Synthesis of a Grease Thickener from Cashew Nut Shell Liquor
Abstract
:1. Introduction
2. Results and Discussion
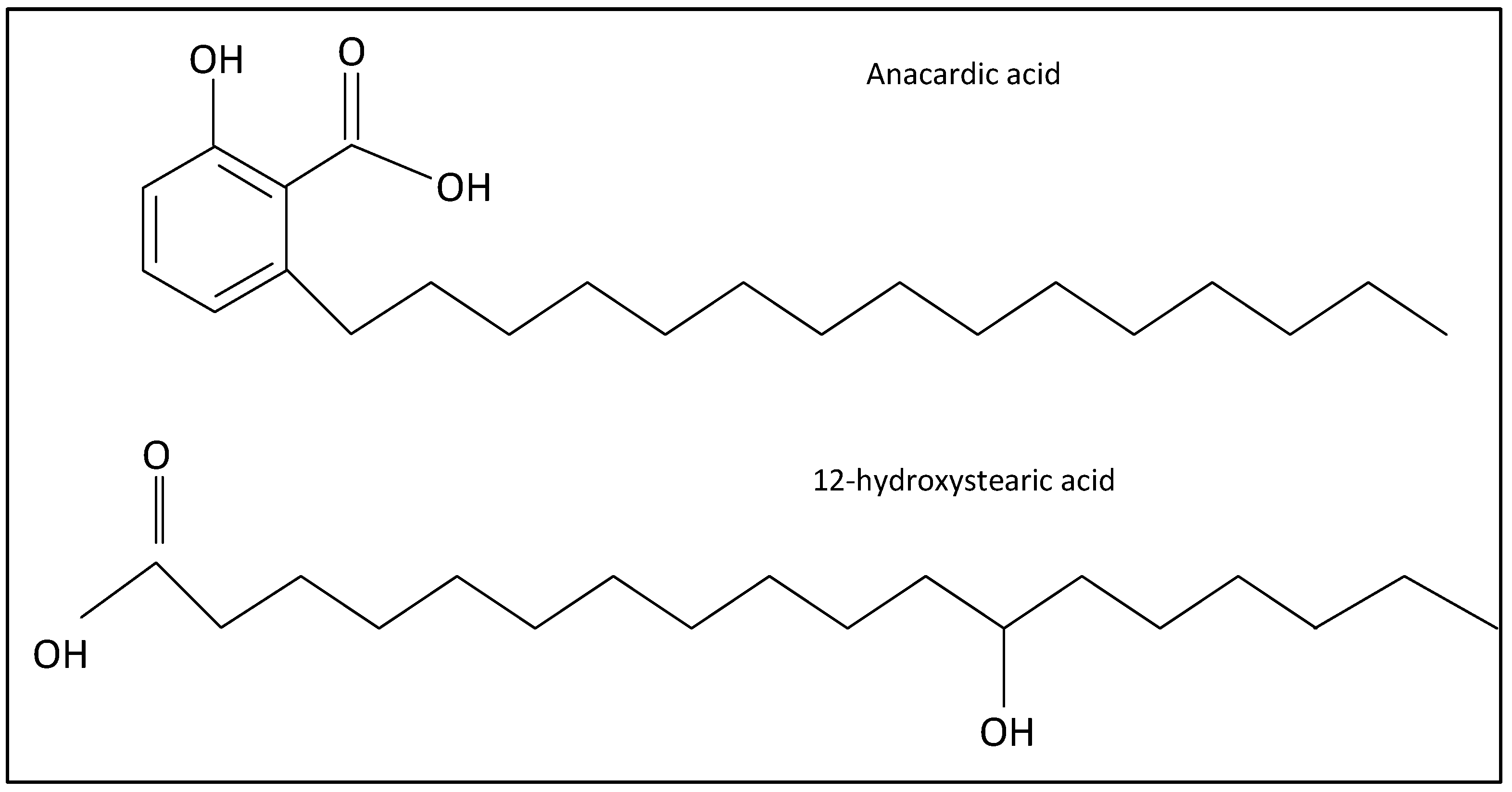
2.1. Separation of Anacardic Acid
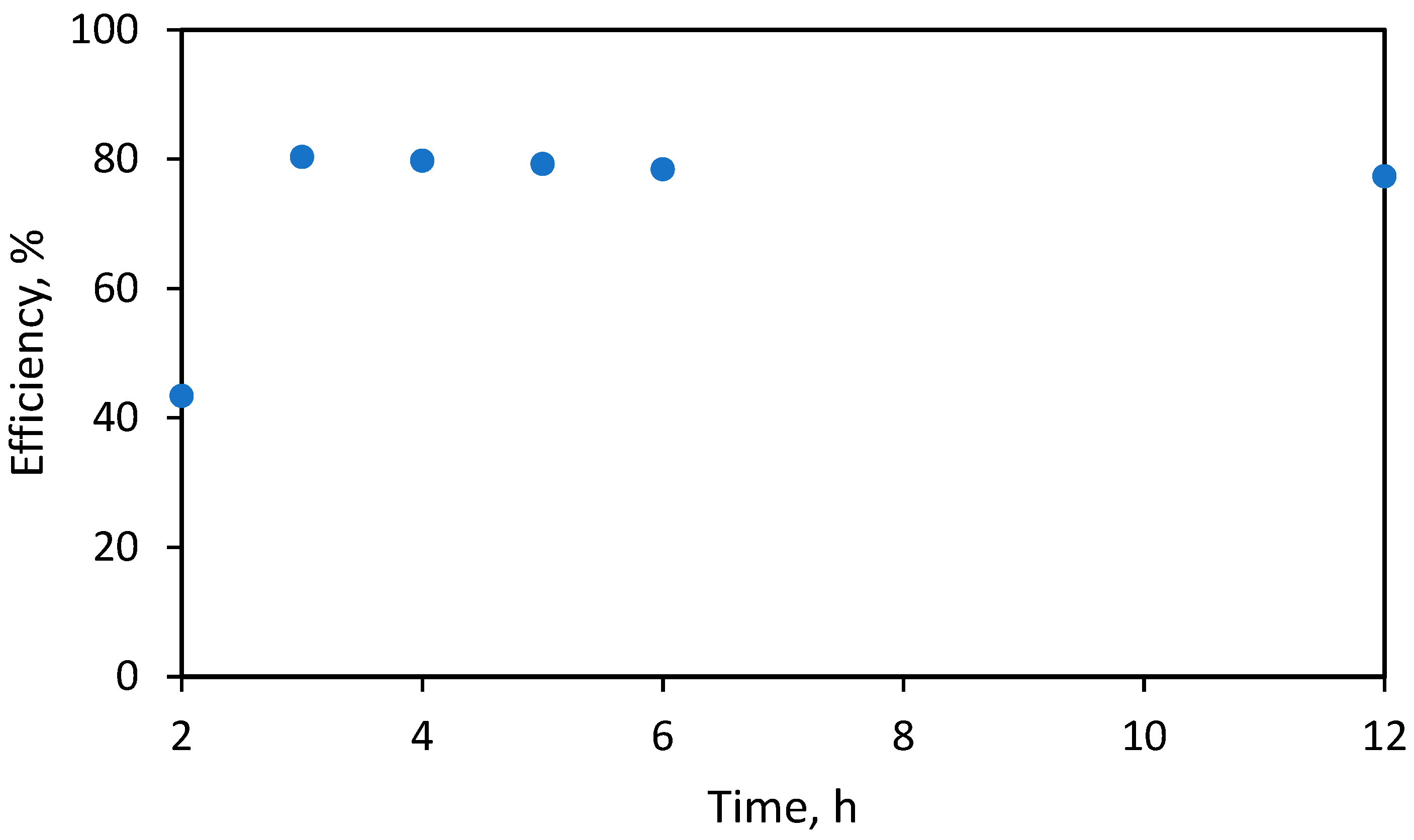
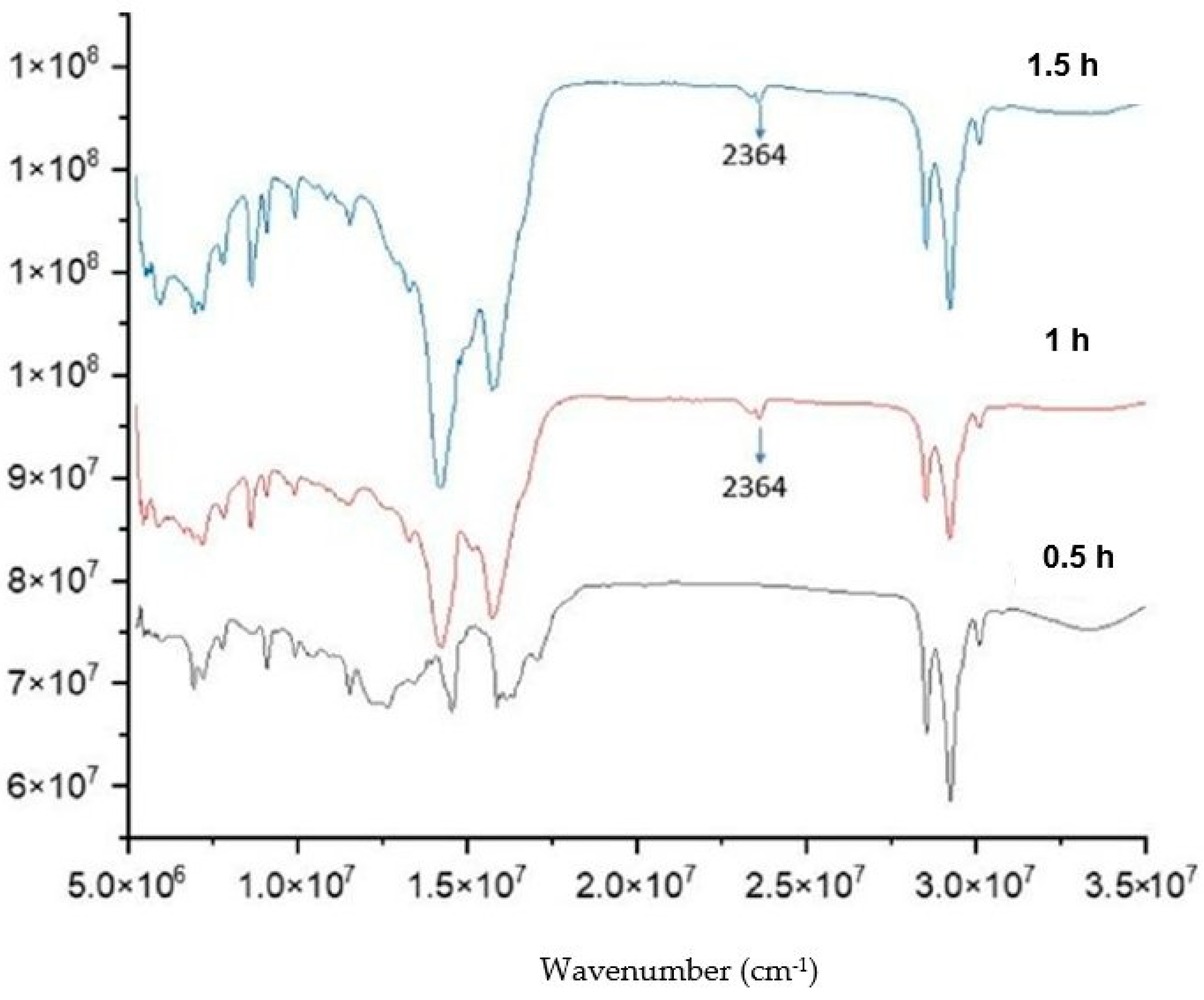
2.2. Synthesis of Lithium Anacardate
3. Experiment
3.1. Materials
3.2. Forming and Extracting Calcium Anacardate from Cashew Nut Shell Liquor
3.3. Forming and Separating Anacardic Acids
3.4. Synthesis of Lithium Anacardate
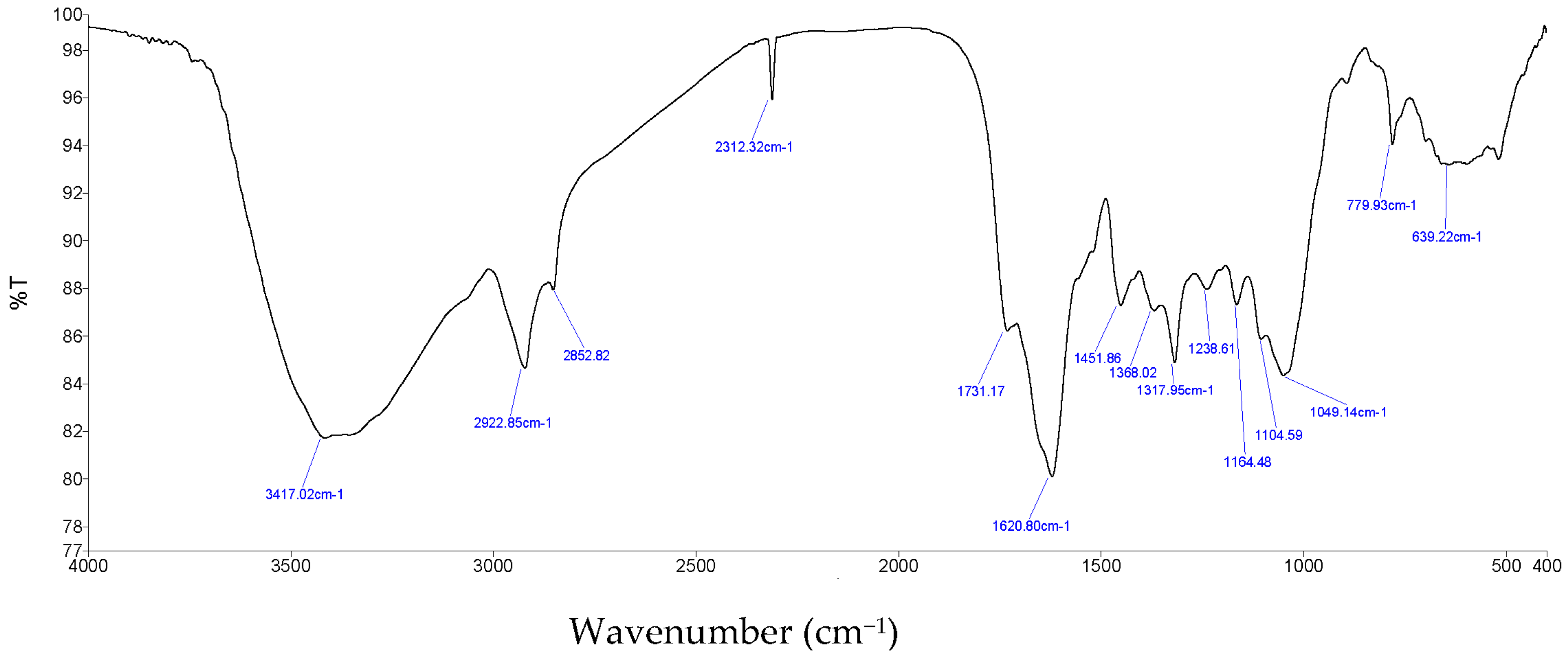
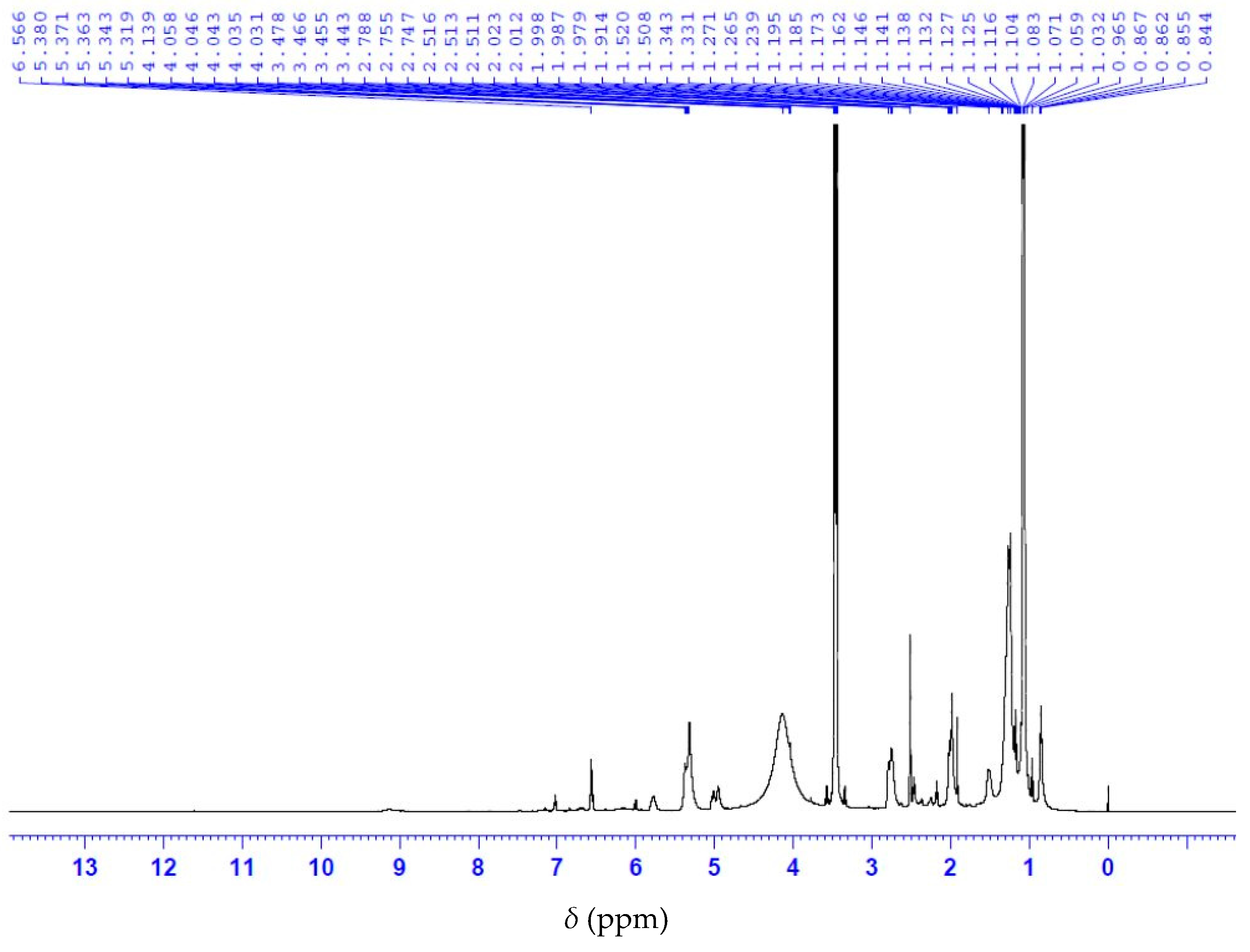
3.5. Characterization
3.6. Grease Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Strategies to Achieve a Carbon Neutral Society: A Review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Donahue, C.J. Lubricating Grease: A Chemical Primer. J. Chem. Educ. 2006, 83, 862–869. [Google Scholar] [CrossRef]
- Pirro, D.M.; Daschner, E. Lubrication Fundamentals; Wessol, A.A., Ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ogunniyi, D.S. Castor Oil: A Vital Industrial Raw Material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.J.; Olsen, C.; Cobbs, G.A.; Stolowich, N.J.; Parrott, M.M. Bioactivity of Anacardic Acid against Colorado Potato Beetle (Leptinotarsa decemlineata) Larvae. J. Agric. Food Chem. 2006, 54, 7522–7529. [Google Scholar] [CrossRef] [PubMed]
- Gedam, P.H.; Sampathkumaran, P.S. Cashew Nut Shell Liquid: Extraction, Chemistry and Applications. Prog. Org. Coat. 1986, 14, 115–157. [Google Scholar] [CrossRef]
- Mgaya, J.; Shombe, G.B.; Masikane, S.C.; Mlowe, S.; Mubofu, E.B.; Revaprasadu, N. Cashew Nut Shell: A Potential Bio-Resource for the Production of Bio-Sourced Chemicals, Materials and Fuels. Green Chem. 2019, 21, 1186–1201. [Google Scholar] [CrossRef]
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional Composition of Raw Fresh Cashew (Anacardium occidentale L.) Kernels from Different Origin. Food Sci. Nutr. 2016, 4, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Trox, J.; Vadivel, V.; Vetter, W.; Stuetz, W.; Scherbaum, V.; Gola, U.; Nohr, D.; Biesalski, H.K. Bioactive Compounds in Cashew Nut (Anacardium occidentale L.) Kernels: Effect of Different Shelling Methods. J. Agric. Food Chem. 2010, 58, 5341–5346. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.; Ghosal, A.; Gupta, A.; Zafar, F.; Kumari, R.; Alam, M.; Nishat, N. Comparative Studies on Coating Materials of Urotropine Modified Furfurylolated-tCNSL and Methylolated-tCNSL Thermoset for Anticorrosive Application: Switching towards a Cleaner Approach. J. Clean. Prod. 2022, 345, 130933. [Google Scholar] [CrossRef]
- Prasannakumar, P.; Sankarannair, S.; Bose, C.; Santhakumari, R.; Jyothi, S.N. Influence of Techniques on Synthesizing Cashew Nut Shell Oil as a Prospective Biolubricant on Its Physicochemical, Tribological, and Thermal Behaviors. J. Clean. Prod. 2023, 401, 136717. [Google Scholar] [CrossRef]
- Hoang, A.S.; Nguyen, H.N.; Quoc Bui, N.; Vu, H.S.; Vo, T.P.; Nguyen, T.V.; Minh Phan, C. Extraction of Gallium from Bayer Liquor Using Extractant Produced from Cashew Nutshell Liquid. Miner. Eng. 2015, 79, 88–93. [Google Scholar] [CrossRef]
- Phan, C.M.; Hoang, S.A.; Vu, S.H.; Nguyen, H.M.; Nguyen, C.V.; Hyde, A.E.; Yusa, S.I. Application of a Cashew-Based Oxime in Extracting Ni, Mn and Co from Aqueous Solution. Chem. Biol. Technol. Agric. 2021, 8, 37. [Google Scholar] [CrossRef]
- Roy, A.; Fajardie, P.; Lepoittevin, B.; Baudoux, J.; Lapinte, V.; Caillol, S.; Briou, B. CNSL, a Promising Building Blocks for Sustainable Molecular Design of Surfactants: A Critical Review. Molecules 2022, 27, 1443. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Phan, C.M.; Hoang, S.A.; Yusa, S. Comparison between Cashew-Based and Petrochemical Hydroxyoximes: Insights from Molecular Simulations. Molecules 2023, 28, 3971. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Atasie, V.N. Chemical Composition and Physicochemical Properties Of Cashew Nut (Anacardium occidentale) Oil and Cashew Nut Shell Liquid. J. Agric. Food Environ. Sci. 2008, 2, 1–10. [Google Scholar]
- Yuliana, M.; Tran-Thi, N.Y.; Ju, Y.H. Effect of Extraction Methods on Characteristic and Composition of Indonesian Cashew Nut Shell Liquid. Ind. Crops Prod. 2012, 35, 230–236. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sebastin Santhosh, M.; Kemparaju, K.; Girish, K.S. Emerging Roles of Anacardic Acid and Its Derivatives: A Pharmacological Overview: Pharmacological Roles Of AA And Its Derivatives. Basic Clin. Pharmacol. Toxicol. 2012, 110, 122–132. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Antibacterial Agents from the Cashew Anacardium occidentale (Anacardiaceae) Nut Shell Oil. J. Agric. Food Chem. 1991, 39, 418–421. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dhanurdhar; Rokhum, S.L. Extraction of a Cardanol Based Liquid Bio-Fuel from Waste Natural Resource and Decarboxylation Using a Silver-Based Catalyst. Renew. Sustain. Energy Rev. 2017, 72, 560–564. [Google Scholar] [CrossRef]
- Grüter, R.; Trachsel, T.; Laube, P.; Jaisli, I. Expected Global Suitability of Coffee, Cashew and Avocado Due to Climate Change. PLoS ONE 2022, 17, e0261976. [Google Scholar] [CrossRef]
- Patel, R.N.; Bandyopadhyay, S.; Ganesh, A. Extraction of Cashew (Anacardium occidentale) Nut Shell Liquid Using Supercritical Carbon Dioxide. Bioresour. Technol. 2006, 97, 847–853. [Google Scholar] [CrossRef]
- Philip, J.Y.N.; Da Cruz Francisco, J.; Dey, E.S.; Buchweishaija, J.; Mkayula, L.L.; Ye, L. Isolation of Anacardic Acid from Natural Cashew Nut Shell Liquid (CNSL) Using Supercritical Carbon Dioxide. J. Agric. Food Chem. 2008, 56, 9350–9354. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Bezerra, T.; Oliveira De Almeida, M.; Maria De Amorim Lima, N.; Lúcia De Castro Rodrigues, N.; Gomes Pereira Ribeiro, V.; Jania Teixeira, M.; Carbone, L.; Mele, G.; Lomonaco, D.; Elaine Mazzetto, S. In Vitro Antileishmanial Activity of Sustainable Anacardic Acid and Cardol Based Silver Nanoparticles on L. Braziliensis. Int. J. Pharm. 2022, 619, 121698. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Tarun Kumar, V.; Krishna, B.B.; Bhaskar, T. Characterization of Slow Pyrolysis Products from Three Different Cashew Wastes. Bioresour. Technol. 2023, 376, 128859. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Barreto, A.C.H.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Sousa, E.M.B.; Fechine, P.B.A. Magnetic Nanoparticles Coated with Anacardic Acid Derived from Cashew Nut Shell Liquid. J. Mater. Sci. 2013, 48, 7875–7882. [Google Scholar] [CrossRef]
- Rush, R.E. A Review of the More Common Standard Grease Tests in Use Today. Lubr. Eng. 1997, 53, 17. [Google Scholar]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of Spent Lithium-Ion Batteries in View of Lithium Recovery: A Critical Review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Grageda, M.; Gonzalez, A.; Quispe, A.; Ushak, S. Analysis of a Process for Producing Battery Grade Lithium Hydroxide by Membrane Electrodialysis. Membranes 2020, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Gaitán-Jiménez, S.-Y.; Restrepo-Sánchez, L.-P.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Cashew (Anacardium occidentale) Nut-Shell Liquid as Antioxidant in Bulk Soybean Oil. Molecules 2022, 27, 8733. [Google Scholar] [CrossRef]
- Ranarijaona, M.M.; Rambala Rakotomena, N.A.H.; Andrianjafy, M.T.; Ramiharimanana, F.D.; Herinirina, L.C.; Ramarosandratana, N.H.; Briou, B.; Fajardie, P.; Mavingui, P.; Métay, E.; et al. Development of Sustainable Chemistry in Madagascar: Example of the Valuation of CNSL and the Use of Chromones as an Attractant for Mosquitoes. Molecules 2021, 26, 7625. [Google Scholar] [CrossRef] [PubMed]
- Selvamuthukumar, M.; Harish Babu, B.; Bobba, S.; Baskar, S.; Joy, N. Investigation on the Lubricating Behavior of Cashew Nut Shell Liquid Oil as a Renewable and Reliable Petrochemical Product. Mater. Today Proc. 2021, 44, 3583–3588. [Google Scholar] [CrossRef]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel Method for Isolation of Major Phenolic Constituents from Cashew (Anacardium occidentale L.) Nut Shell Liquid. J. Agric. Food Chem. 2001, 49, 2548–2551. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, S.A.; Pham, K.D.; Nguyen, N.H.; Tran, H.T.; Hoang, N.; Phan, C.M. Synthesis of a Grease Thickener from Cashew Nut Shell Liquor. Molecules 2023, 28, 7624. https://doi.org/10.3390/molecules28227624
Hoang SA, Pham KD, Nguyen NH, Tran HT, Hoang N, Phan CM. Synthesis of a Grease Thickener from Cashew Nut Shell Liquor. Molecules. 2023; 28(22):7624. https://doi.org/10.3390/molecules28227624
Chicago/Turabian StyleHoang, Son A., Khanh D. Pham, Nhung H. Nguyen, Ha T. Tran, Ngoc Hoang, and Chi M. Phan. 2023. "Synthesis of a Grease Thickener from Cashew Nut Shell Liquor" Molecules 28, no. 22: 7624. https://doi.org/10.3390/molecules28227624






