A Ratiometric Fluorescent Probe for N2H4 Having a Large Detection Range Based upon Coumarin with Multiple Applications
Abstract
:1. Introduction
2. Results and Discussion
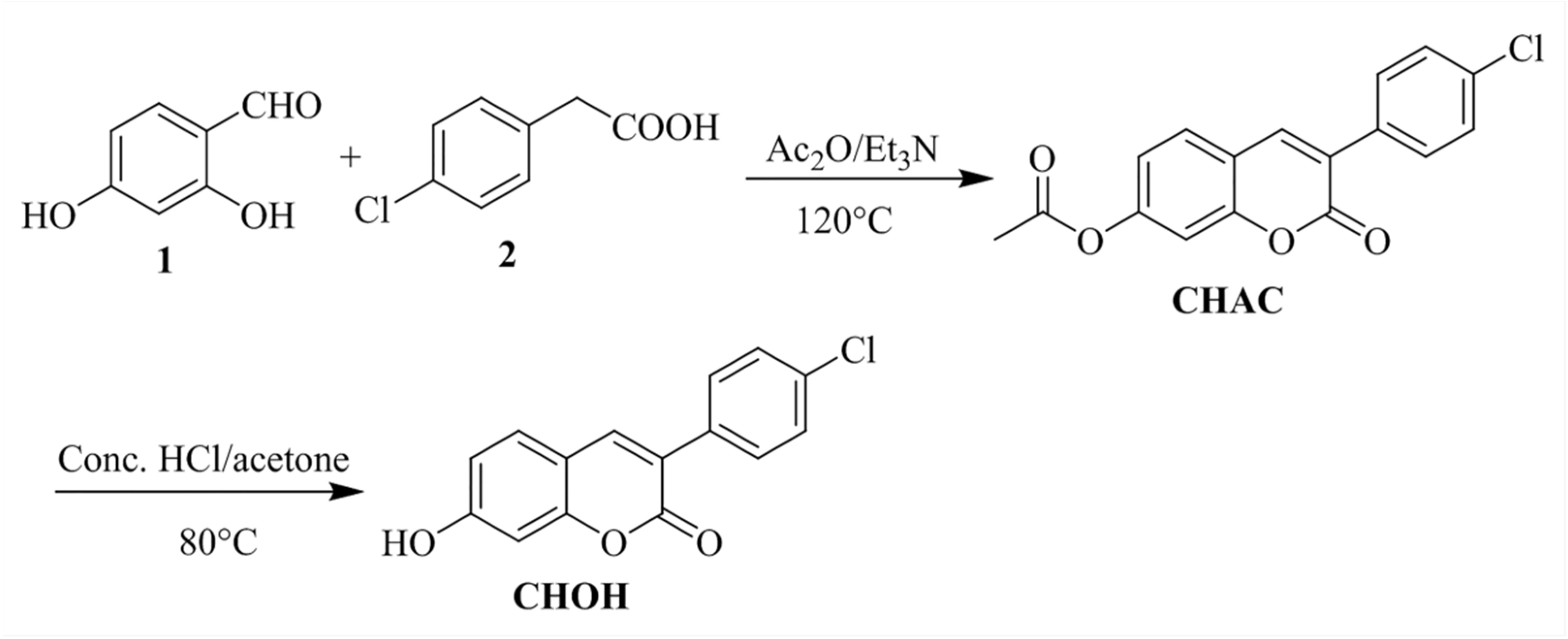
2.1. Design and Synthesis of the Probe CHAC
2.2. Optical Properties
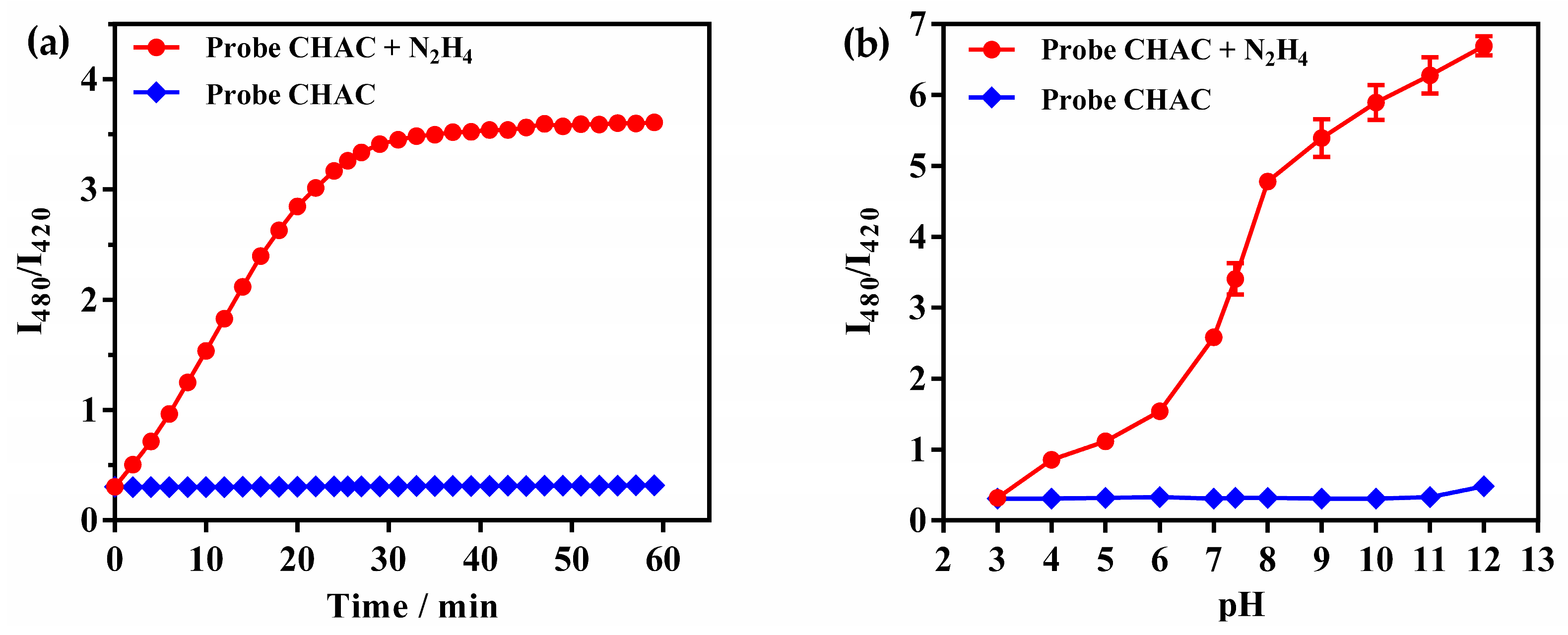
2.3. Reaction Time and pH Effects
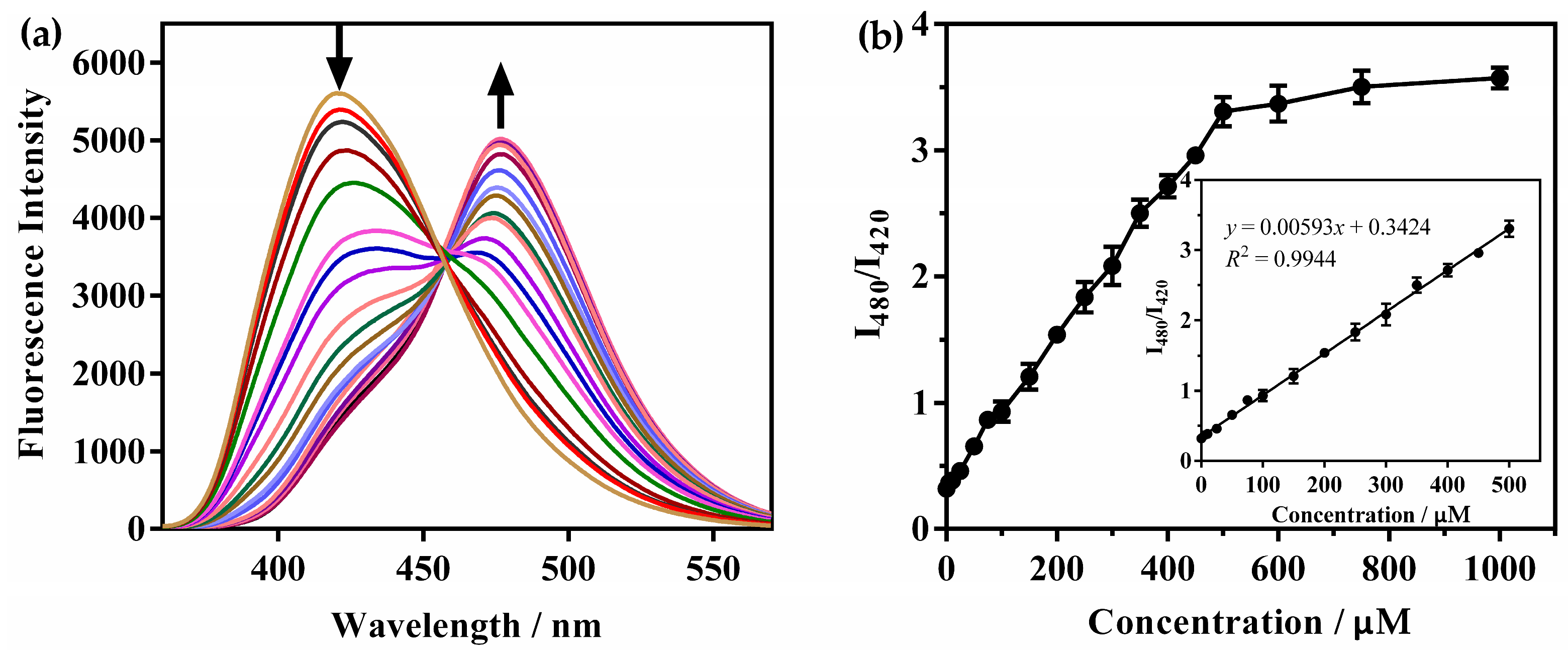
2.4. Fluorescence Spectra (FS) of Probes Titrated with N2H4
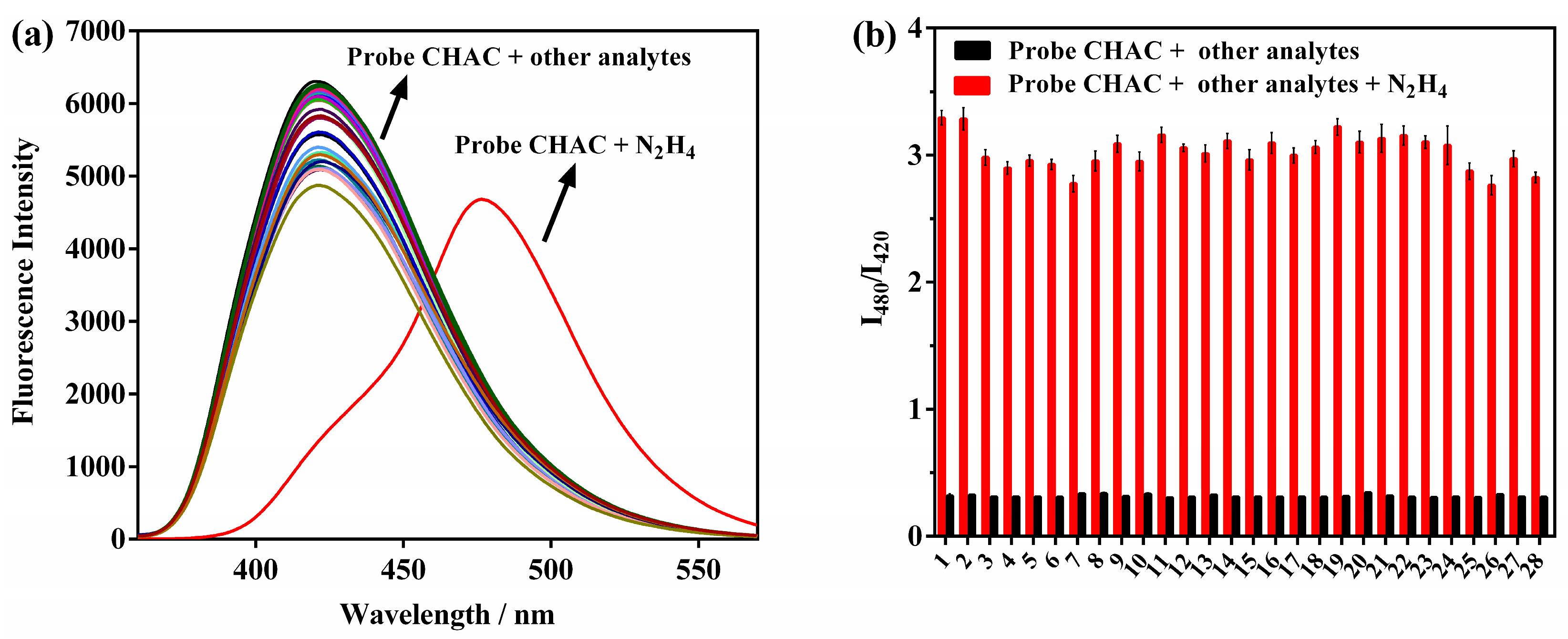
2.5. CHAC Selectivity and Anti-Interference Performance
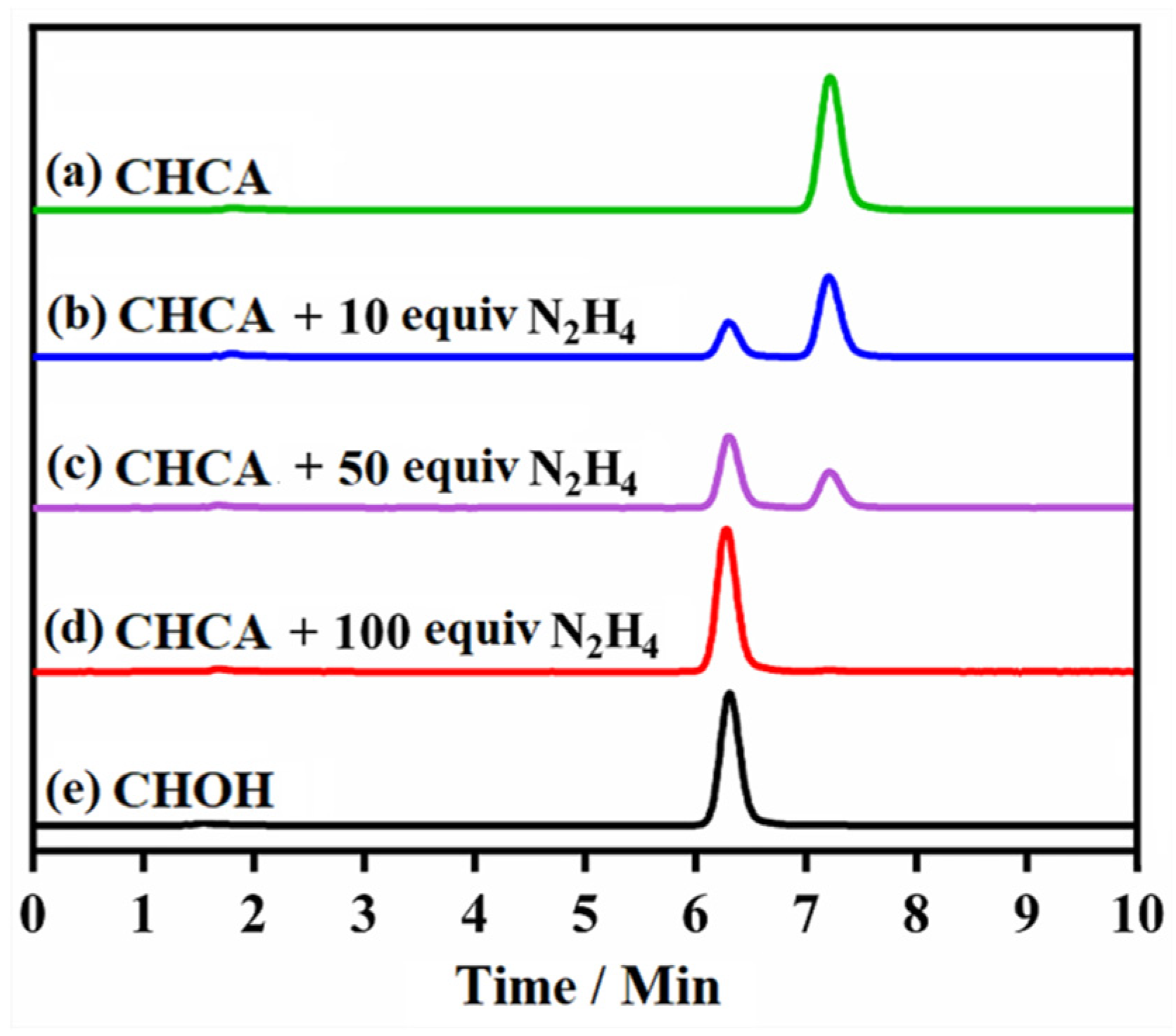
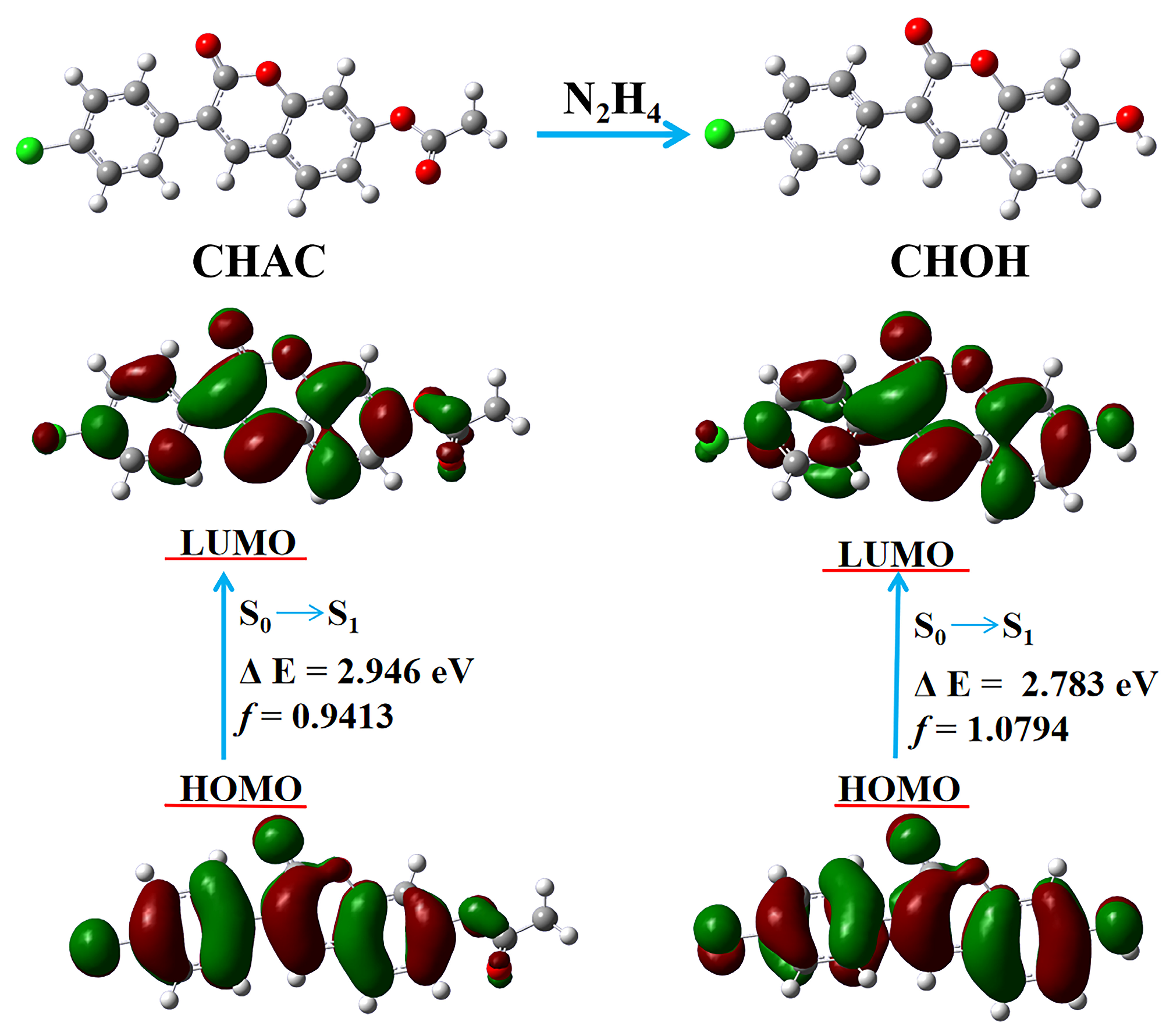
2.6. The Proposed Detection Mechanism
2.7. N2H4 Determinations for Water Samples
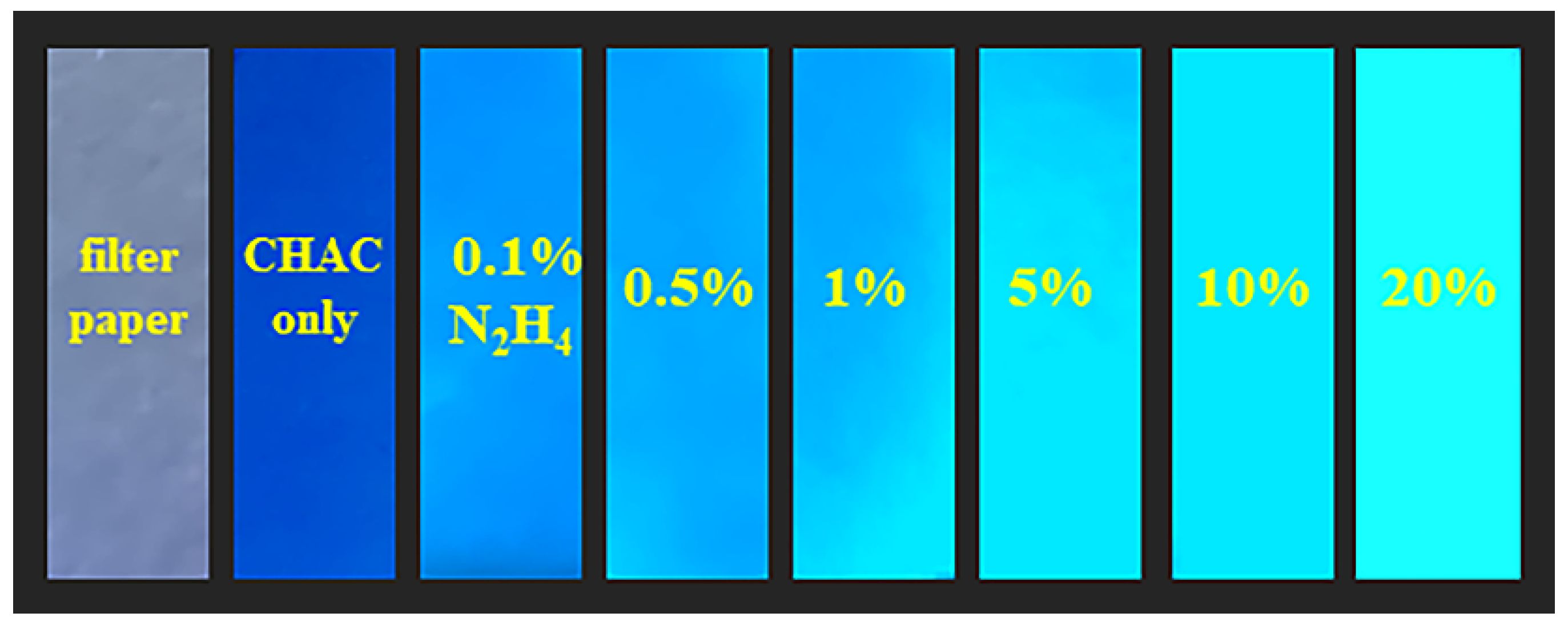
2.8. Gaseous N2H4 Detections
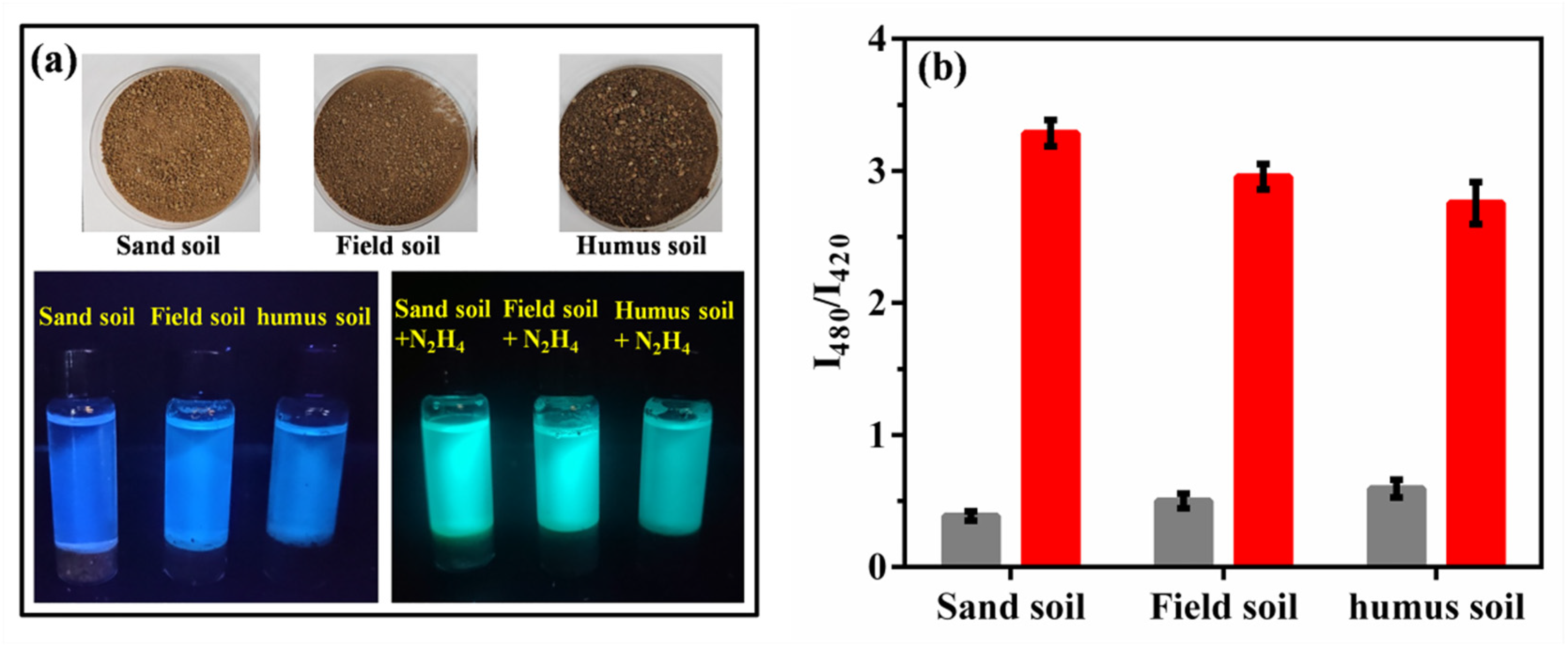
2.9. N2H4 Determinations in Soils
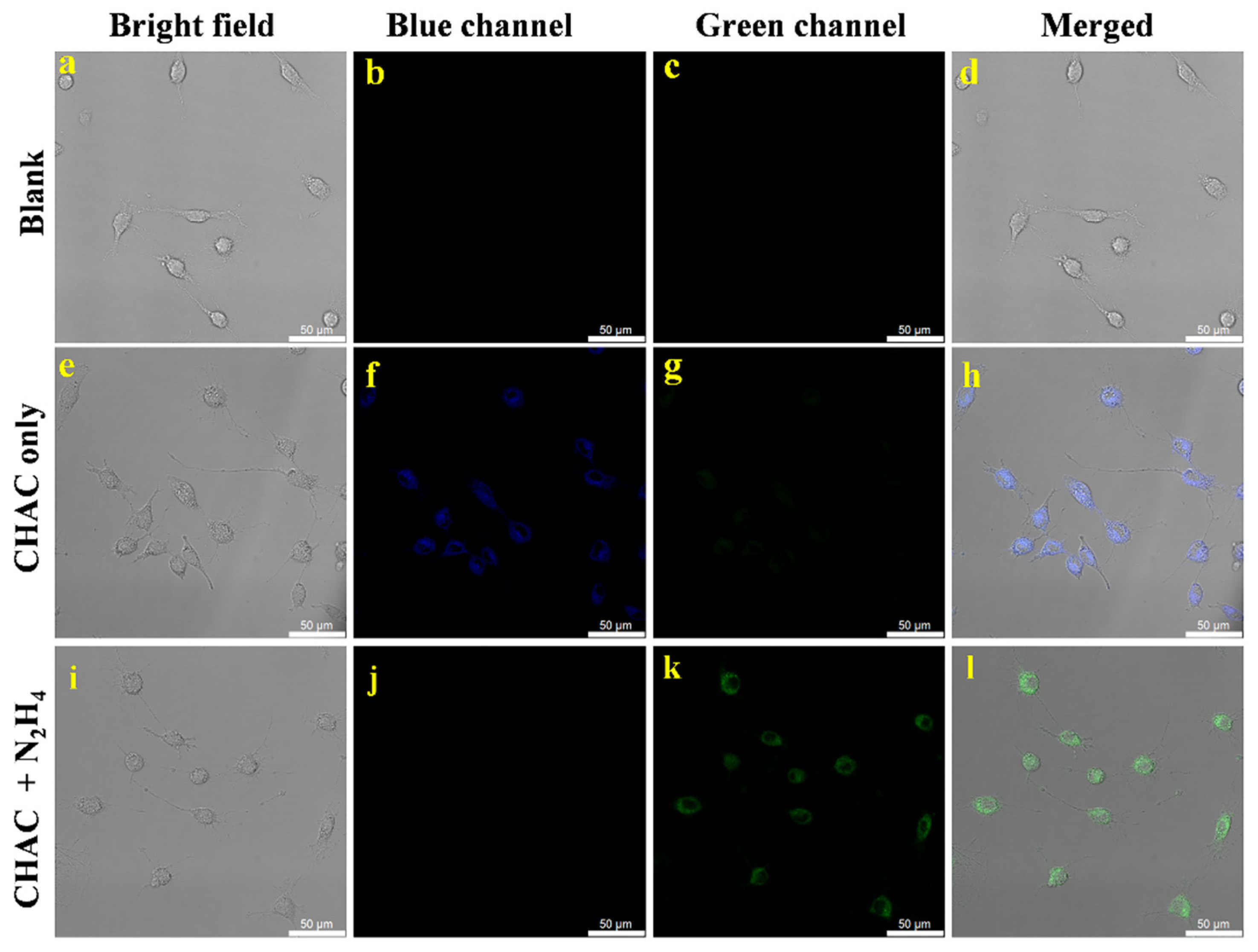
2.10. Toxicity of Probe CHAC and Imaging of Cells
3. Materials and Methods
3.1. Materials and Apparatus
3.2. Synthesis of the Probe CHAC
3.3. Synthesis of the Fluorophore CHOH
3.4. Preparations for Spectral Measurement
3.5. Determination of Quantum Yield (QY)
3.6. HPLC Detection
3.7. Detection of N2H4 in Water Samples
3.8. Cytotoxicity Test
3.9. Living Cell Fluorescence Imaging (FI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Khaled, K.F. Experimental and theoretical study for corrosion inhibition of mild steel in hydrochloric acid solution by some new hydrazine carbodithioic acid derivatives. Appl. Surf. Sci. 2006, 252, 4120–4128. [Google Scholar] [CrossRef]
- Rosca, V.; Koper, M.T.M. Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim. Acta 2008, 53, 5199–5205. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Direct hydrazine fuel cells: A review. Appl. Catal. B Environ. 2010, 98, 1–9. [Google Scholar] [CrossRef]
- Toth, B. Synthetic and naturally occurring hydrazines as possible cancer causative agents. Cancer Res. 1975, 35, 3693–3697. [Google Scholar]
- Garrod, S.; Bollard, M.E.; Nicholls, A.W.; Connor, S.C.; Connelly, J.; Nicholson, J.K.; Holmes, E. Integrated metabonomic analysis of the multiorgan effects of hydrazine toxicity in the rat. Chem. Res. Toxicol. 2005, 18, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Aust, S.D. Peroxidase substrates stimulate the oxidation of hydralazine to metabolites which cause single-strand breaks in DNA. Chem. Res. Toxicol. 1997, 10, 328–334. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Integrated Risk Information System (IRIS) on Hydrazine/hydrazine Sulfate; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999.
- Oh, J.A.; Shin, H.S. Simple and sensitive determination of hydrazine in drinking water by ultra-high-performance liquid chromatography-tandem mass spectrometry after derivatization with naphthalene-2,3-dialdehyde. J. Chromatogr. A 2015, 1395, 73–78. [Google Scholar] [CrossRef]
- George, M.; Nagaraja, K.S.; Balasubramanian, N. Spectrophotometric determination of hydrazine. Talanta 2008, 75, 27–31. [Google Scholar] [CrossRef]
- Maleki, N.; Safavi, A.; Farjami, E.; Tajabadi, F. Palladium nanoparticle decorated carbon ionic liquid electrode for highly efficient electrocatalytic oxidation and determination of hydrazine. Anal. Chim. Acta 2008, 611, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Khan, F.; Ahmed, M.K.; Pandey, S.K. Potentiometric determination of free acidity in presence of hydrolysable ions and a sequential determination of hydrazine. Talanta 2011, 85, 958–963. [Google Scholar] [CrossRef]
- Gu, X.; Camden, J.P. Surface-enhanced raman spectroscopy-based approach for ultrasensitive and selective detection of hydrazine. Anal. Chem. 2015, 87, 6460–6464. [Google Scholar] [CrossRef]
- Abhijna Krishna, R.; Velmathi, S. A review on fluorimetric and colorimetric detection of metal ions by chemodosimetric approach 2013–2021. Coordin. Chem. Rev. 2022, 459, 214401. [Google Scholar] [CrossRef]
- Dou, W.-T.; Han, H.-H.; Sedgwick, A.C.; Zhu, G.-B.; Zang, Y.; Yang, X.-R.; Yoon, J.; James, T.D.; Li, J.; He, X.-P. Fluorescent probes for the detection of disease-associated biomarkers. Sci. Bull. 2022, 67, 853–878. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, S.; Xie, Y.; Mu, X.; Zhu, J. Recent progress in the development of fluorescent probes for the detection of hydrazine (N2H4). Crit. Rev. Anal. Chem. 2022, 52, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Yang, Y.-S.; Wang, W.; Jiao, Q.-C.; Zhu, H.-L. Fluorescent sensors for the detection of hydrazine in environmental and biological systems: Recent advances and future prospects. Coord. Chem. Rev. 2020, 417, 213367. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Zhang, Z.-H.; Qu, J.; Wang, J.-Y. A lysosomal targeted fluorescent probe based on coumarin for monitoring hydrazine in living cells with high performance. Anal. Methods 2021, 14, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-W.; Zhang, M.-H.; Cheng, J.-Y.; Man, R.-J.; Li, D.-D. A berberrubine-derived fluorescent probe for hydrazine and its practical application in water and food samples. Anal. Chim. Acta 2021, 1172, 338504. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, W.; Cheng, Z.; Kong, F.; Chen, C.; Li, X.; Ma, H. A portable system based on turn-on fluorescent probe for the detection of hydrazine in real environment. Dyes Pigm. 2022, 198, 110004. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, L.; Liu, A.; Yan, X.; Zhong, K.; Liu, X.; Gao, X.; Li, J. A new cascade reaction-based colorimetric and fluorescence “turn on” dual-function probe for cyanide and hydrazine detection. Dyes Pigm. 2021, 186, 109034. [Google Scholar] [CrossRef]
- Luo, M.; Li, Q.; Shen, P.; Hu, S.; Wang, J.; Wu, Z.; Su, Z. Coumarin 1,4-enedione for selective detection of hydrazine in aqueous solution and fluorescence imaging in living cells. Anal. Bioanal. Chem. 2021, 413, 7541–7548. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Han, Y.; Zhu, Y.; Sun, Y.; Shan, Y.; Wang, K.-N.; Liu, Q.; Dong, B.; Cao, D.; Lin, W. Two ratiometric fluorescent probes based on the hydroxyl coumarin chalcone unit with large fluorescent peak shift for the detection of hydrazine in living cells. Anal. Chem. 2022, 94, 12836–12844. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Qing, M.; Luo, X.Y.; Xie, J.; Zhang, L.N. A dual-response fluorescent probe for discriminative sensing of hydrazine and bisulfite as well as intracellular imaging with different emission. Spectrochim. Acta A 2022, 270, 120795. [Google Scholar] [CrossRef]
- Hou, J.-T.; Wang, B.; Wang, S.; Wu, Y.; Liao, Y.-X.; Ren, W.X. Detection of hydrazine via a highly selective fluorescent probe: A case study on the reactivity of cyano-substituted C=C bond. Dyes Pigm. 2020, 178, 108366. [Google Scholar] [CrossRef]
- Mu, S.; Gao, H.; Li, C.; Li, S.; Wang, Y.; Zhang, Y.; Ma, C.; Zhang, H.; Liu, X. A dual-response fluorescent probe for detection and bioimaging of hydrazine and cyanide with different fluorescence signals. Talanta 2021, 221, 121606. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, M.; Zhang, Y.; Ruan, S.; Yin, J.; Song, J.; Yang, Y.; Wang, Z.; Wang, S. A smart nopinone-based fluorescent probe for colorimetric and fluorogenic detection of hydrazine in water and plants with high sensitivity and selectivity. J. Lumin. 2020, 226, 117436. [Google Scholar] [CrossRef]
- Samanta, S.K.; Maiti, K.; Ali, S.S.; Guria, U.N.; Ghosh, A.; Datta, P.; Mahapatra, A.K. A solvent directed D-π-A fluorescent chemodosimeter for selective detection of hazardous hydrazine in real water sample and living cell. Dyes Pigm. 2020, 173, 107997. [Google Scholar] [CrossRef]
- Ban, Y.; Wang, R.; Li, Y.; An, Z.; Yu, M.; Fang, C.; Wei, L.; Li, Z. Mitochondria-targeted ratiometric fluorescent detection of hydrazine with a fast response time. New J. Chem. 2018, 42, 2030–2035. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, X.; Rodrigues, J.; Hu, X.; Sheng, R.; Bao, G.-M. A dual-analytes responsive fluorescent probe for discriminative detection of ClO− and N2H4 in living cells. Spectrochim. Acta A 2021, 246, 118953. [Google Scholar] [CrossRef]
- Yan, H.; Huo, F.; Yue, Y.; Chao, J.; Yin, C. A practical pH-compatible fluorescent sensor for hydrazine in soil, water and living cells. Analyst 2020, 145, 7380–7387. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yin, C.; Zhang, Y.; Wen, Y.; Huo, F. A novel ratiometric and colorimetric fluorescent probe for hydrazine based on ring-opening reaction and its applications. Sens. Actuators B 2019, 285, 368–374. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, Z.; Li, H.; Li, D.; Wang, X.; Qin, C. Design, synthesis and application in analytical chemistry of photo-sensitive probes based on coumarin. Crit. Rev. Anal. Chem. 2021, 51, 565–581. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Tian, X.; Li, M.; Zhang, Y.; Gong, S.; Wang, X.; Wang, Z.; Wang, S. A coumarin-based fluorescent probe for hydrazine detection and its applications in real water samples and living cells. J. Photochem. Photobiol. A Chem. 2023, 437, 114467. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, M.; Xu, C.; Fan, F.; Chen, P.; Wang, Y.; Li, D. An ICT-based coumarin fluorescent probe for the detection of hydrazine and its application in environmental water samples and organisms. Front. Bioeng. Biotechnol. 2022, 10, 937489. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Wu, W.-N.; Xu, Z.-Q.; Xu, Z.-H.; Zhao, X.-L.; Fan, Y.-C. A novel ‘turn-on’ coumarin-based fluorescence probe with aggregation-induced emission (AIE) for sensitive detection of hydrazine and its imaging in living cells. Spectrochim. Acta A 2019, 222, 117272. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Wang, J.; Liu, L.; Zhang, Q. A highly selective fluorescent probe based on coumarin for the imaging of N2H4 in living cells. Spectrochim. Acta A 2017, 173, 170–174. [Google Scholar] [CrossRef]
- Choi, M.G.; Hwang, J.; Moon, J.O.; Sung, J.; Chang, S.-K. Hydrazine-selective chromogenic and fluorogenic probe based on levulinated coumarin. Org. Lett. 2011, 13, 5260–5263. [Google Scholar] [CrossRef]
- Shi, X.; Yin, C.; Wen, Y.; Zhang, Y.; Huo, F. A probe with double acetoxyl moieties for hydrazine and its application in living cells. Spectrochim. Acta A 2018, 203, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Yang, L.; Xiong, H.; Han, S.; Zhang, Y.; Wang, J.; Chen, W.; Song, X. Coumarinocoumarin-based fluorescent probe for the sensitive and selective detection of hydrazine in living cells and zebra fish. Chin. Chem. Lett. 2020, 31, 129–132. [Google Scholar] [CrossRef]
- Guo, S.-H.; Guo, Z.-Q.; Wang, C.-Y.; Shen, Y.; Zhu, W.-H. An ultrasensitive fluorescent probe for hydrazine detection and its application in water samples and living cells. Tetrahedron 2019, 75, 2642–2646. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Z.; Shangguan, M.; Yi, S.; Zeng, X.; Zhang, Y.; Hou, L. A fluorescence ‘‘turn-on” sensor for detecting hydrazine in environment. Microchem. J. 2020, 152, 104376. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Xu, C.; Song, H.; Pang, X.; Liu, X. A dual-responsive fluorescent probe for detection of fluoride ion and hydrazine based on test strips. Spectrochim. Acta A 2019, 211, 125–131. [Google Scholar] [CrossRef]
- Jiang, X.; Shangguan, M.; Lu, Z.; Yi, S.; Zeng, X.; Zhang, Y.; Hou, L. A “turn-on” fluorescent probe based on V-shaped bis-coumarin for detection of hydrazine. Tetrahedron 2020, 76, 130921. [Google Scholar] [CrossRef]
- Li, K.; Xu, H.-R.; Yu, K.-K.; Hou, J.-T.; Yu, X.-Q. A coumarin-based chromogenic and ratiometric probe for hydrazine. Anal. Methods 2013, 5, 2653–2656. [Google Scholar] [CrossRef]
- Garazd, M.M.; Garazd, Y.L.; Ogorodniichuk, A.S.; Khilya, V.P. Modified coumarins. 29. Synthesis of structural analogs of natural 6-arylfuro[3,2-g]chromen-7-ones. Chem. Nat. Comp. 2009, 45, 158–163. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ju, I.G.; Choe, Y.H.; Kim, Y.; Park, S.; Hyun, Y.-M.; Oh, M.S.; Kim, D. Hydrazine Exposé: The next-generation fluorescent probe. ACS Sens. 2019, 4, 441–449. [Google Scholar] [CrossRef]
- Wang, B.; Yang, R.; Zhao, W. Construction of a mitochondria-targeted ratiometric fluorescent probe for monitoring hydrazine in soil samples and culture cells. J. Hazard. Mater. 2021, 406, 124589. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xie, R.; Pang, X.; Li, Y.; Zhou, Z.; Li, H. A colorimetric and near-infrared ratiometric fluorescent probe for hydrazine detection and bioimaging. Spectrochim. Acta A 2020, 243, 118764. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Gao, Y.; Wang, Y.; Li, M.; Yang, H.; Song, J.; Wang, Z.; Wang, S. A novel berberine-based colorimetric and fluorimetric probe for hydrazine detection. New J. Chem. 2020, 44, 15752–15757. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Ji, P.; Jin, X.; Liu, J.; Zhu, H. A red-emitting fluorescent probe based on flavone for hydrazine detection and its application in aqueous solution. Anal. Methods 2016, 8, 2267–2273. [Google Scholar] [CrossRef]
- Wang, W.-D.; Hu, Y.; Li, Q.; Hu, S.-L. A carbazole-based turn-on fluorescent probe for the detection of hydrazine in aqueous solution. Inorg. Chim. Acta 2018, 477, 206–211. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yang, S.; Tian, H.; Liu, Y.; Hao, Y.; Zhang, J.; Sun, B. A fluorescent probe for sensitive detection of hydrazine and its application in red wine and water. Anal. Sci. 2018, 34, 329–333. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Z.; Xiao, X.; Xia, L.; Li, G. Aldehyde spiropyran fluorescent probe for rapid determination of hydrazine in environmental water. Luminescence 2022, 37, 1891–1898. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Wang, S.-Q.; Wang, H.-Y.; Zhang, R.-R.; Liu, J.-T.; Zhao, B.-X. Novel pyrazoline-based selective fluorescent probe for the detection of hydrazine. Spectrochim. Acta A 2015, 138, 247–251. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Liu, C.; Yu, M.; Zhang, H.; Guo, L.; Wei, L. A colorimetric and ratiometric fluorescent probe for hydrazine and its application in living cells with low dark toxicity. Sens. Actuators B 2017, 241, 665–671. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Q.; Chen, Y.; Ou, Y.; Li, H.; Li, B. A dual-response ratiometric fluorescent probe for hypochlorite and hydrazine detection and its imaging in living cells. Spectrochim. Acta A 2020, 241, 118672. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Li, X.; Yang, Z.; Guo, Z.; Zhang, J.; Ma, J.; Wei, C. A coumarin-fused ‘off-on’ fluorescent probe for highly selective detection of hydrazine. Spectrochim. Acta A 2020, 230, 118075. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Z.; Chen, S.; Liu, Y.; Sheng, L.; Tian, Z.; Huang, D.; Xu, H. A smart reaction-based fluorescence probe for ratio detection of hydrazine and its application in living cells. Microchem. J. 2020, 156, 104809. [Google Scholar] [CrossRef]

| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 0 | Not detected | — | — |
| 5.00 | 5.36 | 107.2 | 1.1 | |
| 25.00 | 25.51 | 102.0 | 0.8 | |
| 50.00 | 52.07 | 104.1 | 3.6 | |
| Lake water | 0 | Not detected | — | — |
| 5.00 | 5.13 | 102.2 | 2.8 | |
| 25.00 | 24.91 | 99.5 | 0.8 | |
| 50.00 | 51.02 | 102.0 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Sun, X.; Zhang, Y.; Zhang, C.; Liu, S.; Wang, S. A Ratiometric Fluorescent Probe for N2H4 Having a Large Detection Range Based upon Coumarin with Multiple Applications. Molecules 2023, 28, 7629. https://doi.org/10.3390/molecules28227629
Sheng X, Sun X, Zhang Y, Zhang C, Liu S, Wang S. A Ratiometric Fluorescent Probe for N2H4 Having a Large Detection Range Based upon Coumarin with Multiple Applications. Molecules. 2023; 28(22):7629. https://doi.org/10.3390/molecules28227629
Chicago/Turabian StyleSheng, Xiao, Xinfeng Sun, Yiwen Zhang, Chen Zhang, Shuling Liu, and Shouxin Wang. 2023. "A Ratiometric Fluorescent Probe for N2H4 Having a Large Detection Range Based upon Coumarin with Multiple Applications" Molecules 28, no. 22: 7629. https://doi.org/10.3390/molecules28227629






