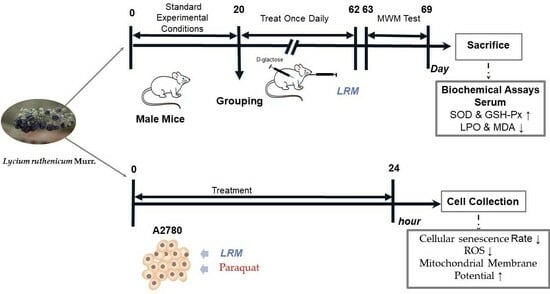
The Ethanolic Extract of Lycium ruthenicum Ameliorates Age-Related Physiological Damage in Mice
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of LRM
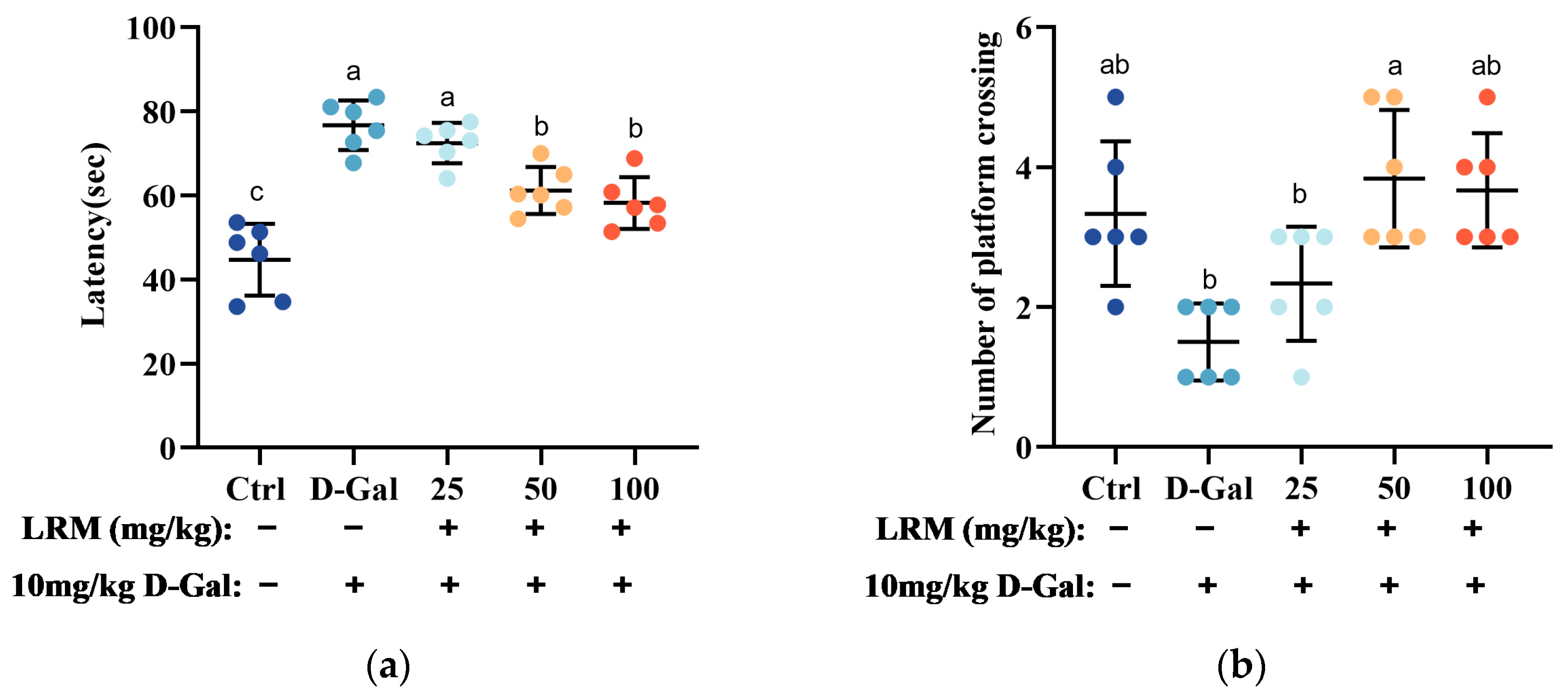
2.2. Effect of LRM Pretreatment on D-Gal-Induced Cognitive Ability in Mice
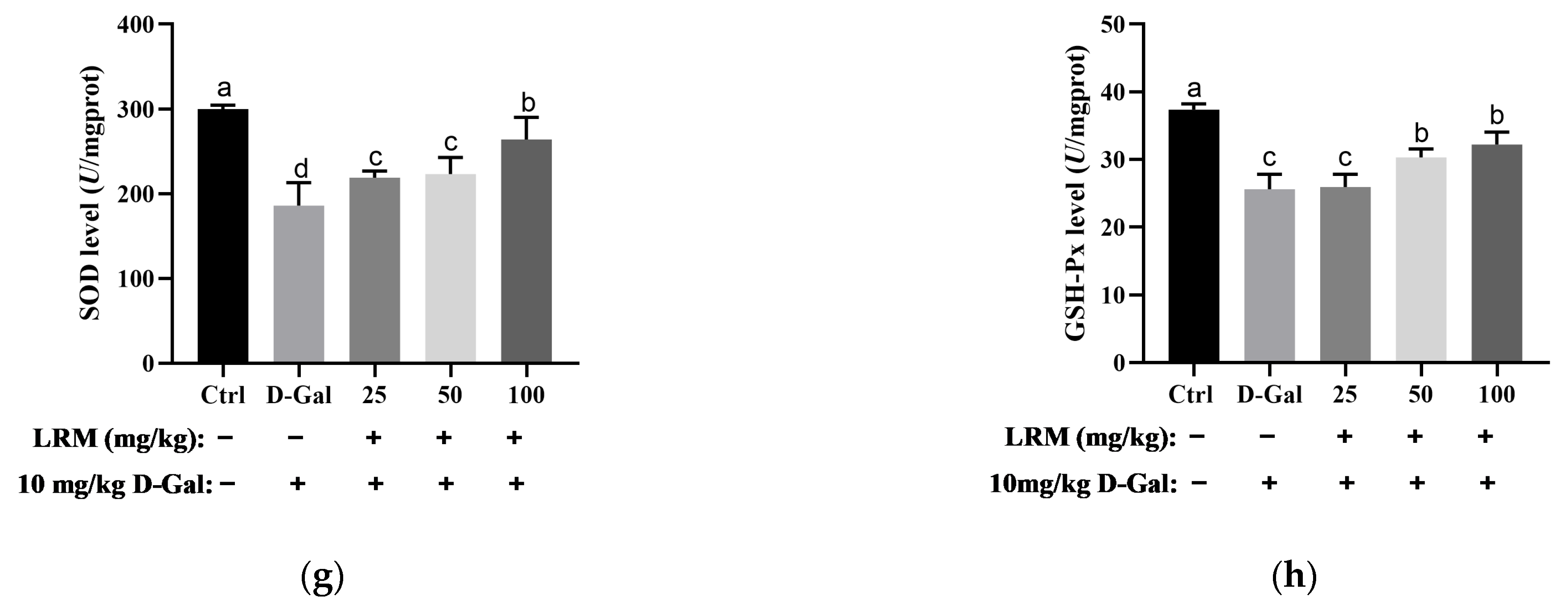
2.3. Effect of LRM Treatment on Oxidative/Antioxidant Status in Mice
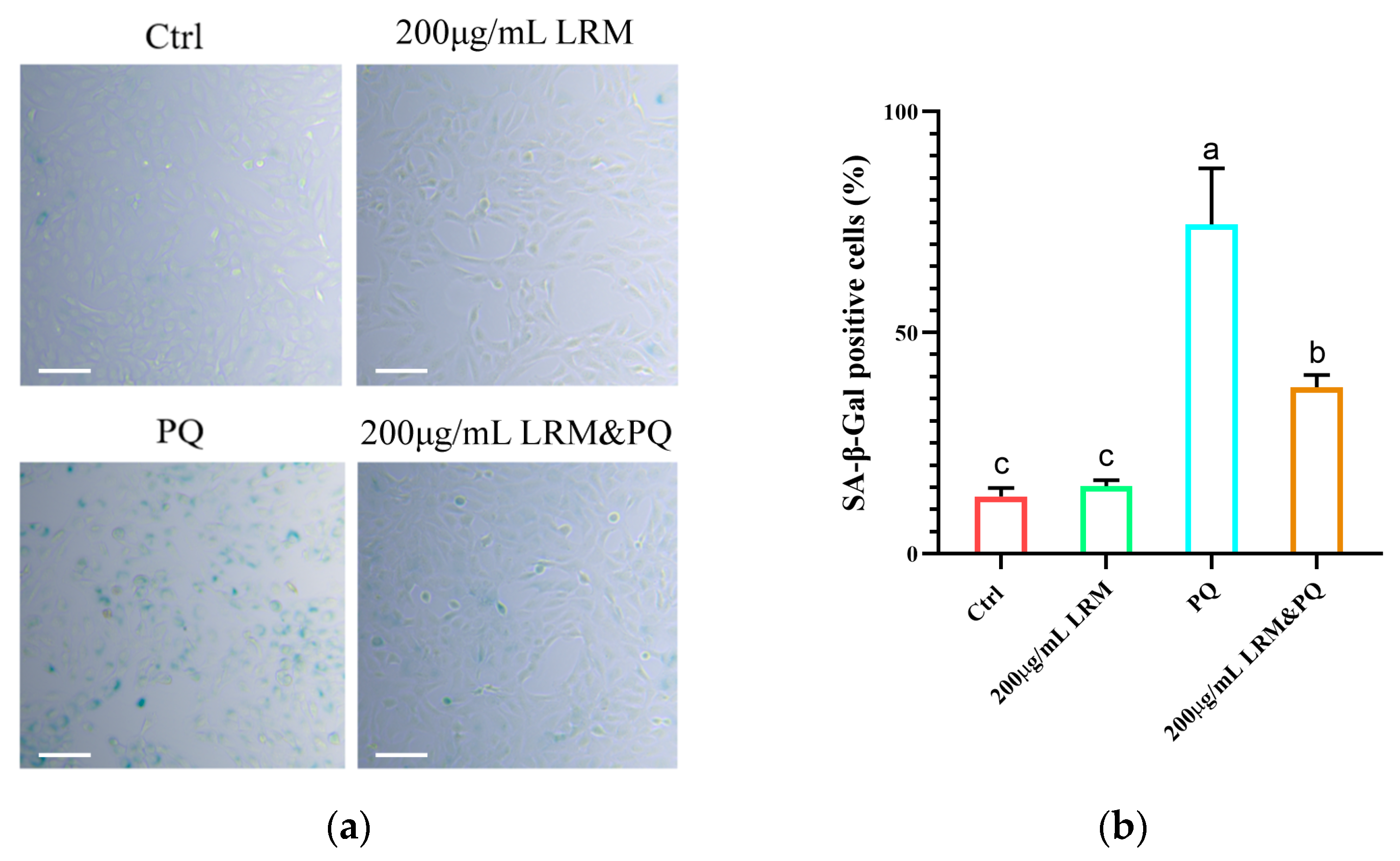
2.4. Effects of LRM Pretreatment on A2780 Cellular Senescence Induced by PQ
2.5. Effects of LRM Pretreatment on ROS Production in A2780 Cells Induced by PQ
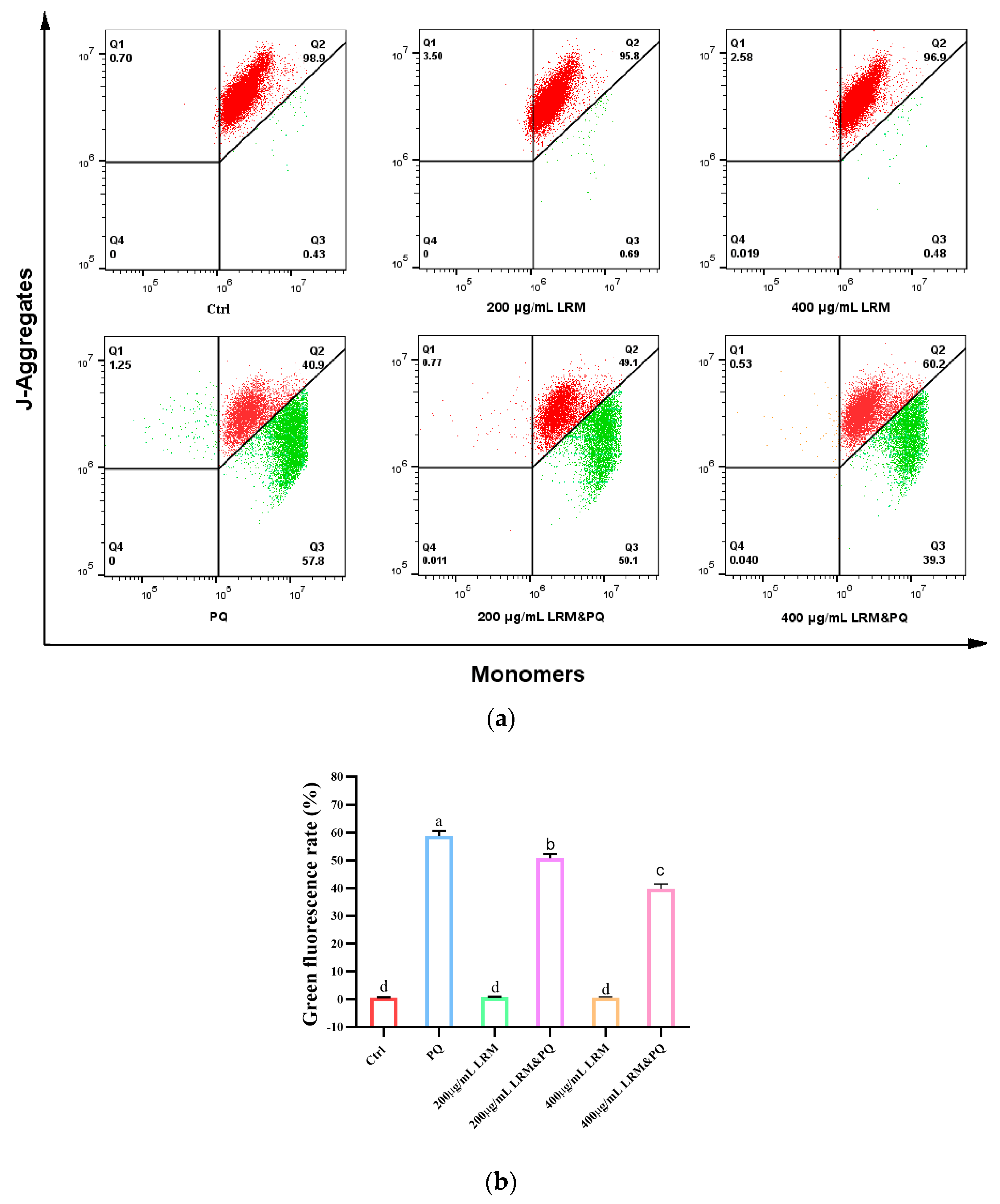
2.6. Effects of LRM Pretreatment on Mitochondrial Membrane Potential (MMP) of A2780 Induced by PQ
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Materials
4.3. HPLC-DAD Analysis
4.4. Q-TOF MS Conditions
4.5. Animals and Treatment
4.6. Morris Water Maze (MWM) Test
4.7. Anesthetization and Sample Collection
4.8. Determination of the Contents of LPO and MDA and the Enzyme Activities of SOD and GSH-Px
4.9. Cell Culture and Treatment
4.10. Cellular Senescence Assay
4.11. Cell ROS Assay
4.12. Mitochondrial Transmembrane Potential Assay
4.13. Statistical Analysis and Other Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of Ageing and Role of Dietary Antioxidants. BioMed Res. Int. 2014, 2014, 831–841. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 18071544. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 18010096. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.A.; Shaban, A.; Diab, H.A.; Elsaghayer, W.A.; Mjedib, M.D.; Hnesh, A.M.; Sahu, R.P. Phoenix dactylifera protects against oxidative stress and hepatic injury induced by paracetamol intoxication in rats. Biomed. Pharmacother. 2018, 104, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Gawas, M.; Bains, A.; Janghu, S.; Kamat, P.; Chawla, P. A Comprehensive Review on Varicose Veins: Preventive Measures and Different Treatments. J. Am. Nutr. Assoc. 2022, 41, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.-A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 25173809. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, P.; Liu, Y.; Zha, L.; Ling, W.; Guo, H. A dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: A randomized controlled trial. Nutrition 2020, 74, 110745. [Google Scholar] [CrossRef]
- Piovezana Gomes, J.V.; Buttow Rigolon, T.C.; da Silveira Souza, M.S.; Alvarez-Leite, J.I.; Della Lucia, C.M.; Duarte Martino, H.S.; Barbosa Rosa, C.d.O. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: A systematic review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Steed, L.E.; Truong, V.D. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J. Food Sci. 2008, 73, S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Bolondi, C.; Teti, G.; Falconi, M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effects of cyanidin 3-O-glucopyranoside on amyloid beta (25–35) oligomer-induced toxicity. Neurosci. Lett. 2010, 473, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Feng, Z.; Chen, N. Spermidine as a target for cancer therapy. Pharmacol. Res. 2020, 159, 104–943. [Google Scholar] [CrossRef]
- Levin, R.A.; Miller, J.S. Relationships within tribe Lycieae (Solanaceae): Paraphyly of Lycium and multiple origins of gender dimorphism. Am. J. Bot. 2005, 92, 2044–2053. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Zeng, S.H.; Wu, M.; Zou, C.Y.; Liu, X.M.; Shen, X.F.; Hayward, A.; Liu, C.Z.; Wang, Y. Comparative analysis of anthocyanin biosynthesis during fruit development in two Lycium species. Physiol. Plant. 2014, 150, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, N.; Wang, H.; Li, G. The major anthocyanin of Lycium ruthenicum Murr. relieves cognitive deficits, oxidative stress, neuroinflammation, and hippocampal metabolome alterations in aging rats. J. Funct. Foods 2022, 94, 105104. [Google Scholar] [CrossRef]
- Yun, D.; Yan, Y.; Liu, J. Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum Murr: A review. Carbohydr. Polym. 2022, 291, 119618. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Lin, S.; Wang, Z.; Zong, Y.; Wang, Y. The health-promoting anthocyanin petanin in Lycium ruthenicum fruit: A promising natural colorant. Crit. Rev. Food Sci. Nutr. 2023, 2225192. [Google Scholar] [CrossRef] [PubMed]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Black Goji Berry Anthocyanins: Extraction, Stability, Health Benefits, and Applications. Acs Food Sci. Technol. 2021, 1, 1360–1370. [Google Scholar] [CrossRef]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Q.; Fan, H.-X.; He, R.-R.; Xiao, J.; Tsoi, B.; Lan, K.-H.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Lycibarbarspermidines A–O, New Dicaffeoylspermidine Derivatives from Wolfberry, with Activities against Alzheimer’s Disease and Oxidation. J. Agric. Food Chem. 2016, 64, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Liu, Y.; Yang, F.; Wang, J.; Fu, D.; Zhang, X.; Peng, X.; Liang, X. Characterization of anthocyanins in wild Lycium ruthenicum Murray by HPLC-DAD/QTOF-MS/MS. Anal. Methods 2015, 7, 4947–4956. [Google Scholar] [CrossRef]
- Redish, A.D.; Touretzky, D.S. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998, 10, 73–111. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and D-galactose induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef]
- Alia, M.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Bravo, L.; Goya, L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006, 212, 110–118. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 21093289. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Metur, S.P.; Klionsky, D.J. The curious case of polyamines: Spermidine drives reversal of B cell senescence. Autophagy 2020, 16, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Ghisletta, P.; Rodrigue, K.M.; Kennedy, K.M.; Lindenberger, U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. Neuroimage 2010, 51, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, Y.; Xu, Q.; He, Y.; Tian, S.; Zhu, S.; Zhu, Y.; Dong, X. Mussel oligopeptides ameliorate cognition deficit and attenuate brain senescence in D-galactose-induced aging mice. Food Chem. Toxicol. 2013, 59, 412–420. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimer’s Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, H.; Vaisi-Raygani, A.; Rahimi, Z.; Tavilani, H.; Aminian, M.; Pourmotabbed, T. Association between enzymatic and non-enzymatic antioxidant defense mechanism with apolipoprotein E genotypes in Alzheimer disease. Clin. Biochem. 2008, 41, 932–936. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Javadi, B.; Sahebkar, A. The Underlying Role of Oxidative Stress in Neurodegeneration: A Mechanistic Review. CNS Neurol. Disord.-Drug Targets 2018, 17, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Baar, M.P.; de Keizer, P.L.J. Senescent Cells Drive Frailty through Systemic Signals. Trends Mol. Med. 2018, 24, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Chen, X.J. Adenine Nucleotide Translocase, Mitochondrial Stress, and Degenerative Cell Death. Oxidative Med. Cell. Longev. 2013, 2013, 146860. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.-P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Xie, Z.; Zeng, H.; Wang, P.; Li, J.; Zheng, G.; Wang, S.; Cao, Q.; Li, M.; Liu, W.; et al. Oxidative stress-mediated mitochondrial dysfunction facilitates mesenchymal stem cell senescence in ankylosing spondylitis. Cell Death Dis. 2020, 11, 775. [Google Scholar] [CrossRef]
- Chen, Q.; Vasse, G.F.; Nwozor, K.O.; Bekker, N.J.; van den Berge, M.; Brandsma, C.-A.; de Vries, M.; Heijink, I.H. FAM13A regulates cellular senescence marker p21 and mitochondrial reactive oxygen species production in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L460–L466. [Google Scholar] [CrossRef] [PubMed]
- Song, I.Y.; Snyder, A.M.; Kim, Y.; Neely, E.B.; Wade, Q.W.; Connor, J.R. The Nrf2-mediated defense mechanism associated with HFE genotype limits vulnerability to oxidative stress-induced toxicity. Toxicology 2020, 441, 152525. [Google Scholar] [CrossRef] [PubMed]
- Dilberger, B.; Baumanns, S.; Schmitt, F.; Schmiedl, T.; Hardt, M.; Wenzel, U.; Eckert, G.P. Mitochondrial Oxidative Stress Impairs Energy Metabolism and Reduces Stress Resistance and Longevity of C. elegans. Oxidative Med. Cell. Longev. 2019, 2019, 6840540. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xue, X.; Gong, N.; Jiang, J. Ginsenoside Rg1 suppresses paraquat-induced epithelial cell senescence by enhancing autophagy in an ATG12-dependent manner. Environ. Toxicol. 2022, 37, 2302–2313. [Google Scholar] [CrossRef]
- Eftekhari, A.; Hasanzadeh, A.; Khalilov, R.; Hosainzadegan, H.; Ahmadian, E.; Eghbal, M.A. Hepatoprotective role of berberine against paraquat-induced liver toxicity in rat. Environ. Sci. Pollut. Res. 2020, 27, 4969–4975. [Google Scholar] [CrossRef]
- Boulanger, E.; Puisieux, F.; Gaxatte, C.; Wautier, J.L. Aging: Role and control of glycation. Rev. Med. Interne 2007, 28, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Park, W.J.; Lee, Y.J. Recent Advances in Anti-Aging Medicine. Korean J. Fam. Med. 2019, 40, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Wang, J.; Li, J.E.; Xiong, L.; Chen, H.; Liu, X.; Wang, N.; Ouyang, K.H.; Wang, W.J. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr. Polym. 2018, 183, 11–20. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, M.; Zheng, H.; Wang, Y.; Wu, S.; Gao, Y.; Chen, J. Resveratrol inhibits the progression of premature senescence partially by regulating v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA) and sirtuin 1 (SIRT1). Ren. Fail. 2022, 44, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Zhang, H.; Su, Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012, 38, 250–257. [Google Scholar] [CrossRef]
- Manna, A.; De Sarkar, S.; De, S.; Bauri, A.K.; Chattopadhyay, S.; Chatterjee, M. The variable chemotherapeutic response of Malabaricone-A in leukemic and solid tumor cell lines depends on the degree of redox imbalance. Phytomedicine 2015, 22, 713–723. [Google Scholar] [CrossRef]
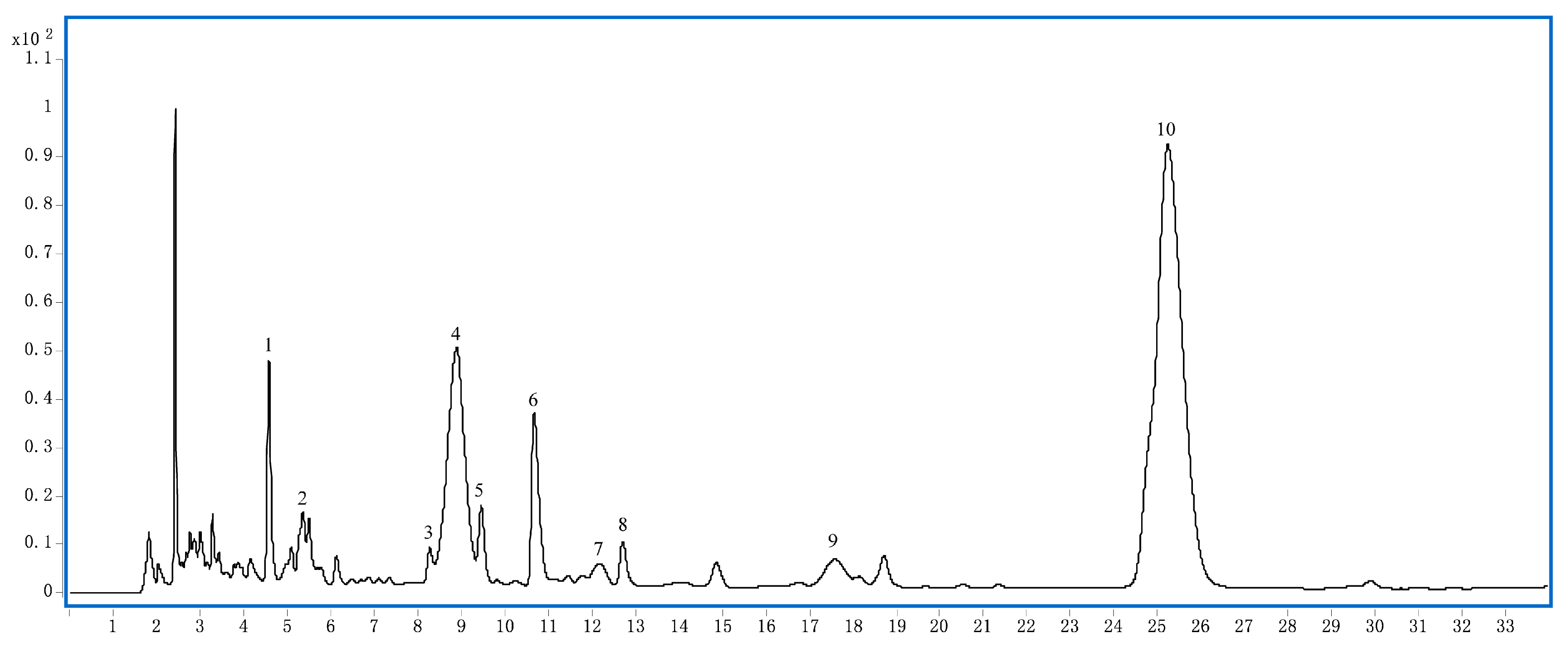


| No. | Compound | tR(min) | [M + H]+ | MS/MS [m/z] |
|---|---|---|---|---|
| 1 | Lycibarbar spermidine B&D | 4.8 | 634.2930 | 163/220/310/ 382/472/634/ |
| 2 | Delphinidin-3-O-rutinoside(glucosyl-trans-p-coumaroyl)-5-O-glucoside | 5.4 | 1081.2907 | 303/465/919/ 1081 |
| 3 | N1, N10-dihydrocaffeoyl spermidine | 8.3 | 474.2595 | 165/222/474 |
| 4 | Petunidin-3-O-rutinoside(glucosyl-cis-p-coumaroyl)-5-O-glucoside | 8.8 | 1095.3116 | 317/479/933/ 1095 |
| 5 | N1-trans-Caffeoyl, N10-dihydrocaffeoyl spermidine | 9.5 | 472.2434 | 163/220/310/ 472 |
| 6 | N1-Dihydrocaffeoyl, N10-trans-caffeoyl -spermidine | 10.7 | 472.2436 | 163/222/293 472 |
| 7 | Petunidin-3-O-rutinoside(glucosyl-trans-p-coumaroyl)-5-O-glucoside | 12.1 | 1095.3112 | 317/479/933/ 1095 |
| 8 | N1, N10-dicaffeoyl-spermidine | 12.7 | 470.2267 | 163/220/308/ 470 |
| 9 | Delphinidin-3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | 17.6 | 919.2449 | 303/465/757/ 919 |
| 10 | Petunidin-3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | 25.2 | 933.2616 | 317/479/771/ 933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Liu, L.; Shi, T.; Yin, M.; Feng, X.; Shan, Y. The Ethanolic Extract of Lycium ruthenicum Ameliorates Age-Related Physiological Damage in Mice. Molecules 2023, 28, 7615. https://doi.org/10.3390/molecules28227615
Cui B, Liu L, Shi T, Yin M, Feng X, Shan Y. The Ethanolic Extract of Lycium ruthenicum Ameliorates Age-Related Physiological Damage in Mice. Molecules. 2023; 28(22):7615. https://doi.org/10.3390/molecules28227615
Chicago/Turabian StyleCui, Boya, Lanying Liu, Tao Shi, Min Yin, Xu Feng, and Yu Shan. 2023. "The Ethanolic Extract of Lycium ruthenicum Ameliorates Age-Related Physiological Damage in Mice" Molecules 28, no. 22: 7615. https://doi.org/10.3390/molecules28227615
APA StyleCui, B., Liu, L., Shi, T., Yin, M., Feng, X., & Shan, Y. (2023). The Ethanolic Extract of Lycium ruthenicum Ameliorates Age-Related Physiological Damage in Mice. Molecules, 28(22), 7615. https://doi.org/10.3390/molecules28227615