, J = 7.7, 1.5 Hz, 1H), 7.90 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.74 (qd, J = 7.2, 1.3 Hz, 2H), 7.66 (td, J = 7.7, 1.4 Hz, 2H), 7.59 (td, J = 7.6, 1.4 Hz, 1H), 7.43 (dd, J = 7.6, 1.3 Hz, 1H), 7.32 (dd, J = 7.5, 1.4 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ175.64, 165.27, 147.91, 147.12, 134.47, 131.86, 131.05, 130.90, 130.19, 130.16, 129.12, 128.75, 128.47, 128.33, 127.38, 127.20, 126.26, 121.59, 118.88; HR-ESI-MS: m/z = 394.1180 [M + H]+, calculated for C24H16N3O3: 394.1186.
Compound B24: Yield 51%; white solid; mp: 264–266 °C; 1H NMR (400 MHz, DMSO-d6) δ12.57 (s, 1H), 9.00 (dd, J = 4.2, 1.7 Hz, 1H), 8.57 (d, J = 1.8 Hz, 1H), 8.53 (dd, J = 8.3, 1.7 Hz, 1H), 8.25 (dd, J = 7.9, 1.4 Hz, 1H), 8.20–8.11 (m, 2H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H), 7.73 (dd, J = 7.6, 1.4 Hz, 1H), 7.69–7.62 (m, 3H), 7.59 (td, J = 7.6, 1.4 Hz, 1H), 7.42 (dd, J = 7.7, 1.3 Hz, 1H), 7.32 (dd, J = 7.5, 1.4 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ176.67, 168.16, 167.59, 152.73, 149.09, 143.04, 141.55, 137.48, 132.87, 132.17, 131.29, 131.23, 131.19, 130.57, 130.25, 129.83, 128.50, 128.32, 128.27, 128.16, 127.38, 124.48, 123.02, 122.83; HR-ESI-MS: m/z = 394.1205 [M + H]+, calculated for C24H16N3O3: 394.1186.
Compound B25: Yield 51%; white solid; mp: 169–171 °C; 1H NMR (400 MHz, DMSO-d6) δ12.55 (s, 1H), 8.60–8.45 (m, 1H), 8.29–8.24 (m, 1H), 8.22 (dd, J = 7.8, 1.4 Hz, 1H), 8.07–7.96 (m, 2H), 7.73 (td, J = 7.5, 1.4 Hz, 1H), 7.69–7.54 (m, 5H), 7.41 (dd, J = 7.6, 1.3 Hz, 1H), 7.34 (dd, J = 7.5, 1.4 Hz, 1H), 7.10 (d, J = 8.3 Hz, 1H), 4.05 (s, 3H); 13C NMR (176 MHz, DMSO-d6) δ174.95, 168.55, 168.16, 157.97, 143.00, 141.72, 132.65, 132.18, 131.29, 131.20, 131.19, 131.11, 130.84, 130.29, 129.65, 128.53, 128.39, 128.32, 126.39, 125.97, 125.39, 122.94, 122.50, 115.42, 104.52, 56.49; HR-ESI-MS: m/z = 421.1184 [M-H]-, calculated for C26H17N2O4: 421.1183.
The procedure for the synthesis of intermediate I-4. Compound B21 (1.0 eq) and methyl 3-aminopropionate hydrochloride (1.2 eq) were dissolved in 20 mL N,N-dimethylformamide, then HATU (1.5 eq) and triethylamine (2 eq) were added. The mixture was stirred at 60 °C for 10 h, and the solvent was evaporated in a vacuum. The reaction mixture was poured into water (50 mL). The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to I-4.
The procedure for the synthesis of compound B26. The intermediate I-4 (1.0 eq) and lithium hydroxide monohydrate (2.0 eq) were dissolved in 20 mL of a mixed solvent of ethanol: H2O (4:1). The mixture was stirred at room temperature for 8 h, and the solvent was evaporated in a vacuum. The reaction mixture was poured into water (50 mL). 1 M HCl solution was added dropwise to adjust the pH to 5. The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to B26.
Compound B26: Yield 53%; white solid; mp: 240–242 °C; 1H NMR (400 MHz, DMSO-d6) δ8.84 (s, 1H), 8.70 (d, J = 5.0 Hz, 1H), 8.14 (dd, J = 7.8, 1.4 Hz, 1H), 8.00 (s, 1H), 7.82 (d, J = 5.0 Hz, 1H), 7.70 (td, J = 7.6, 1.4 Hz, 1H), 7.61 (td, J = 7.6, 1.4 Hz, 1H), 7.54–7.44 (m, 3H), 7.39 (dd, J = 7.7, 1.3 Hz, 1H), 7.29 (dd, J = 7.8, 1.7 Hz, 1H), 3.03 (d, J = 7.0 Hz, 2H), 1.83 (t, J = 7.4 Hz, 2H); 13C NMR (176 MHz, DMSO-d6) δ176.54, 167.71, 165.22, 151.16, 149.12, 141.97, 138.92, 136.97, 132.92, 131.63, 130.70, 130.19, 129.86, 129.47, 128.47, 128.23, 127.46, 125.18, 122.89, 37.00, 36.91; HR-ESI-MS: m/z = 449.1032 [M + H]+, calculated for C23H18ClN4O4: 449.1011.
The procedure for the synthesis of compound B27. The compound B21 (1.0 eq) and 4-bromobenzenesulfonamide (1.2 eq) were dissolved in 20 mL N, N-dimethylformamide in an ice bath, then 4-dimethylaminopyridine (1.5 eq) and EDCI (1.2 eq) were added. The mixture was stirred at room temperature for 6 h. The solvent was evaporated in a vacuum. The residue was purified by column chromatography to give B27.
Compound B27: Yield 49%; white solid; mp: 188–190 °C; 1H NMR (400 MHz, DMSO-d6) δ8.84 (d, J = 0.5 Hz, 1H), 8.70 (d, J = 5.0 Hz, 1H), 8.11–8.04 (m, 1H), 7.86 (dd, J = 4.9, 0.6 Hz, 1H), 7.82–7.77 (m, 1H), 7.60–7.46 (m, 2H), 7.42–7.30 (m, 6H), 7.21–7.14 (m, 1H), 7.07–7.00 (m, 1H); 13C NMR (176 MHz, DMSO-d6) δ175.62, 170.76, 163.81, 150.05, 147.93, 144.64, 143.32, 139.34, 138.44, 131.99, 131.26, 130.27, 129.59, 129.25, 128.62, 128.34, 128.17, 127.88, 127.51, 126.30, 126.15, 124.19, 122.23, 121.45; HR-ESI-MS: m/z = 594.9864 [M + H]+, calculated for C26H17BrClN4O4S: 594.9837.
The procedure for the synthesis of Intermediate I-5a. Iodine substituted benzoic acid (1.2 eq) and intermediate I-3a (1.0 eq) were dissolved in 20 mL dichloromethane, then HATU (1.5 eq) and triethylamine (2 eq) were added. The mixture was stirred at room temperature for 10 h, and the solvent was evaporated in a vacuum. The residue was purified by column chromatography to give intermediate I-5a.
The procedure for the synthesis of intermediate I-6a. Intermediate I-5a (1.0 eq) and cesium carbonate (1.0 eq) were dissolved in 20 mL dimethyl sulfoxide. The mixture was stirred at room temperature for 10 h, the reaction mixture was poured into water (50 mL), the mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to intermediate I-6a.
General preparation of compounds B28 and B29. The intermediate I-6a (1.0 eq), substituted phenylboronic acids (1.0 eq), potassium carbonate (3.0 eq), and tetrakis(triphenylphosphine)palladium (0.2 eq) were added into 20 mL of a mixed solvent of toluene: 95%ethanol (1:1). The mixture was stirred at 110 °C under N2 for 12 h. The solvent was then evaporated in a vacuum, the reaction mixture was poured into water (50 mL). 1 M HCl solution was added dropwise to adjust the pH to 5. The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to B28 and B29.
Compound B28: Yield 55%; white solid; mp: 288–289 °C; 1H NMR (400 MHz, DMSO-d6) δ13.07 (s, 1H), 8.82–8.73 (m, 2H), 8.17 (dd, J = 7.8, 1.4 Hz, 1H), 7.98 (dt, J = 6.8, 1.9 Hz, 1H), 7.88–7.78 (m, 4H), 7.71 (td, J = 7.6, 1.3 Hz, 1H), 7.64 (dd, J = 7.7, 1.3 Hz, 1H), 7.60–7.52 (m, 2H); 13C NMR (176 MHz, DMSO-d6) δ176.20, 166.46, 165.81, 150.32(2C), 140.64, 139.18, 132.65, 132.52, 130.74, 130.23, 128.81, 128.09, 128.07, 128.02, 121.42, 120.26(2C); HR-ESI-MS: m/z = 344.1041 [M + H]+, calculated for C20H14N3O3: 344.1030.
Compound B29: Yield 54%; white solid; mp: 258–260 °C; 1H NMR (400 MHz, DMSO-d6) δ8.85–8.75 (m, 2H), 8.08 (dd, J = 7.8, 1.4 Hz, 1H), 7.94–7.89 (m, 2H), 7.86–7.82 (m, 2H), 7.78 (td, J = 7.6, 1.4 Hz, 1H), 7.65 (td, J = 7.6, 1.3 Hz, 1H), 7.60 (dd, J = 7.8, 1.2 Hz, 1H), 7.19–7.14 (m, 2H); 13C NMR (176 MHz, DMSO-d6) δ176.78, 167.92, 165.81, 150.30(2C), 141.97, 140.00, 138.65, 132.74, 132.23, 130.56, 130.22, 128.38(2C), 127.33, 126.77(2C), 121.56, 120.34(2C); HR-ESI-MS: m/z = 344.1043 [M + H]+, calculated for C20H14N3O3: 344.1030.
The procedure for the synthesis of intermediate I-7. The isoniazid (1.0 eq) and 2-bromobenzoic acid (1.05 eq) were dissolved in 20 mL phosphorus oxychloride. The mixture was stirred at 110 °C for 10 h. Saturated sodium bicarbonate solution was added dropwise until there were no bubbles in the reaction solution. The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to obtain I-7.
The procedure for the synthesis of intermediate I-8. The intermediate I-7 (1.0 eq), substituted phenylboronic acid (1.0 eq), potassium carbonate (3.0 eq), and tetrakis(triphenylphosphine)palladium (0.2 eq) were added into 20 mL of a mixed solvent of toluene: 95%ethanol (1:1). The mixture was stirred at 110 °C under N2 for 12 h. The solvent was then evaporated in a vacuum and the residue was purified by column chromatography to intermediate I-8.
The procedure for the synthesis of compound B30. The intermediate I-8 (1.0 eq) and lithium hydroxide monohydrate (2.0 eq) were dissolved in 20 mL of a mixed solvent of ethanol: H2O (4:1). The mixture was stirred at room temperature for 8 h, and the solvent was evaporated in a vacuum. The reaction mixture was poured into water (50 mL). 1 M HCl solution was added dropwise to adjust the pH to 5. The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic solution was washed with saturated aqueous sodium chloride solution (30 mL × 3) and dried over anhydrous Na2SO4. The residue was purified by column chromatography to B30.
Compound B30: Yield 58%; white solid; mp: 237–239 °C; 1H NMR (400 MHz, DMSO-d6) δ12.60 (s, 1H), 8.80–8.67 (m, 2H), 8.19 (dt, J = 7.7, 1.8 Hz, 1H), 7.95 (dt, J = 7.7, 1.8 Hz, 1H), 7.72–7.56 (m, 4H), 7.51–7.46 (m, 2H), 7.40 (dd, J = 7.6, 1.4 Hz, 1H), 7.33 (dd, J = 7.6, 1.5 Hz, 1H); 13C NMR (176 MHz, DMSO-d6) δ168.24, 165.58, 162.46, 151.35, 142.00, 141.48, 132.12, 132.10, 131.36, 131.22, 131.10, 130.54, 130.11, 128.96, 128.45, 128.33, 122.26, 120.16; HR-ESI-MS: m/z = 344.1026 [M + H]+, calculated for C20H14N3O3: 344.1030.
Spectral data, including HR-ESI-MS, 1H-NMR and 13C-NMR, for compounds A1–A21 and B1–B30 are provided in Supplementary Materials.
3.2. Biology
In Vitro URAT1 Inhibitory Assay. A transient expression system of human URAT1 in HEK293T cells was constructed to measure 14C-uric acid transport. The HEK293T cells were obtained from ATCC (catalogue No.: CRL-11268). The cell cryovials were removed from liquid nitrogen storage, and immediately placed on dry ice prior to thawing. The frozen cell vials were placed briefly (30 s to 1 min) in a 37 °C water bath, until only small ice crystals left and the cell pellet was almost completely thawed. The cell suspension was transferred into a 15 mL centrifuge tube, and 10 mL complete medium (DMEM +10% FBS +500 μg/mL G418 +1% P/S) was added. After centrifugation at 1000 rpm for 5 min, the supernatant was discarded, and cell precipitation was resuspended in 5 mL complete medium and transferred to a T75 culture flask. 15 mL of the assay complete medium was added into a T75 flask and incubated at 37 °C and 5% CO2. The uptake assay test could be carried out after cell culture adherent to the well in the 96-well plate. Then, the cells were washed with 200 µL/well of pre-warmed Cl- free HBSS buffer, and all buffer was removed at the last washing. 50 µL/well of Cl- free HBSS Buffer containing Uric acid [8-14C] (2.5 μCi/mL) and samples were added to the cells, and incubated for 8 min. Then, the cells were washed three times with Cl- free HBSS Buffer and all buffer was removed at the last washing. 50 µL/well of lysis buffer (100 mM NaOH) was added to the lysate cells and stirred at 900 rpm for 5 min. 150 µL/well MicroScint™-40 cocktail was added and agitated at 900 rpm for 5 min. Finally, the 96-plate was counted in a MicroBeta2 (PerkinElmer). The data were analyzed and IC50 values was calculated with GraphPad Prism 6 software.
The IC50 curves for the compounds are provided in Supplementary Materials.
4. Conclusions
Based on the known URAT1 inhibitors Epaminurad and Telmisartan, two series of biphenyl carboxylic acid-based URAT1 inhibitors were recognized, and SAR clues were provided. Both series afforded potent URAT1 inhibitors, and A1 and B21 exhibited IC50 values of 0.93 μM and 0.17 μM, respectively. These new inhibitors not only represent a new chemical prototype of potent URAT1 inhibitors, but are expected to have reduced toxicity and improved chemical accessibility compared to Epaminurad and Telmisartan. The results confirmed that ligand-based approaches could be an effective way to identify novel and potent URAT1 inhibitors.
5. Patents
A Chinese patent (202211251941.3) derived from results presented herein was applied in 2022.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28217415/s1.
Author Contributions
Conceptualization, Z.X.; methodology, X.H., Y.W. and Y.Y.; investigation, X.H. and Y.W.; resources, Z.X.; data curation, X.H. and Y.W.; writing—original draft preparation, X.H.; writing—review and editing, Z.X.; supervision, Z.X.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (21871295) and CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-069).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Data for URAT1 inhibition were provided by ChemPartner as contract services.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.W.; Glynn, R.J.; DeLabry, L.O. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am. J. Med. 1987, 82, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Eraly, S.A.; Vallon, V.; Rieg, T.; Gangoiti, J.A.; Wikoff, W.R.; Siuzdak, G.; Barshop, B.A.; Nigam, S.K. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genom. 2008, 33, 180–192. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ohya, K.; Shimada, S.; Anzai, N.; Tamai, I. Functional cooperation of URAT1 (SLC22A12) and URATv1 (SLC2A9) in renal reabsorption of urate. Nephrol. Dial. Transpl. 2013, 28, 603–611. [Google Scholar] [CrossRef]
- Nigam, S.K. The SLC22 transporter family: A paradigm for the impact of drug transporters on metabolic pathways, signaling, and disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef]
- Woodward, O.M.; Kottgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Kottgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef]
- Sattui, S.E.; Gaffo, A.L. Treatment of hyperuricemia in gout: Current therapeutic options, latest developments and clinical implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 145–159. [Google Scholar] [CrossRef]
- Song, D.; Zhao, X.; Wang, F.; Wang, G. A brief review of urate transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout: Current therapeutic options and potential applications. Eur. J. Pharmacol. 2021, 907, 174291. [Google Scholar] [CrossRef]
- Stamp, L.K.; Merriman, T.R.; Singh, J.A. Expert opinion on emerging urate-lowering therapies. Expert Opin. Emerg. Drugs 2018, 23, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, T.; da Silva-Junior, E.F.; Zhang, J.; Xu, S.; Gao, S.; Liu, X.; Zhan, P. Novel urate transporter 1 (URAT1) inhibitors: A review of recent patent literature (2020-present). Expert. Opin. Ther. Pat. 2022, 32, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.O.; Ohtomo, S.; Kiyokawa, J.; Nakagawa, T.; Yamane, M.; Lee, K.J.; Kim, K.H.; Kim, B.H.; Tanaka, J.; Kawabe, Y.; et al. Stronger uricosuric effects of the novel selective URAT1 inhibitor UR-1102 lowered plasma urate in tufted capuchin monkeys to a greater extent than benzbromarone. J. Pharmacol. Exp. Ther. 2016, 357, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ohe, T.; Umezawa, R.; Kitagawara, Y.; Yasuda, D.; Takahashi, K.; Nakamura, S.; Abe, A.; Sekine, S.; Ito, K.; Okunushi, K.; et al. Synthesis of novel benzbromarone derivatives designed to avoid metabolic activation. Bioorg. Med. Chem. Lett. 2018, 28, 3708–3711. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Lesinurad: First Global Approval. Drugs 2016, 76, 509–516. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, D.; Shen, Z.; Wilson, D.M.; Renner, M.; Miner, J.N.; Girardet, J.L.; Lee, C.A. Characterization of stereoselective metabolism, inhibitory effect on uric acid uptake transporters, and pharmacokinetics of Lesinurad atropisomers. Drug Metab. Dispos. 2019, 47, 104–113. [Google Scholar] [CrossRef]
- Kuriyama, S. Dotinurad: A novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia. Clin. Exp. Nephrol. 2020, 24 (Suppl. 1), 6–7. [Google Scholar] [CrossRef]
- Uda, J.; Kobashi, S.; Miyata, S.; Ashizawa, N.; Matsumoto, K.; Iwanaga, T. Discovery of Dotinurad (FYU-981), a new phenol derivative with highly potent uric acid lowering activity. ACS Med. Chem. Lett. 2020, 11, 2017–2023. [Google Scholar] [CrossRef]
- Hosoya, T.; Sano, T.; Sasaki, T.; Fushimi, M.; Ohashi, T. Dotinurad versus benzbromarone in Japanese hyperuricemic patient with or without gout: A randomized, double-blind, parallel-group, phase 3 study. Clin. Exp. Nephrol. 2020, 24 (Suppl. 1), 62–70. [Google Scholar] [CrossRef]
- Felser, A.; Lindinger, P.W.; Schnell, D.; Kratschmar, D.V.; Odermatt, A.; Mies, S.; Jeno, P.; Krahenbuhl, S. Hepatocellular toxicity of benzbromarone: Effects on mitochondrial function and structure. Toxicology 2014, 324, 136–146. [Google Scholar] [CrossRef]
- Lee, M.H.; Graham, G.G.; Williams, K.M.; Day, R.O. A benefit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best interest of patients? Drug Saf. 2008, 31, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.A.; Jiang, Y.; Li, L.; Chen, Y.Y.; Li, Y.M.; Lan, Q.S.; Wu, T.; Lin, C.T.; Cao, Y.; Nandakumar, K.S.; et al. Structural insights into the atomistic mechanisms of uric acid recognition and translocation of human urate anion transporter 1. ACS Omega 2020, 5, 33421–33432. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kong, L.D. Urate transporter URAT1 inhibitors: A patent review (2012–2015). Expert. Opin. Ther. Pat. 2016, 26, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Ouchi, M.; Kudo, H.; Tsuruoka, S.; Hisatome, I.; Anzai, N. Recent approaches to gout drug discovery: An update. Expert Opin. Drug Discov. 2020, 15, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, T.; Ai, W.; Zalloum, W.A.; Kang, D.; Wu, T.; Liu, X.; Zhan, P. Novel urate transporter 1 (URAT1) inhibitors: A review of recent patent literature (2016–2019). Expert Opin. Ther. Pat. 2019, 29, 871–879. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Gu, C.; Liu, B.; Jin, F.; Yuan, J.; Feng, J.; Zhang, L.; Lan, J.; Dong, Q.; et al. Discovery of potent and orally bioavailable inhibitors of human uric acid transporter 1 (hURAT1) and binding mode prediction using homology model. Bioorg Med. Chem. Lett. 2016, 26, 277–282. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X.; Ding, H.; Ye, P.; Gu, J.; Wang, X.; Jiang, Z.; Li, D.; Wang, Z.; Long, W.; et al. Efficacy and safety of a selective URAT1 inhibitor SHR4640 in Chinese subjects with hyperuricaemia: A randomized controlled phase II study. Rheumatology 2021, 60, 5089–5097. [Google Scholar] [CrossRef]
- Tang, H.; Cui, B.; Chen, Y.; Chen, L.; Wang, Z.; Zhang, N.; Yang, Y.; Wang, X.; Xie, X.; Sun, L.; et al. Safety and efficacy of SHR4640 combined with febuxostat for primary hyperuricemia: A multicenter, randomized, double-blind, phase II study. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X211067304. [Google Scholar] [CrossRef]
- Ahn, S.O.; Horiba, N.; Ohtomo, S.; Lee, K.J.; Kim, K.H.; Kim, B.H.; Kiyokawa, J.; Yamane, M.; Ikegami, H.; Nakagawa, T.; et al. The therapeutic efficacy of the novel uricosuric agent UR-1102 for hyperuricemia and gout. Ann. Rheum. Dis. 2013, 72, 704. [Google Scholar] [CrossRef]
- Pascart, T.; Richette, P. Investigational drugs for hyperuricemia, an update on recent developments. Expert Opin. Investig. Drugs 2018, 27, 437–444. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Anzai, N.; Jutabha, P.; Sato, H.; Sakurai, H.; Ichida, K. Concentration-dependent inhibitory effect of irbesartan on renal uric acid transporters. J. Pharmacol. Sci. 2010, 114, 115–118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).