The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-κB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Aβ-Exposed THP-1 Cells
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterization of Cinnamon/Curcumin/Turmeric Spice Blend (CCSB)
2.2. CCSB Possesses Antioxidant Properties
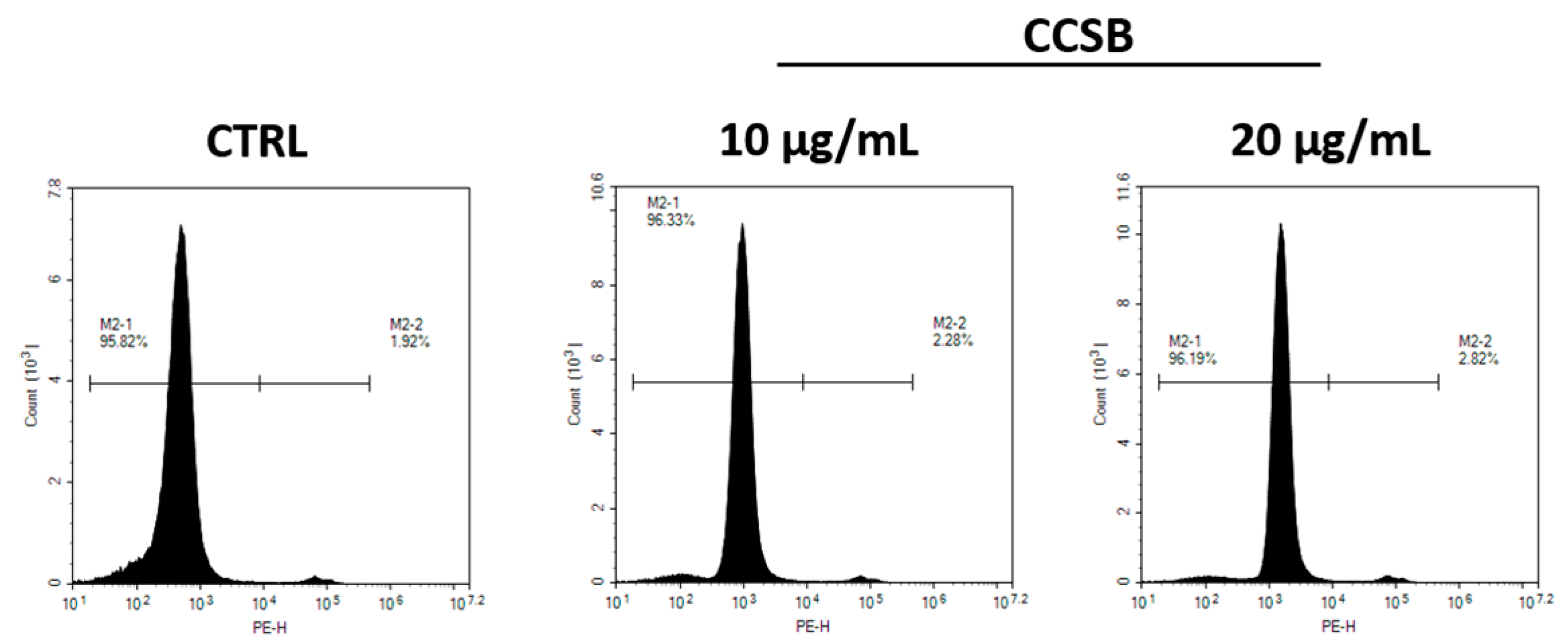
2.3. CCSB Does Not Exert Cytotoxic Effects in THP-1 Cells
2.4. CCSB Counteracts ROS Production in Aβ-Stressed THP-1 Cells
2.5. CCSB Hinders the Release of Pro-Inflammatory Cytokines in Aβ-Stressed THP-1 Cells
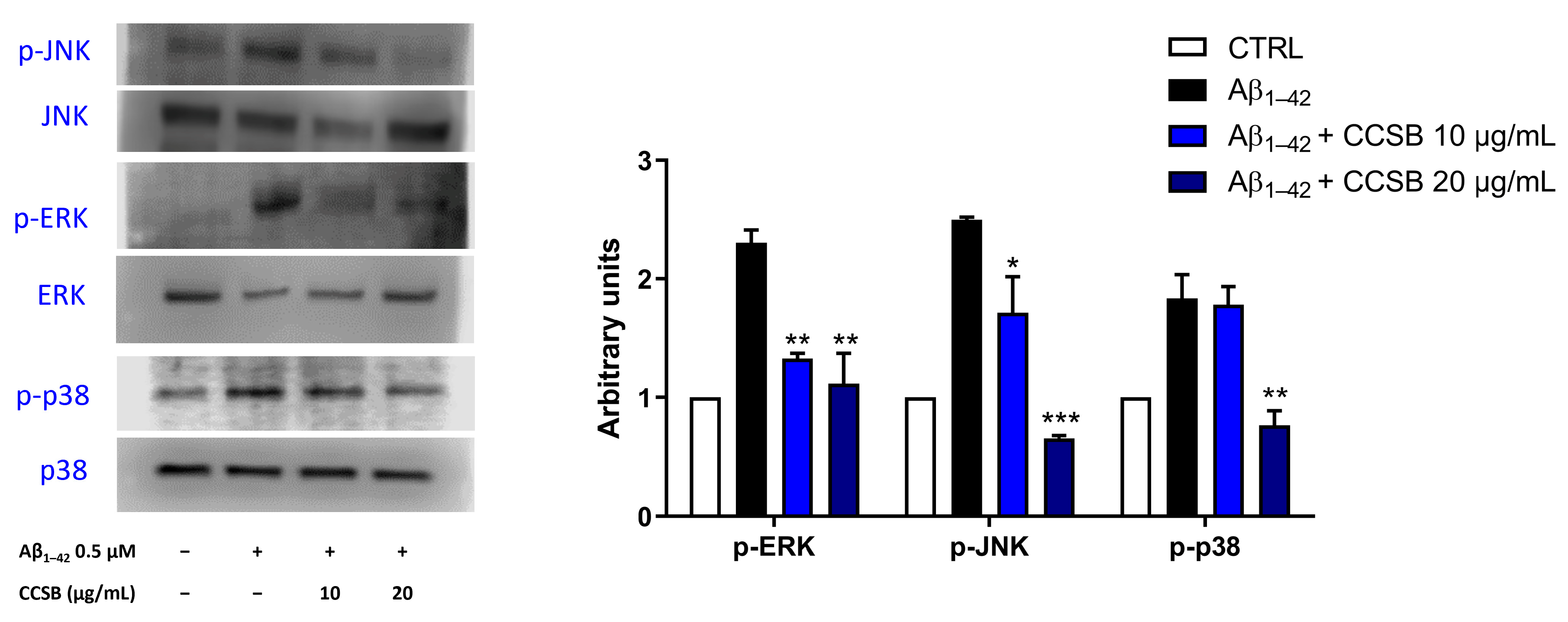
2.6. CCSB Inhibits Mitogen-Activated Protein Kinases (MAPKs) Phosphorylation in Aβ-Stressed THP-1 Cells
2.7. CCSB Counteracts NF-κB Activation in Aβ-Stressed THP-1 Cells
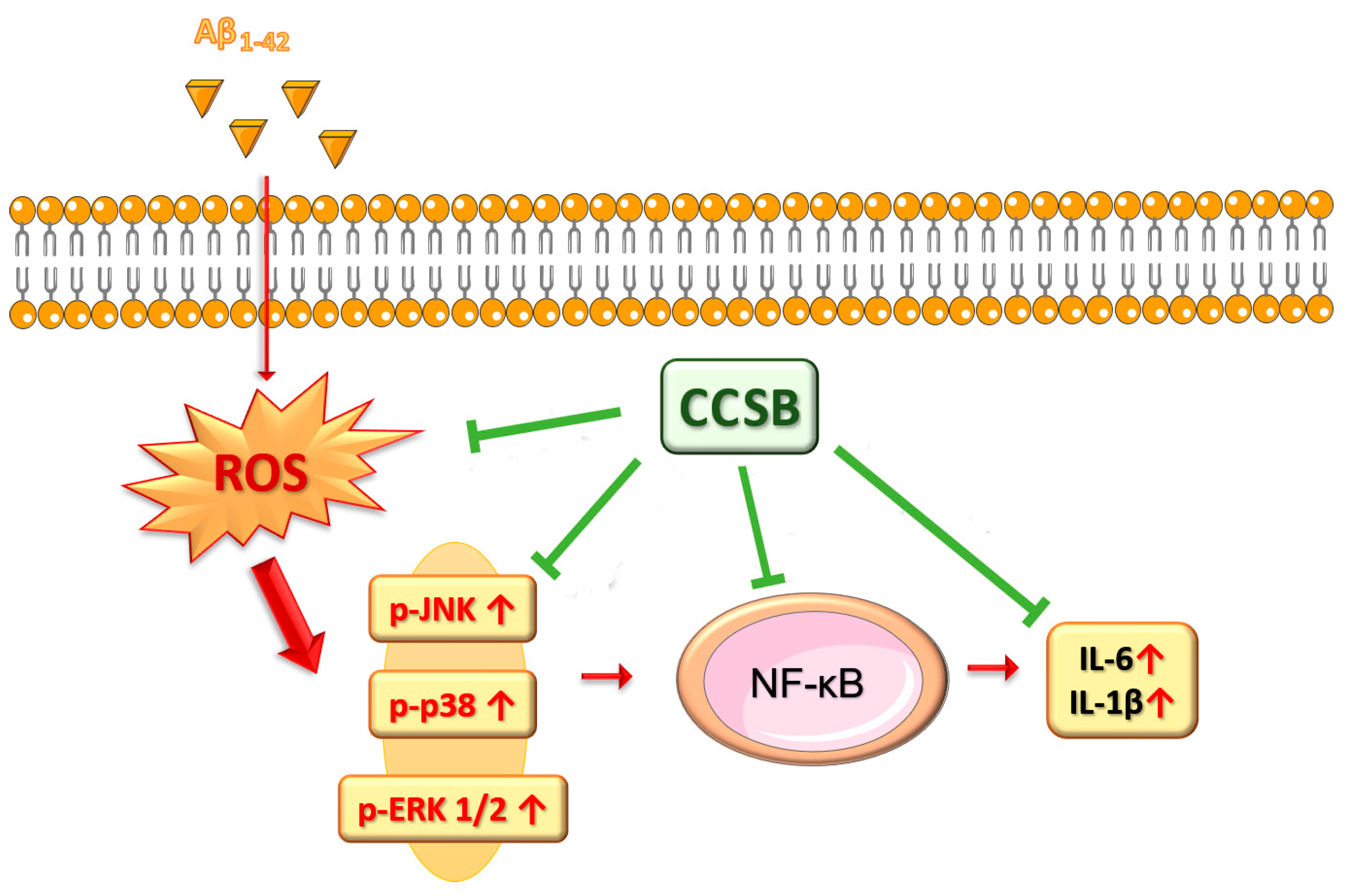
3. Discussion
4. Materials and Methods
4.1. Chemical Characterization
4.1.1. Reagents and Standard Solutions
4.1.2. Preparation of Methanol Extract
4.1.3. RP-DAD-HPLC Separation and Identification
4.1.4. Standard Solutions
4.1.5. Calibration and Linearity
4.2. Cell-Free Antioxidant Capacity Evaluation
4.2.1. Folin–Ciocalteu Method
4.2.2. Quenching of the Stable 2,2-Diphenylpicrylhydrazyl (DPPH) Radical
4.2.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
4.2.4. Reducing Power
4.3. Cell (In Vitro) Studies
4.3.1. Cell Culture
4.3.2. Cytotoxicity Evaluation of CCSB
4.3.3. Determination of the Reactive Oxygen Species (ROS)
4.3.4. Evaluation of Cytokine Secretion and Expression
4.3.5. Protein Expression Studies
4.3.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Balon, K.; Wiatrak, B. PC12 and THP-1 Cell Lines as Neuronal and Microglia Model in Neurobiological Research. Appl. Sci. 2021, 11, 3729. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, K.L.C.; de Aquino, T.M.; da Silva-Junior, E.F. Natural Compounds as Inhibitors of Abeta Peptide and Tau Aggregation. CNS Neurol. Disord. Drug Targets 2023. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Shao, R.; Xiao, J. Natural Products for Treatment of Alzheimer’s Disease and Related Diseases: Understanding their Mechanism of Action. Curr. Neuropharmacol. 2013, 11, 337. [Google Scholar] [CrossRef]
- Wu, W.; Huang, J.; Han, P.; Zhang, J.; Wang, Y.; Jin, F.; Zhou, Y. Research Progress on Natural Plant Molecules in Regulating the Blood–Brain Barrier in Alzheimer’s Disease. Molecules 2023, 28, 7631. [Google Scholar]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Ali, A.; Saqib, F.; Aleem, A.; Bibi, S.; Afzal, K.; Ali, A.; Baig, A.; Khan, S.A.; Bin Asad, M.H.H. Pharmacological exploration of traditional plants for the treatment of neurodegenerative disorders. Phytother Res. 2020, 34, 3089–3112. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. The Association of Palmitoylethanolamide with Luteolin Decreases Neuroinflammation and Stimulates Autophagy in Parkinson’s Disease Model. CNS Neurol. Disord. Drug Targets 2015, 14, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Munch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed]
- Frydman-Marom, A.; Levin, A.; Farfara, D.; Benromano, T.; Scherzer-Attali, R.; Peled, S.; Vassar, R.; Segal, D.; Gazit, E.; Frenkel, D.; et al. Orally administrated cinnamon extract reduces beta-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS ONE 2011, 6, e16564. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Hassani, S.; Khan, F.; Ziaee, M.; Abdollahi, M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018, 130, 241–258. [Google Scholar] [CrossRef]
- Young, H.; Clayton, D.; Masis, N.; Gaylor, C.; Benton, D. Effects of a cinnamon, curcumin/turmeric dietary supplement on glucose, lipid, and cognitive measures. Curr. Dev. Nutr. 2020, 4, 493. [Google Scholar] [CrossRef]
- Lee, M.S.; Wahlqvist, M.L.; Chou, Y.C.; Fang, W.H.; Lee, J.T.; Kuan, J.C.; Liu, H.Y.; Lu, T.M.; Xiu, L.; Hsu, C.C.; et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014, 23, 581–591. [Google Scholar] [CrossRef]
- Wahlqvist, M.L.; Lee, M.S.; Lee, J.T.; Hsu, C.C.; Chou, Y.C.; Fang, W.H.; Liu, H.Y.; Xiu, L.; Andrews, Z.B. Cinnamon users with prediabetes have a better fasting working memory: A cross-sectional function study. Nutr. Res. 2016, 36, 305–310. [Google Scholar] [CrossRef]
- Nakhaee, S.; Kooshki, A.; Hormozi, A.; Akbari, A.; Mehrpour, O.; Farrokhfall, K. Cinnamon and cognitive function: A systematic review of preclinical and clinical studies. Nutr. Neurosci. 2023, 1–15. [Google Scholar] [CrossRef]
- Voulgaropoulou, S.D.; van Amelsvoort, T.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- van Galen, E.; Kroes, B.; Llorente, G. Assessment Report on Curcuma longa L., Rhizome; European Medicines Agency: Amsterdam, The Netherlands, 2018.
- Zachariah, T.J.; Leela, N.K. 11—Volatiles from herbs and spices. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Sawston, UK, 2006; pp. 177–218. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and Combined Antioxidant Activity of Spices and Spice Phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Curro, M.; Risitano, R.; Ferlazzo, N.; Cirmi, S.; Gangemi, C.; Caccamo, D.; Ientile, R.; Navarra, M. Citrus bergamia Juice Extract Attenuates beta-Amyloid-Induced Pro-Inflammatory Activation of THP-1 Cells Through MAPK and AP-1 Pathways. Sci. Rep. 2016, 6, 20809. [Google Scholar] [CrossRef] [PubMed]
- Grzanna, R.; Phan, P.; Polotsky, A.; Lindmark, L.; Frondoza, C.G. Ginger extract inhibits beta-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes. J. Altern. Complement. Med. 2004, 10, 1009–1013. [Google Scholar] [CrossRef]
- Brown, G.C.; Vilalta, A. How microglia kill neurons. Brain Res. 2015, 1628, 288–297. [Google Scholar] [CrossRef]
- Giri, R.K.; Selvaraj, S.K.; Kalra, V.K. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J. Immunol. 2003, 170, 5281–5294. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Tremblay, M.E.; Petratos, S.; Zoupi, L.; Boziki, M.; Kesidou, E.; Simeonidou, C.; Theotokis, P. Origin and Emergence of Microglia in the CNS-An Interesting (Hi)story of an Eccentric Cell. Curr. Issues Mol. Biol. 2023, 45, 2609–2628. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Forloni, G.; Balducci, C. Alzheimer’s Disease, Oligomers, and Inflammation. J. Alzheimers Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, S.; Maurya, N.S.; Kushwaha, S.; Scotti, L.; Chawade, A.; Mani, A. Herbal Therapeutics for Alzheimer’s Disease: Ancient Indian Medicine System from the Modern Viewpoint. Curr. Neuropharmacol. 2023, 21, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Venigalla, M.; Raju, R.; Munch, G. Pharmacological considerations for treating neuroinflammation with curcumin in Alzheimer’s disease. J. Neural. Transm. 2022, 129, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Hwang, J.S.; Lee, C.G.; So, J.S.; Sahoo, A.; Im, C.R.; Jeon, W.K.; Ko, B.S.; Lee, S.H.; Park, Z.Y.; et al. Cinnamon extract suppresses experimental colitis through modulation of antigen-presenting cells. World J. Gastroenterol. 2011, 17, 976–986. [Google Scholar] [CrossRef]
- Gravandi, M.M.; Abdian, S.; Tahvilian, M.; Iranpanah, A.; Moradi, S.Z.; Fakhri, S.; Echeverria, J. Therapeutic targeting of Ras/Raf/MAPK pathway by natural products: A systematic and mechanistic approach for neurodegeneration. Phytomedicine 2023, 115, 154821. [Google Scholar] [CrossRef]
- Kheiri, G.; Dolatshahi, M.; Rahmani, F.; Rezaei, N. Role of p38/MAPKs in Alzheimer’s disease: Implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 2018, 30, 9–30. [Google Scholar] [CrossRef]
- Kim, M.E.; Na, J.Y.; Lee, J.S. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol. Immunotoxicol. 2018, 40, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, P.; Zhang, J.; Hong, T. Bisdemethoxycurcumin attenuates OVA-induced food allergy by inhibiting the MAPK and NF-kappaB signaling pathways. Exp. Ther. Med. 2022, 23, 401. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Motolani, A.; Campos, L.; Lu, T. The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8972. [Google Scholar] [CrossRef] [PubMed]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-kappaB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Azelmat, J.; Vaillancourt, K.; Grenier, D. A polyphenolic cinnamon fraction exhibits anti-inflammatory properties in a monocyte/macrophage model. PLoS ONE 2021, 16, e0244805. [Google Scholar] [CrossRef]
- Bai, X.; Oberley-Deegan, R.E.; Bai, A.; Ovrutsky, A.R.; Kinney, W.H.; Weaver, M.; Zhang, G.; Honda, J.R.; Chan, E.D. Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection. Respirology 2016, 21, 951–957. [Google Scholar] [CrossRef]
- Silverberg, G.D.; Mayo, M.; Saul, T.; Rubenstein, E.; McGuire, D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: A hypothesis. Lancet Neurol. 2003, 2, 506–511. [Google Scholar] [CrossRef]
- Gholampour, S.; Bahmani, M.; Shariati, A. Comparing the Efficiency of Two Treatment Methods of Hydrocephalus: Shunt Implantation and Endoscopic Third Ventriculostomy. Basic Clin. Neurosci. 2019, 10, 185–198. [Google Scholar] [CrossRef]
- Karimy, J.K.; Reeves, B.C.; Damisah, E.; Duy, P.Q.; Antwi, P.; David, W.; Wang, K.; Schiff, S.J.; Limbrick, D.D., Jr.; Alper, S.L.; et al. Inflammation in acquired hydrocephalus: Pathogenic mechanisms and therapeutic targets. Nat. Rev. Neurol. 2020, 16, 285–296. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Kokotkiewicz, A.; Miceli, N.; Taviano, M.F.; Maugeri, A.; Cirmi, S.; Synowiec, A.; Gniewosz, M.; Elansary, H.O.; et al. Production of Verbascoside, Isoverbascoside and Phenolic Acids in Callus, Suspension, and Bioreactor Cultures of Verbena officinalis and Biological Properties of Biomass Extracts. Molecules 2020, 25, 5609. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Albergamo, A.; Dugo, G.; Navarra, M.; Cirmi, S. Protective Effects of a Red Grape Juice Extract against Bisphenol A-Induced Toxicity in Human Umbilical Vein Endothelial Cells. Toxics 2023, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Russo, C.; Musumeci, L.; Navarra, M.; Lombardo, G.E. Oleacein Attenuates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages by the Inhibition of TLR4/MyD88/NF-kappaB Pathway. Int. J. Mol. Sci. 2022, 23, 1206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bali, A.; Chaudhari, B.B. Synthesis of methanesulphonamido-benzimidazole derivatives as gastro-sparing antiinflammatory agents with antioxidant effect. Bioorg. Med. Chem. Lett. 2017, 27, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Askari, B.; Rudbari, H.A.; Micale, N.; Schirmeister, T.; Maugeri, A.; Navarra, M. Anticancer study of heterobimetallic platinum(II)-ruthenium(II) and platinum(II)-rhodium(III) complexes with bridging dithiooxamide ligand. J. Organomet. Chem. 2019, 900, 120918. [Google Scholar] [CrossRef]
- Maugeri, A.; Russo, C.; Musumeci, L.; Lombardo, G.E.; De Sarro, G.; Barreca, D.; Cirmi, S.; Navarra, M. The Anticancer Effect of a Flavonoid-Rich Extract of Bergamot Juice in THP-1 Cells Engages the SIRT2/AKT/p53 Pathway. Pharmaceutics 2022, 14, 2168. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Russo, C.; Gangemi, S.; Calapai, G.; Cirmi, S.; Navarra, M. Bergamottin and 5-Geranyloxy-7-methoxycoumarin Cooperate in the Cytotoxic Effect of Citrus bergamia (Bergamot) Essential Oil in Human Neuroblastoma SH-SY5Y Cell Line. Toxins 2021, 13, 275. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa Oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-kappaB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef]
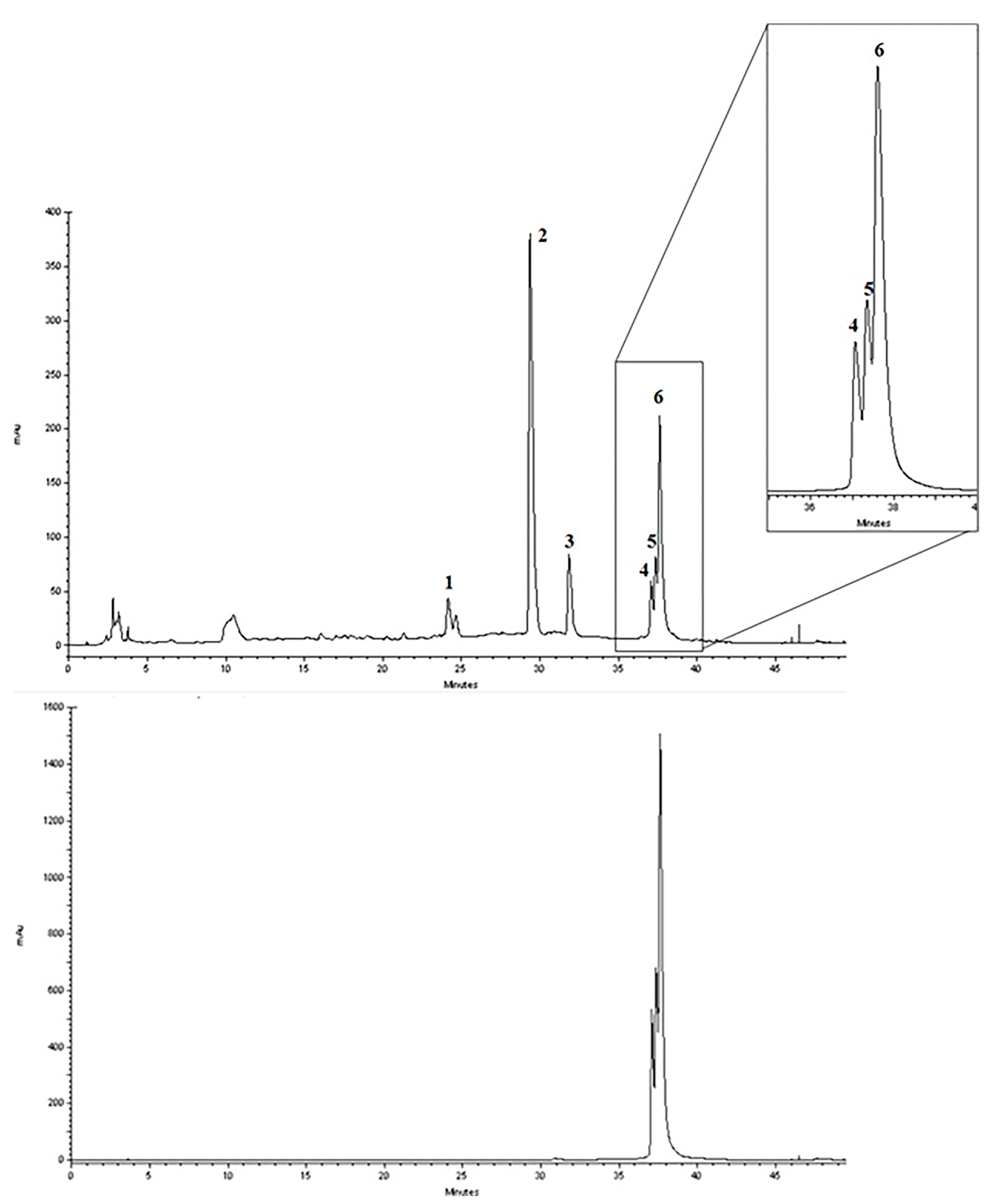




| mg/g | |||
|---|---|---|---|
| Mean | SD | ||
| Coumarin | 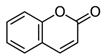 | 0.018 | 0.010 |
| Cinnamaldehyde | 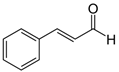 | 0.125 | 0.023 |
| 2-Methoxycinnamaldehyde | 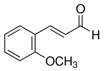 | 0.037 | 0.011 |
| Bisdemethoxycurcumin | 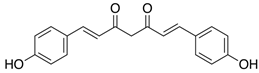 | 8.763 | 1.058 |
| Desmethoxycurcumin | 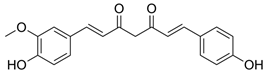 | 19.156 | 1.930 |
| Curcumin |  | 173.022 | 20.091 |
| ORAC (µmol TE/g) | 6787.1 ± 325.1 |
| DPPH (mg TE/g) | 312.1 ± 5.9 |
| Folin–Ciocalteu (mg GAE/g) | 149.2 ± 2.1 |
| Reducing Power (mg AAE/g) | 289.9 ± 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, A.; Russo, C.; Patanè, G.T.; Barreca, D.; Mandalari, G.; Navarra, M. The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-κB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Aβ-Exposed THP-1 Cells. Molecules 2023, 28, 7949. https://doi.org/10.3390/molecules28247949
Maugeri A, Russo C, Patanè GT, Barreca D, Mandalari G, Navarra M. The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-κB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Aβ-Exposed THP-1 Cells. Molecules. 2023; 28(24):7949. https://doi.org/10.3390/molecules28247949
Chicago/Turabian StyleMaugeri, Alessandro, Caterina Russo, Giuseppe Tancredi Patanè, Davide Barreca, Giuseppina Mandalari, and Michele Navarra. 2023. "The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-κB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Aβ-Exposed THP-1 Cells" Molecules 28, no. 24: 7949. https://doi.org/10.3390/molecules28247949
APA StyleMaugeri, A., Russo, C., Patanè, G. T., Barreca, D., Mandalari, G., & Navarra, M. (2023). The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-κB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Aβ-Exposed THP-1 Cells. Molecules, 28(24), 7949. https://doi.org/10.3390/molecules28247949










