Design of Experiments for Optimizing Ultrasound-Assisted Extraction of Bioactive Compounds from Plant-Based Sources
Abstract
:1. Introduction
2. Extraction Methods of Bioactive Compounds from Plant Sources
3. Ultrasound-Assisted Extraction
4. Common Statistical Tools Used for Optimizing and Modeling UAE of Bioactive Compounds form Plat-Based Materials
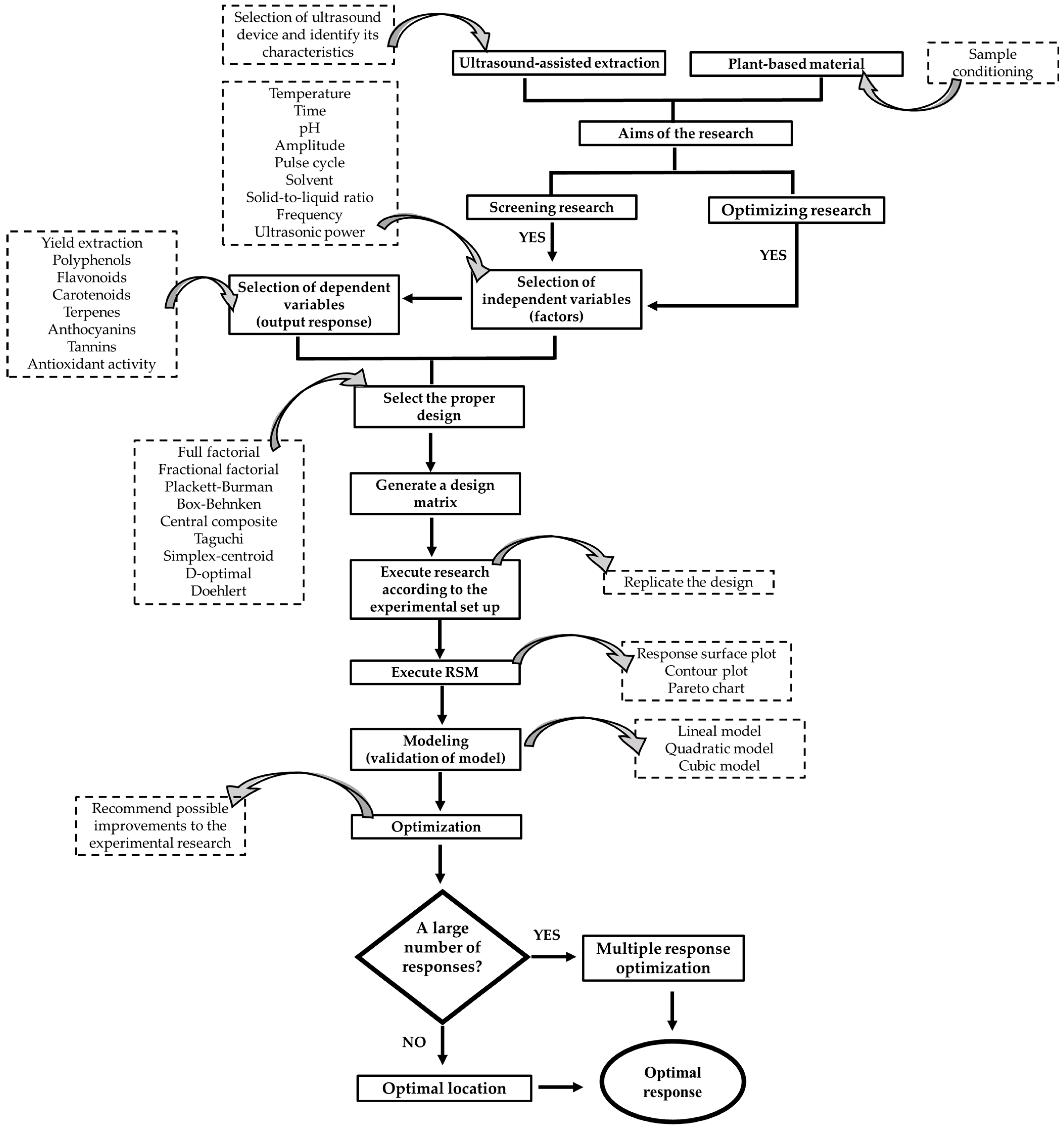
4.1. Response Surface Methodology
- Select the independent variables and their respective levels, along with potential response variables. At this stage, a screening design of experiments (DOE) can be employed.
- Choose the appropriate DOE.
- Conduct experiments and record the results.
- Develop a model equation based on the experimental data, which can be visualized as a contour plot or a 3D surface and Paret chart.
- Validate the model. This step often employs analysis of variance to identify the most significant factors in the model and assess their reliability.
- Determine the optimal conditions.
4.2. Full Factorial Design
4.2.1. Fractional Factorial Design
4.2.2. Plackett–Burman Design
4.3. Box-Behnken Design
4.4. Central Composite Design
4.5. Taguchi Design
4.6. Mixture Designs
4.7. D-Optimal Design
4.8. Doehlert Design
4.9. Combined Designs
5. Recommendations to Select an Analytical DOE: Advantages and Limitations
- How many independent variables (factors) and levels per variable are there?
- How many experimental runs will be there?
- Is it a screening or an optimizing experiment?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leichtweis, M.G.; Molina, A.K.; Petropoulos, S.A.; Carocho, M.; Pires, T.C.S.P.; Dias, M.I.; Calhelha, R.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Valorization of Pumpkin Peel as a Source of Bioactive Compounds: Optimization of Heat- and Ultrasound-Assisted Extraction. Molecules 2023, 28, 3168. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, H.; Damar, I. Valorization of Spinach Roots for Recovery of Phenolic Compounds by Ultrasound-Assisted Extraction: Characterization, Optimization, and Bioaccessibility. Eur. Food Res. Technol. 2023, 249, 1899–1913. [Google Scholar] [CrossRef]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial By-Products: Valuable Sources of Bioactive Compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.G.; Troter, D.Z.; Savić, I.M.; Savić Gajić, I.M.; Zvezdanović, J.B.; Konstantinović, I.B.; Konstantinović, S.S. Design and Optimization of “Greener” and Sustainable Ultrasound-Assisted Extraction of Valuable Bioactive Compounds from Common Centaury (Centaurium erythraea Rafn) Aerial Parts: A comparative Study Using Aqueous Propylene Glycol and Ethanol. Ind. Crops Prod. 2023, 192, 116070. [Google Scholar] [CrossRef]
- United Nations. Global Sustainable Development Report 2023. Department of Economic and Social Affairs. Sustainable Development. 2023. Available online: https://sdgs.un.org/gsdr/gsdr2023 (accessed on 25 October 2023).
- Anaya-Esparza, L.M.; de la Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5431. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Din-Shirahighe, L.; Ceccato-Antonini, S.R. Agro-Industrial Wastes as Sources of Bioactive Compounds for Food and Fermentation Industries. Ciência Rural 2020, 50, e20190857. [Google Scholar] [CrossRef]
- Gry, J.; Black, L.; Eriksen, F.D.; Pilegaard, K.; Plumb, J.; Rhodes, M.; Sheehan, D.; Kiely, M.; Kroon, P.A. EuroFIR-BASIS—A Combined Composition and Biological Activity Database for Bioactive Compounds in Plant-Based Foods. Trends Food Sci. Technol. 2007, 18, 434–444. [Google Scholar] [CrossRef]
- Montalvo-González, E.; Villagán, Z.; González-Torres, S.; Iñiguez-Muñoz, L.E.; Isiordia-Espinoza, M.A.; Ruvalcaba-Gómez, J.M.; Arteaga-Garibay, R.I.; Acosta, J.L.; González-Silva, N.; Anaya-Esparza, L.M. Physiological Effects and Human Health Benefits of Hibiscus sabdariffa: A Review of Clinical Trials. Pharmaceuticals 2022, 15, 464. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective Extraction of Bioactive Compounds from Plants Using Recent Extraction Techniques: A Review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of Emerging Technologies for the Extraction of Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-malek, Z.; Roobab, U.; Abdullah-Munir, M.; Naderipour, A.; Imran-Qureshi, M.; Bekhit, A.E.D.; Liu, Z.W.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative Towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Yang, L.; Zhang, S.; Jiang, H. Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach. Foods 2023, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.N.A.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Dzah, C.S.; Dzigbor, A. Ultrasound Assisted Extraction: A Relook at Solvent to Material Ratio, its Effects on Process Efficiency and How It Can Be Exploited for Different Uses. J. Food Process Eng. 2023, 46, e14339. [Google Scholar] [CrossRef]
- Shekhar, S.; Prakash, P.; Singha, P.; Prasad, K.; Singh, S.K. Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm. Foods 2023, 12, 1925. [Google Scholar] [CrossRef]
- Teixeira, B.A.; Vidigal, M.C.T.R.; de Castro Leite Júnior, B.; Vieira, É.N.R.; Martins, E.M.F.; Stringheta, P.C. Optimization, Kinetic and Phenomenological Modeling of Ultrasound-Assisted Extraction Process of Bioactive Compounds from Raspberries (Rubus idaeus L.). Food Anal. Methods 2023, 16, 759–770. [Google Scholar]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds: Oleuropein, Phenolic Acids, Phenolic Alcohols and Flavonoids from Olive Leaves and Evaluation of Its Antioxidant Activities. Ind. Crop. Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Insang, S.; Kijpatanasilp, I.; Jafari, S.; Assatarakul, K. Ultrasound-Assisted Extraction of Functional Compound from Mulberry (Morus alba L.) Leaf Using Response Surface Methodology and Effect of Microencapsulation by Spray Drying on Quality of Optimized Extract. Ultrason. Sonochem. 2022, 82, 105806. [Google Scholar] [CrossRef] [PubMed]
- Salacheep, S.; Kasemsiri, P.; Pongsa, U.; Okhawilai, M.; Chindaprasirt, P.; Hiziroglu, S. Optimization of Ultrasound-Assisted Extraction of Anthocyanins and Bioactive Compounds from Butterfly Pea Petals Using Taguchi Method and Grey Relational Analysis. J. Food Sci. Technol. 2020, 57, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Factorial Optimization of Ultrasound-Assisted Extraction of Phycocyanin from Synechocystis salina: Towards a Biorefinery Approach. Life 2022, 12, 1389. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional Versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Mathialagan, R.; Mansor, N.; Shamsuddin, M.R.; Uemura, Y.; Majeed, Z. Optimisation of Ultrasonic-Assisted Extraction (UAE) of Allicin from Garlic (Allium sativum L.). Chem. Eng. Trans. 2017, 56, 1747–1752. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC—Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green Extraction Techniques in Green Analytical Chemistry. TrAC—Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- González-Silva, N.; Nolasco-Gozález, Y.; Aguilar-Hernádez, G.; Sáyago-Ayerdí, S.G.; Villagrán, Z.; Acosta, J.L.; Montalvo-González, E.; Anaya-Esparza, L.M. Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium cattleianum Leaves: Optimization Using the Response. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef]
- de Lima, A.S.; Maia, D.V.; Haubert, L.; Oliveira, T.L.; Fiorentini, Â.M.; Rombaldi, C.V.; da Silva, W.P. Action Mechanism of Araçá (Psidium cattleianum Sabine) Hydroalcoholic Extract against Staphylococcus aureus. LWT-Food Sci. Technol. 2020, 119, 108884. [Google Scholar] [CrossRef]
- Mesomo Bombardelli, M.C.; Machado, C.S.; Kotovicz, V.; Kruger, R.L.; Santa, O.R.D.; Torres, Y.R.; Corazza, M.L.; da Silva, E.A. Extracts from Red Araçá (Psidium cattleianum) Fruits: Extraction Process, Modelling and Assessment of the Bioactivity Potentialities. J. Supercrit. Fluids 2021, 176, 105278. [Google Scholar] [CrossRef]
- Zandoná, G.P.; Bagatini, L.; Woloszyn, N.; de Souza Cardoso, J.; Hoffmann, J.F.; Moroni, L.S.; Stefanello, F.M.; Junges, A.; Rombaldi, C.V. Extraction and Characterization of Phytochemical Compounds from Araçazeiro (Psidium cattleianum) Leaf: Putative Antioxidant and Antimicrobial Properties. Food Res. Int. 2020, 137, 109573. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Lee, S.; Jeong, S.; Kim, J.C.; Ahn, K.S.; Mosaddik, A.; Cho, S.K. Free Radical-Scavenging Activities and Cytoprotective Effect of Polyphenol-Rich Ethyl Acetate Fraction of Guava (Psidium cattleianum) Leaves on H2O2-Treated HepG2 Cell. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 687–694. [Google Scholar] [CrossRef]
- Dacoreggio, M.V.; Moroni, L.S.; Kempka, A.P. Antioxidant, Antimicrobial and Allelopathic Activities and Surface Disinfection of the Extract of Psidium cattleianum Sabine Leaves. Biocatal. Agric. Biotechnol. 2019, 21, 101295. [Google Scholar] [CrossRef]
- Coelho, J.M.P.; Johann, G.; da Silva, E.A.; Palú, F.; Vieira, M.G.A. Extraction of Natural Antioxidants from Strawberry Guava Leaf by Conventional and Non-Conventional Techniques. Chem. Eng. Commun. 2021, 208, 1131–1142. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds from Plants to Drug Development, 1st ed.; Domínguez-González, H., González-Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 291–316. [Google Scholar]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops–A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Gueffai, A.; Gonzalez-Serrano, D.J.; Christodoulou, M.C.; Orellana-Palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-Christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Ultrasound-Assisted Extraction of Antioxidant Bioactive Compounds from Wastes of Rapeseed Industry and Their Application in Delaying Rapeseed Oil Oxidation. Environ. Technol. Innov. 2023, 30, 103081. [Google Scholar] [CrossRef]
- López-Romero, B.A.; Luna-Bárcenas, G.; García-Magaña, M.L.; Anaya-Esparza, L.M.; Zepeda-vallejo, L.G.; López-García, U.M.; Ortiz-basurto, R.I.; Aguilar-Hernández, G.; Pérez-Larios, A.; Montalvo-González, E. Extraction of Acetogenins Using Thermosonication-Assisted Extraction from Annona muricata Seeds and Their Antifungal Activity. Molecules 2022, 27, 6045. [Google Scholar] [CrossRef]
- Görgüç, A.; Özer, P.; Yilmaz, F.M. Simultaneous Effect of Vacuum and Ultrasound Assisted Enzymatic Extraction on the Recovery of Plant Protein and Bioactive Compounds from Sesame Bran. J. Food Compos. Anal. 2020, 87, 103424. [Google Scholar] [CrossRef]
- Nenciu, F.; Fatu, V.; Arsenoaia, V.; Persu, C.; Voicea, I.; Vladut, N.V.; Matache, M.G.; Gageanu, I.; Marin, E.; Biris, S.S.; et al. Bioactive Compounds Extraction Using a Hybrid Ultrasound and High-Pressure Technology for Sustainable Farming Systems. Agriculture 2023, 13, 899. [Google Scholar] [CrossRef]
- Sharif, T.; Bhatti, H.N.; Bull, I.D.; Bilal, M. Recovery of High-Value Bioactive Phytochemicals from Agro-Waste of Mango (Mangifera indica L.) Using Enzyme-Assisted Ultrasound Pretreated Extraction. Biomass Convers. Biorefin. 2023, 13, 6591–6599. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined Impact of Pulsed Electric Field and Ultrasound on Bioactive Compounds and FT-IR Analysis of Almond Extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Viganó, J.; Assis, B.F.P.; Náthia-Neves, G.; dos Santos, P.; Meireles, M.A.A.; Carvalho-Veggi, P.; Martínez, J. Extraction of Bioactive Compounds from Defatted Passion Fruit Bagasse (Passiflora edulis sp.) Applying Pressurized Liquids Assisted by Ultrasound. Ultrason. Sonochem. 2020, 64, 104999. [Google Scholar] [CrossRef] [PubMed]
- Macedo, C.; Silva, A.M.; Ferreira, A.S.; Moreira, M.M.; delerue-Matos, C.; Rodriuges, F. Microwave- and Ultrasound-Assisted Extraction of Cucurbita pepo Seeds: A Comparison Study of Antioxidant Activity, Phenolic Profile, and In Vitro Cells Effects. Appl. Sci. 2022, 12, 1763. [Google Scholar] [CrossRef]
- Razola-Díaz, M.D.C.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; García-Villanova, B.; Verardo, V. Optimization of ultrasound-assisted extraction via sonotrode of phenolic compounds from orange by-products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Londoño, J.; de Lima, V.R.; Lara, O.; Gil, A.; Pasa, T.B.C.; Arango, G.J.; Pineda, J.R.R. Clean Recovery of Antioxidant Flavonoids from Citrus Peel: Optimizing an Aqueous Ultrasound-Assisted Extraction Method. Food Chem. 2010, 119, 81–87. [Google Scholar] [CrossRef]
- Shahidi, S.A. Effect of Solvent Type on Ultrasound-Assisted Extraction of Antioxidant Compounds from Ficaria kochii: Optimization by Response Surface Methodology. Food Chem. Toxicol. 2022, 163, 112981. [Google Scholar] [CrossRef]
- Martín-García, B.; Aznar-Ramos, M.J.; Verardo, V.; Gómez-Caravaca, A.M. The Establishment of Ultrasonic-Assisted Extraction for the Recovery of Phenolic Compounds and Evaluation of Their Antioxidant Activity from Morus alba Leaves. Foods 2022, 11, 314. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound Assisted Extraction of Phenolic Compounds from Peaches and Pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef]
- Menezes Maciel Bindes, M.; Hespanhol Miranda Reis, M.; Luiz Cardoso, V.; Boffito, D.C. Ultrasound-Assisted Extraction of Bioactive Compounds from Green Tea Leaves and Clarification with Natural Coagulants (Chitosan and Moringa oleífera seeds). Ultrason. Sonochem. 2019, 51, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Paraíso, C.M.; Siqueira, S.; Corre, V.G.; Magon, T.; Peralta, R.M.; Visentainer, J.V.; Scaramal, G. Ultrasound Assisted Extraction of Hibiscus (Hibiscus sabdariffa L.) Bioactive Compounds for Application as Potential Functional Ingredient. J. Food Sci. Technol. 2019, 56, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of Ultrasound-Assisted Extraction Conditions for Recovery of Phenolic Compounds and Antioxidant Capacity from Banana (Musa cavendish) Peel. J. Food Process. Preserv. 2017, 41, e13148. [Google Scholar] [CrossRef]
- Fernández-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; García-Barroso, C. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Jabuticaba (Myrciaria cauliflora) Fruit Through a Box-Behnken Experimental Design. Food Sci. Technol. 2019, 39, 1018–1029. [Google Scholar] [CrossRef]
- Fumić, B.; Jug, M.; Končić, M.Z. Multi-Response Optimization of Ultrasound-Assisted Extraction of Bioactive Components from Medicago sativa L. Croat. Chem. Acta 2017, 90, 481–491. [Google Scholar] [CrossRef]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of Ultrasonic-Assisted Extraction of Major Phenolic Compounds from Olive Leaves (Olea europaea L.) Using Response Surface Methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Eun, J.B. Effect of Ultrasound-Assisted Extraction of Moringa stenopetala Leaves on Bioactive Compounds and Their Antioxidant Activity. Food Technol. Biotechnol. 2019, 57, 77–86. [Google Scholar] [CrossRef]
- Rathnakumar, K.; Anal, A.K.; Lakshmi, K. Optimization of Ultrasonic Assisted Extraction of Bioactive components from different Parts of Pineapple Waste. Int. J. Agric. Environ. Biotechnol. 2017, 10, 553. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Gomez-Delgado, E.; López-Córdoba, A. Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology. Foods 2022, 11, 2425. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of Ultrasound-Assisted Extraction (UAE) Process for the Recovery of Bioactive Compounds from Bitter Gourd Using Response Surface Methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods 2022, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Sanou, A.; Konaté, K.; Kabakdé, K.; Dakuyo, R.; Bazié, D.; Hemayoro, S.; Dicko, M.H. Modelling and Optimisation of Ultrasound-Assisted Extraction of Roselle Phenolic Compounds Using the Surface Response Method. Sci. Rep. 2023, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, A.; Chaudhary, G.; Goia, F. Designing the Design of Experiments (DOE)—An Investigation on the Influence of Different Factorial Designs on the Characterization of Complex Systems. Energy Build. 2021, 250, 111298. [Google Scholar] [CrossRef]
- Cerqueira, U.M.F.M.; Bezerra, M.A.; Ferreira, S.L.C.; de Jesus Araújo, R.; da Silva, B.N.; Novaes, C.G. Doehlert Design in the Optimization of Procedures Aiming Food Analysis—A Review. Food Chem. 2021, 364, 130429. [Google Scholar] [CrossRef] [PubMed]
- Vera Candioti, L.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental Design and Multiple Response Optimization Using the Desirability Function in Analytical Methods Development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Prakash Maran, J.; Manikandan, S.; Vigna Nivetha, C.; Dinesh, R. Ultrasound Assisted Extraction of Bioactive Compounds from Nephelium lappaceum L. Fruit Peel Using Central Composite Face Centered Response Surface Design. Arab. J. Chem. 2017, 10, S1145–S1157. [Google Scholar] [CrossRef]
- Ravanfar, R.; Tamadon, A.M.; Niakousari, M. Optimization of Ultrasound Assisted Extraction of Anthocyanins from Red Cabbage Using Taguchi Design Method. J. Food Sci. Technol. 2015, 52, 8140–8147. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Saibua, S.; Limmatvapirat, C. Optimization of Ultrasound-Assisted Extraction of Yields and Total Methoxyflavone Contents from Kaempferia parviflora Rhizomes. Molecules 2022, 27, 4162. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Fei, S.; Gu, H.; Yang, L. Optimization of Ultrasonic Circulating Extraction of Samara Oil from Acer saccharum Using Combination of Plackett–Burman Design and Box–Behnken Design. Ultrason. Sonochem. 2017, 35, 161–175. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of Solvent Composition and Its Interaction with Ultrasonic Energy on the Ultrasound-Assisted Extraction of Phenolic Compounds from Mango Peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Patil, S.S.; Deshannavar, U.B.; Ramasamy, M.; Hegde, P.G. Modeling and Optimisation Studies on the Ultrasound-Assisted Extraction of Phenolic Compounds from Azadirachta indica. Chem. Eng. Commun. 2022, 209, 1423–1438. [Google Scholar] [CrossRef]
- Bi, Y.; Lu, Y.; Yu, H.; Luo, L. Optimization of Ultrasonic-Assisted Extraction of Bioactive Compounds from Sargassum henslowianum Using Response Surface Methodology. Pharmacogn. Mag. 2017, 13, 179–188. [Google Scholar] [CrossRef]
- Santos, M.R.M.; Fetsch, V.T.; Santos, K.A.; Tavares, F.; Silva, E.A. Ultrasound-Assisted Extraction of Bioactive Compounds from Sete Capote Leaves (Campomanesia Guazumifolia Cambess). J. Multidiscip. Eng. Sci. Technol. 2020, 7, 11782–11790. [Google Scholar]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing By-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Yeom, S.H.; Gam, D.H.; Kim, J.H.; Kim, J.W. Development of Ultrasound-Assisted Extraction to Produce Skin-Whitening and Anti-Wrinkle Substances from Safflower Seed. Molecules 2022, 27, 1296. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Broeckx, G.; Bijttebier, S.; Naessens, T.; Fransen, E.; Kiekens, F.; Caballero-George, C.; Vander Heyden, Y.; Apers, S.; Pieters, L.; et al. Ultrasound-Assisted Extraction Optimization and Validation of an HPLC-DAD Method for the Quantification of Polyphenols in Leaf Extracts of Cecropia species. Sci. Rep. 2019, 9, 2028. [Google Scholar] [CrossRef]
- Kim, J.H.; Yeom, S.H.; Hwang, Y.S.; Kim, S.H.; Kim, J.W. Ultrasound-Assisted Extraction of Polyphenols from Carthamus tinctorius Seeds: Optimization of Process Variables. Biotechnol. Bioprocess Eng. 2022, 27, 843–852. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Costa, P.; Lorenzo, J.M. Modeling Approaches to Optimize the Recovery of Polyphenols Using Ultrasound-Assisted Extraction. In Design Optimization of Innovative Food Processing Techniques Assisted by Ultrasound. Developing Healthier and Sustainable Food Products, 1st ed.; Barba, F.J., Chemat, F., Sichetti-Munekata, P.E., Cravotto, G., Lorenzo-Rodríguez, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 1, pp. 15–38. [Google Scholar]
- Gajic, I.S.; Savic, I.; Boskov, I.; Žerajić, S.; Markovic, I.; Gajic, D. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Black Locust (Robiniae pseudoacaciae) Flowers and Comparison with Conventional Methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green Solvents and Ultrasound-Assisted Extraction of Bioactive Orange (Citrus sinensis) Peel Compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Domokos, E.; Coşarcă, S.; Miklos, A.; Imre, S.; Domokos, J.; Dehelean, C.A. Study of the Ultrasound-Assisted Extraction of Polyphenols from Beech (Fagus sylvatica L.) Bark. BioResources 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.M.; Tomuta, I.; Popa, D.S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut (Juglans regia L.) Male Flowers Based on Process Optimization of Ultrasonic Assisted-Extraction: Phytochemical Profile and Biological Activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Flores, A.; Hérnandez-Almanza, A.; Sáenz-Galindo, A.; Morlett-Chávez, J.; Aguilar, C.N.; Ascacio-Valdés, J. Ultrasound-Assisted Extraction of Antioxidant Polyphenolic Compounds from Nephelium lappaceum L. (Mexican Variety) Husk. Asian Pac. J. Trop. Med. 2018, 11, 676–681. [Google Scholar]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Spruce Wood Bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Detti, C.; dos Santos, B.; Brunetti, C.; Ferrini, F.; Gori, A. Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus L. Leaves Using a Response Surface Methodology. Plants 2020, 9, 1482. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Haskap Berries (Lonicera caerulea L.) Using Response Surface Methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef]
- Ali, A.; Lim, X.Y.; Chong, C.H.; Mah, S.H.; Chua, B.L. Optimization of Ultrasound-Assisted Extraction of Natural Antioxidants from Piper betle Using Response Surface Methodology. LWT-Food Sci. technol. 2018, 89, 681–688. [Google Scholar] [CrossRef]
- Jeong, I.J.; Kim, K.J. An Interactive Desirability Function Method to Multiresponse Optimization. Eur. J. Oper. Res. 2009, 195, 412–426. [Google Scholar] [CrossRef]
- Costa, N.R.; Lourenço, J.; Pereira, Z.L. Desirability Function Approach: A Review and Performance Evaluation in Adverse Conditions. Chemom. Intell. Lab. Syst. 2011, 107, 234–244. [Google Scholar] [CrossRef]
- Pierlot, C.; Pawlowski, L.; Bigan, M.; Chagnon, P. Design of Experiments in Thermal Spraying: A Review. Surf. Coat. Technol. 2008, 202, 4483–4490. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, H. Ultrasonic Treatment Increases Extraction Rate of Common Bean (Phaseolus vulgaris L.) Antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of Ultrasound-Assisted Wxtraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- dos Anjos, G.L.; Moreira, G.C.; Carneiro, C.N.; Caldas, J.d.C.; Silva, I.M.d.J.; dos Santos, W.N.L.; Dias, F.d.S. Multivariate Optimization of an Ultrasound-Assisted Extraction Method of Bioactive Phenolic Compounds in Malagueta Peppers (Capsicum frutescens). Food Anal. Methods 2021, 14, 2607–2616. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Contreras, M.D.M.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Romero, I.; Castro, E. Recovery of Bioactive Compounds from Industrial Exhausted Olive Pomace Through Ultrasound-Assisted Extraction. Biology 2021, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in Pharmaceutical Development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Cavalaro, R.I.; da Cruz, R.G.; Dupont, S.; de Moura Bell, J.M.L.N.; Vieira, T.M.F. de S. In Vitro and In Vivo Antioxidant Properties of Bioactive Compounds from Green Propolis Obtained by Ultrasound-Assisted Extraction. Food Chem. X 2019, 4, 100054. [Google Scholar] [CrossRef]
- Rashad, S.; El-Chaghaby, G.; Lima, E.C.; Simoes dos Reis, G. Optimizing the Ultrasonic-Assisted Extraction of Antioxidants from Ulva lactuca Algal Biomass Using Factorial Design. Biomass Convers. Biorefinery 2021, 13, 5681–5690. [Google Scholar] [CrossRef]
- Chen, Q.H.; Fu, M.L.; Liu, J.; Zhang, H.F.; He, G.Q.; Ruan, H. Optimization of Ultrasonic-Assisted Extraction (UAE) of Betulin from White Birch Bark Using Response Surface Methodology. Ultrason. Sonochem. 2009, 16, 599–604. [Google Scholar] [CrossRef]
- Matsumoto, S.; Varela, R.M.; Palma, M.; Molinillo, J.M.G.; Lima, I.S.; Barroso, C.G.; Macías, F.A. Bio-Guided Optimization of the Ultrasound-Assisted Extraction of Compounds from Annona glabra L. Leaves Using the Etiolated Wheat Coleoptile Bioassay. Ultrason.—Sonochemistry 2014, 21, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of Ultrasound-Assisted Extraction of Moringa peregrina Oil with Response Surface Methodology and Comparison with Soxhlet Method. Ind. Crop. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Milić, A.; Daničić, T.; Horecki, A.T.; Šumić, Z.; Kovačević, D.B.; Putnik, P.; Pavlić, B. Maximizing Contents of Phytochemicals Obtained from Dried Sour Cherries by Ultrasound-Assisted Extraction. Separations 2021, 8, 155. [Google Scholar] [CrossRef]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of Ultrasound-Assisted Extraction (UAE) of Flavonoids Compounds (FC) from Hawthorn Seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Ćućuz, V.; Cvejić, J.; Torović, L.; Gojković-Bukarica, L.; Acevska, J.; Dimitrovska, A.; Aldawoud, T.M.S.; Galanakis, C.M. Design of Experiments (DoE) to Model Phenolic Compounds Recovery from Grape Pomace Using Ultrasounds. J. Food Sci. Technol. 2022, 59, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Li, Z.H.; Wang, Z.J.; Liu, L.L.; Sun, T.T.; Ma, J.Z.; Zhang, Y. Ultrasound-Assisted Extraction of Total Anthocyanins from Rubia sylvatica Nakai Fruit and Radical Scavenging Activity of the Extract. Ind. Crop. Prod. 2020, 150, 112420. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Zhao, Y. Phenolic Compounds of “Blue Food” Porphyra haitanensis: Chemical Fingerprints, Antioxidant Activities, and In Vitro Antiproliferative Activities Against HepG2 Cells. Food Res. Int. 2022, 162, 112139. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef]
- Rohilla, S.; Mahanta, C.L. Optimization of Extraction Conditions for Ultrasound-Assisted Extraction of Phenolic Compounds from Tamarillo Fruit (Solanum betaceum) Using Response Surface Methodology. J. Food Meas. Charact. 2021, 15, 1763–1773. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ramos-Aguirre, D.; Zamora-Gasga, V.M.; Yahia, E.; Montalvo-González, E. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds from Justicia spicigera Leaves. Food Sci. Biotechnol. 2018, 27, 1093–1102. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; García-Magaña, M.L.; Vivar-Vera, M.A.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A.; Morales-Castro, J.; Anaya-Esparza, L.M.; González, E.M. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Annona muricata By-Products and Pulp. Molecules 2019, 24, 904. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srinivasa Rao, P. Optimization of Pulsed-Mode Ultrasound Assisted Extraction of Bioactive Compounds from Pomegranate Peel Using Response Surface Methodology. J. Food Meas. Charact. 2020, 14, 3493–3507. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Comparison and Optimization of Conventional and Ultrasound Assisted Extraction for Bioactive Compounds and Antioxidant Activity from Agro-Industrial Acerola (Malpighia emarginata DC) Residue. Lwt-Food Sci. Technol. 2017, 85, 158–169. [Google Scholar] [CrossRef]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Panneerselvam, T.; Govindaraj, S.; Kannan, S.; Parasuraman, P.; Arunachalam, S.; Sankaranarayanan, M.; Baskararaj, S.; Palanisamy, P.; Ammunje, D.N. Optimization and Analysis of Ultrasound-Assisted Extraction of Bioactive Polyphenols from Garcinia indica Using RSM and ANFIS Modeling and Its Anticancer Activity. J. Iran. Chem. Soc. 2020, 17, 789–801. [Google Scholar] [CrossRef]
- Šavikin, K.; Živković, J.; Janković, T.; Ćujić-Nikolić, N.; Zdunić, G.; Menković, N.; Drinić, Z. Optimization of Ultrasound-Assisted Extraction of Phenolics from Sideritis raeseri Using Response Surface Methodology. Molecules 2021, 26, 3949. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.M.; Savic Gajic, I.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Wheatgrass (Triticum aestivum L.). J. Food Sci. Technol. 2020, 57, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Nachiappan, G.; Mishra, H.N. Ultrasound-Assisted Extraction of Flavonoids and Phenolic Compounds from Ocimum tenuiflorum Leaves. Food Sci. Biotechnol. 2015, 24, 1951–1958. [Google Scholar] [CrossRef]
- Giordano, M.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Stojković, D.; Soković, M.; Tavares, D.; Cánepa, A.L.; Ferreira, I.C.F.R.; Caleja, C.; et al. Ultrasound-Assisted Extraction of Flavonoids from Kiwi Peel: Process Optimization and Bioactivity Assessment. Appl. Sci. 2021, 11, 6416. [Google Scholar] [CrossRef]
- Bouaoudia-Madi, N.; Boulekbache-Makhlouf, L.; Madani, K.; Silva, A.M.S.; Dairi, S.; Oukhmanou-Bensidhoum, S.; Cardoso, S.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Myrtus communis L. Pericarp. Antioxidants 2019, 8, 205. [Google Scholar] [CrossRef]
- Dal Prá, V.; Lunelli, F.C.; Vendruscolo, R.G.; Martins, R.; Wagner, R.; Lazzaretti, A.P.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Mazutti, M.A.; et al. Ultrasound-Assisted Extraction of Bioactive Compounds from Palm Pressed Fiber with High Antioxidant and Photoprotective Activities. Ultrason. Sonochem. 2017, 36, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, F.; Tan, J.K.; Mohd Faudzi, S.M.; Abdul Rahman, M.B.; Ashari, S.E. Ultrasound-Assisted Extraction Conditions Optimisation Using Response Surface Methodology from Mitragyna speciosa (Korth.) Havil Leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Arsenijević, D.; Planojević, N.; Terzić, J.; Stefanović, O. Bioactive Compounds from Taraxacum officinale Extracts Obtained by Optimized Ultrasound-Assisted Extraction. Kragujev. J. Sci. 2022, 44, 169–187. [Google Scholar] [CrossRef]
- Chen, X.; Ding, J.; Ji, D.; He, S.; Ma, H. Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components from Coffee Leaves Using the Taguchi Design and Response Surface Methodology. J. Food Sci. 2020, 85, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.K.S.; Joseph, J.; George, D. Optimization of Extraction Parameters of Bioactive Components from Moringa oleifera Leaves Using Taguchi Method. Biomass Convers. Biorefinery 2023, 13, 11973–11982. [Google Scholar] [CrossRef]
- Ku, K.J.; Rao, S.S.; Chen, L. Taguchi-Aided Search Method for Design optimization of engineering systems. Eng. Optim. 1998, 30, 1–23. [Google Scholar] [CrossRef]
- Mandal, V.; Dewanjee, S.; Sahu, R.; Mandal, S.C. Design and Optimization of Ultrasound Assisted Extraction of Curcumin as an Effective Alternative for Conventional Solid Liquid Extraction of Natural Products. Nat. Prod. Commun. 2009, 4, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sánchez, M.C.; Aguilar-Zárate, P.; Michel-Michel, M.R.; Ascacio-Valdés, J.A.; Reyes-Munguía, A. The Ultrasound-Assisted Extraction of Polyphenols from Mexican Firecracker (Hamelia patens Jacq.): Evaluation of Bioactivities and Identification of Phytochemicals by HPLC-ESI-MS. Molecules 2022, 27, 8845. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.H.; Annamalai, K.K.; Idris, F.N.; Kamaruddin, A.H.; Nadzir, M.M. Dataset of Ultrasound-Assisted Extraction of Anthocyanin from the Petals of Clitoria ternatea Using Taguchi Method and Effect of Storage Conditions on the Anthocyanin Stability. Data Br. 2022, 40, 107803. [Google Scholar] [CrossRef]
- Yazıcı, S.Ö. Optimization of All Extraction Process for Phenolic Compounds with Maximum Antioxidant Activity from Extract of Taraxacum assemanii by Statistical Strategies. J. Food Meas. Charact. 2021, 15, 4388–4402. [Google Scholar] [CrossRef]
- Silva, D.S.N.; Silva, M.d.S.; Coelho, T.L.S.; Dantas, C.; Lopes Júnior, C.A.; Caldas, N.M.; Vieira, E.C. Combining High Intensity Ultrasound and Experimental Design to Improve Carotenoid Extraction Efficiency from Buriti (Mauritia flexuosa). Ultrason. Sonochem. 2022, 88, 106076. [Google Scholar] [CrossRef] [PubMed]
- Soussi, M.; Fadil, M.; Al Yaagoubi, W.; Benjelloun, M.; El Ghadraoui, L. Simultaneous Optimization of Phenolic Compounds and Antioxidant Abilities of Moroccan Pimpinella anisum Extracts Using Mixture Design Methodology. Processes 2022, 10, 2580. [Google Scholar] [CrossRef]
- Moreira, G.C.; de Souza Dias, F. Mixture Design and Doehlert Matrix for Optimization of the Ultrasonic Assisted Extraction of Caffeic Acid, Rutin, Catechin and Trans-Cinnamic Acid in Physalis angulata L. and Determination by HPLC DAD. Microchem. J. 2018, 141, 247–252. [Google Scholar] [CrossRef]
- Coelho, T.L.S.; Silva, D.S.N.; dos Santos Junior, J.M.; Dantas, C.; Nogueira, A.R.d.A.; Lopes Júnior, C.A.; Vieira, E.C. Multivariate Optimization and Comparison Between Conventional Extraction (CE) and Ultrasonic-Assisted Extraction (UAE) of Carotenoid Extraction from Cashew Apple. Ultrason. Sonochem. 2022, 84, 105980. [Google Scholar] [CrossRef] [PubMed]
- Zampar, G.G.; Zampar, I.C.; Beserra da Silva de Souza, S.; da Silva, C.; Bolanho Barros, B.C. Effect of Solvent Mixtures on the Ultrasound-Assisted Extraction of Compounds from Pineapple By-Product. Food Biosci. 2022, 50, 102098. [Google Scholar] [CrossRef]
- Ferraz Bezerra, I.C.; de Moraes Ramos, R.T.; Assunção Ferreira, M.R.; Lira Soares, L.A. Optimization Strategy for Extraction of Active Polyphenols from Leaves of Eugenia uniflora Linn. Food Anal. Methods 2020, 13, 735–750. [Google Scholar] [CrossRef]
- Jones, B.; Allen-Moyer, K.; Goos, P. A-Optimal Versus D-Optimal Design of Screening Experiments. J. Qual. Technol. 2021, 53, 369–382. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Aras, O.; Pinto, C.A.; Bayramoglu, M.; Kirbaslar, S.I.; Lorenzo, J.M.; Barba, F.J.; Saraiva, J.A.; Sahin, S. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Grapefruit (Citrus paradisi Macf.) Leaves Via D-Optimal Design and Artificial Neural Network Design with Categorical and Quantitative Variables. J. Sci. Food Agric. 2018, 98, 4584–4596. [Google Scholar] [CrossRef]
- Vural, N.; Algan Cavuldak, Ö.; Akay, M.A. D-Optimal Design and Multi-Objective Optimization for Green Extraction Conditions Developed with Ultrasonic Probe for Oleuropein. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100279. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Gâvan, A.; Iacoviță, C.; Pinela, J.; Barros, L.; Ferreira, I.C.F.R.; Zhang, L.; Lucini, L.; Rocchetti, G.; et al. Optimized Ultrasound-Assisted Extraction of Phenolic Compounds from Thymus comosus Heuff. ex Griseb. et Schenk (wild thyme) and Their Bioactive Potential. Ultrason. Sonochem. 2022, 84, 105954. [Google Scholar] [CrossRef]
- Sobhani, A.; Noormohammadi, N.; Moradi, K.; Ebrahimi, M.; Khanahmadi, M. Optimization of Heat and Ultrasound Assisted Extraction of Bioactive Compounds from Echinacea purpurea Using Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100399. [Google Scholar] [CrossRef]
- Turker, I.; Isleroglu, H. Optimization of Extraction Conditions of Bioactive Compounds by Ultrasonic-Assisted Extraction from Artichoke Wastes. Acta Chim. Slov. 2021, 63, 658–666. [Google Scholar] [CrossRef]
- Farias, A.S.; Santos, H.M.; da Silva Junior, A.L.S.; da Silva, V.H.C.; Mendonça, R.B.E.S.; Coutinho, J.P.; Lôbo, I.P.; de Jesus, R.M. Multivariate Approaches Applied to Optimization of an Ultrasound-Assisted Extraction Procedure for Determination of Essential Elements in Guarana Samples by ICP OES. Food Sci. Technol. 2022, 42, e01321. [Google Scholar] [CrossRef]
- De Sousa, C.C.d.C.; dos Anjos, G.L.; Nóbrega, R.S.A.; Magaton, A.d.S.; de Miranda, F.M.; Dias, F.d.S. Greener Ultrasound-Assisted Extraction of Bioactive Phenolic Compounds in Croton heliotropiifolius Kunth Leaves. Microchem. J. 2020, 159, 105525. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; Vivar-Vera, M.A.; García-Magaña, M.L.; González-Silva, N.; Pérez-Larios, A.; Montalvo-González, E. Ultrasound-Assisted Extraction of Total Acetogenins from the Soursop Fruit by Response Surface Methodology. Molecules 2020, 25, 1139. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, K.; Rani, S. Design of Experiments: Concept and Applications of Plackett Burman Design. Clin. Res. Regul. Aff. 2007, 1, 1–23. [Google Scholar] [CrossRef]
- Rakić, T.; Kasagić-Vujanović, I.; Jovanović, M.; Jančić-Stojanović, B.; Ivanović, D. Comparison of Full Factorial Design, Central Composite Design, and Box-Behnken Design in Chromatographic Method Development for the Determination of Fluconazole and Its Impurities. Annal. Lett. 2014, 47, 1334–1347. [Google Scholar] [CrossRef]
- Dos Santos, C.; Mizobucchi, A.L.; Escaramboni, B.; Pereira-Lopes, B.; Figueiredo-Angolini, C.F.; Nogueira-Eberlin, M.; Alves de Toledo, K.; Fernández-Nuñez, E.G. Optimization of Eugenia punicifolia (Kunth) D. C. Leaf Extraction Using a Simplex Centroid Design Focused on Extracting Phenolics with Antioxidant and Antiproliferative Activities. BMC Chem. 2020, 14, 34. [Google Scholar] [CrossRef]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science, 1st ed.; Kayarogaman, P., Ed.; IntechOpen: London, UK, 2021; Volume 1, pp. 1–19. [Google Scholar]
- Davis, R.; John, P. Application of Taguchi-Based Design of Experiments for Industrial Chemical Processes. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; IntechOpen: London, UK, 2018; Volume 1, p. 137. [Google Scholar]
| Extraction Method | Solvent | Extraction Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|
| Maceration | Ethanol-Water | 120 | 0.31 | [32] |
| Soxhlet | Petroleum ether | 360 | 0.49 | [33] |
| Shaking | Methanol-Acetone-Water | 120 | 6.52 | [31] |
| Hydrodistillation | Water | 180 | 0.40 | [33] |
| Aqueous infusion | Water | 10 | <0.01 | [34] |
| * Stirring | Methanol | 4320 | 15.72 | [35] |
| Supercritical fluid | CO2 | 180 | 0.03 | [33] |
| Pressurized fluid | Water | 20 | 0.44 | [34] |
| Enzymatic | Water | 360 | 12.1 | [36] |
| Ultrasound bath | Water | 180 | 10.1 | [36] |
| Ultrasound (sonicator tip) | Hexane | 5 | 2.55 | [37] |
| Ultrasound (sonicator tip) | Methanol-Acetone-Water | 4 | 15.81 | [31] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fagus sylvatica bark | Polyphenols | Ultrasonic bath at 40 kHz of frequency | Factorial 33 | Single | Ethanol (50%, 70%, and 100 v/v), extraction time (15, 30, and 45 min), temperature (50, 60, and 80 °C) | 27 | Solvent concentration and temperature exhibited significant effects on UAE yield | [86] |
| Lime, orange, and tangerine peels | Phenolic compounds | Ultrasonic bath at 60 kHz of frequency | Factorial 22 | Single | Water content of peel (0 and 75%) and extraction time (30 and 90 min) | Four runs for each citrus peel | Extraction time had no significant effect on UAE yield | [50] |
| Common bean | Phenolic compounds | Ultrasonic bath | Two-level factorial (2k) | Single | Extraction time (40 and 80 min), temperature (30 and 50 °C), ultrasonic power (400 and 560 W), liquid-to-solid ratio (30 and 40 mL/g), Acetone concentration (40 and 60%) | 16 | Extraction time, acetone concentration, and liquid-to-solid ratio were the top three factors that influenced the UAE yield | [96] |
| Mango by-products (peel, endocarp, and kernel) | Polyphenols and flavonoids | Ultrasonic bath at 80 kHz | Factorial (23) with three central points | Single | Liquid-to-solvent ratio (0, 50, and 100%), amplitude (30, 60, and 90%) | 11 runs by each product | Solvent relation and extraction time significantly influenced the UAE yield | [97] |
| Nephelium lappaceum husk | Phenolic compounds | NI | Factorial 33 | Single | Solid-to-liquid ratio (1:3, 1:5, and 1:7), extraction time (10, 15, and 20 min), and ethanol concentration (10, 30, and 50%) | 27 | Solid-to-liquid ratio significantly influenced the UAE process | [88] |
| Strawberry-guava leaves | Phenolic compounds | Ultrasonic probe at 20 kHz of frequency coupled with a titanium tip of 4 mm diameter | Factorial 23 and central points | Single | Temperature (40, 50, and 60 °C), ultrasonic power (100, 300, and 500 W), and leaf: solvent ratio (1:10, 1:15, and 1:20 g/mL) | 11 | Power and solid-to-liquid ratio exhibited significant effects in UAE yield | [37] |
| Malagueta peppers | Phenolics and flavonoids | NI | Factorial (23) with three central points | Multiple | Solvent volume (5 and 15 mL), extraction time (2 and 20 min), temperature (30 and 50 °C) | 11 | Factorial design was used for the preliminary evaluation of extraction conditions | [98] |
| Olive pomace | Phenolic compounds | Ultrasonic probe | Factorial (2k) with five central points | Single | Amplitude (30, 50, and 70%) and extraction time (2, 7, and 12 min) | 9 | Two-level factorial design was used to reduce optimal extraction time obtained previously form a Box-Behnken design | [99] |
| Fresh green olive leaves | Phenolic compounds | Ultrasonic bath at 37 kHz of frequency | Factorial | Single | Solvent concentration (20, 50, 70, and 90% v/v), extraction time (10 to 120 min), and temperature (30 to 65 °C) | 15 | Solvent concentration and extraction time significantly influenced the UAE process | [22] |
| Spruce wood bark | Polyphenols | Ultrasonic bath at 35 kHz of frequency and power of 320 W | Complete factorial (32·2) with three central points | Single | Temperature (40, 50, and 60 °C), time (30, 45, and 60 mi), and ethanol concentration (50 and 70% v/v) | 18 | Ethanol concentration and extraction time were the most significant factors that improving extraction yield | [89] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Annona glabra leaves | Terpenes | Ultrasonic probe 2- and 7-mm diameter, 24 kHz of frequency and 200 W of power, pulse cycle of 0.1 to 1 s | 27−3 | Single | Temperature (5 and 25 °C), volume (25 and 50 mL), time (5 and 15 min), probe (2- and 7-mm diameter), solvent (methanol and acetone), amplitude (30 and 70%), cycle (0.2 and 0.8 s) | 16 | Temperature ad solvent volume were the most notable factors for increasing UAE yield | [104] |
| Moringa peregrina | Phenolic compounds | Ultrasonic bath at 20 kHz of frequency and 580 W of power | 24−1 | Single | Liquid-to-solid ratio (5 and 15 mL/g), ultrasound power (30 and 100%), time (5 and 25 min), temperature (30 and 60 °C) | 8 | The liquid-to-solid ratio and extraction time had significant effects on UAE yield | [105] |
| White birch bark | Betulin | Ultrasonic probe 12.7 mm diameter, 20 kHz of frequency and 450 W of power | 25−1 | Single | Ethanol concentration (65, 80 and 95% v/v), solid-to-liquid ratio (1:40, 1:25, and 1:10), extraction temperature (40, 50, and 60 °C), ultrasonic frequency (2, 5. And 8 kHz), extraction time (1, 3, and 5 min) | 16 | Ethanol concentration and solid-to-liquid ration significantly influenced the UAE yield | [103] |
| Sour cherries | Total phenolics, flavonoids, and anthocyanins | Ultrasonic bath at 40 kHz frequency | 25−1 | Single | Temperature 40 and 60 °C, extraction time 20 and 40 min, ethanol concentration 40 and 60% v/v, ultrasonic power 30 and 60 W/L, and liquid-solid ratio 10 and 20 mg/L | 16 | Temperature, liquid-to-solid ratio, and ethanol concentration had significant effects on UAE yield | [106] |
| Cecropia species, leaves | Phenols, flavonoids, and anthocyanins | Ultrasonic bath at 42 kHz frequency and 100 W of power | 27−3 | Single | Methanol concentration (50 and 90%, v/v), extraction time (30 and 90 min), number of extractions with methanol (1 and 3), extraction temperature (20 and 60 °C), plant-solvent ratio (1:20 and 1:100, m/v), number of extractions with acetone (0 and 2), and particle size (≤710 and ≤125 µm) | 16 | Methanol concentration and extraction temperature had significant effects on UAE yield | [81] |
| Pistacia lentiscus leaves | Total phenols, flavonoids, and tannins | Ultrasonic bath at 39 kHz frequency and 100 W of power | 24−1 with a central point | Single | Temperature (5 and 25 °C), time (15 and 30 min), solvent ratio (0.06 and 0.1 L/g), ethanol concentration (50 and 75%) | 9 | The solvent ratio is the most important factor affecting positively the UAE process | [90] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hawthorn seed | Flavonoids | Ultrasonic bath at 40 kHz of frequency and 100 W of power | L15 (+1, 0, −1) | Simple | Ultrasound temperature (55 and 75 °C), time (30 ad 50 min), ethanol concentration (55 and 85%), solid-liquid ratio (1:14 and 1:22), extraction temperature (82 and 98 °C), and extraction time (1 and 2 h) | 15 | Ultrasonic time, ethanol concentration, and temperature were the most significant variables that influenced the UAE process | [107] |
| Lonicera caerulea | Anthocyanins | Ultrasonic bath at 40 kHz of frequency and 100 W of power | L15 (+1, −1) | Single | Solvent-liquid ratio (5:1 and 25:1), ethanol concentration (70 and 100%), formic acid concentration (0 and 1%), ultrasound bath temperature (25 and 45 °C), extraction time (10 and 30 min) | 15 | Liquid-solid ratio, solvent concentration, and extraction time were the most significant factors that affects the yield recovering od anthocyanins | [91] |
| Grape pomace | Phenolic compounds | Ultrasonic bath at 28 kHz of frequency and 600 W of power | L11 (+1, −1) | Single | Ethanol concentration (0, 40, and 80%), solid-to-liquid ratio (1:10, 1:35, and 1:60 g/mL) | 11 | Solvent concentration significantly influenced the extraction yield | [108] |
| Ceratonia siliqua | Polyphenols | Ultrasonic bath operating in continuous mode | L11 (+1, −1) | Multiple | Extraction time (5 and 60 °C), temperature (15 and 50 °C), solid: solvent ratio (0.05 and 0.2 g/mL), solvent concentration (0 and 100%), sonication frequency (37 and 80 kHz), sonication power (30 and 100 W), particle size (0.3 and 2 mm) | 11 | Extraction time and temperature were the most important factors that influenced the recovering yield of polyphenols | [73] |
| Rubia sylvatica Nakai fruit | Total anthocyanins ad total phenolics | Ultrasonic bath at 40 kHz of frequency and 600 W of power at 30 °C for 20 min | L12 (+1, −1) | Single | Ethanol concentration (30 and 40%), liquid: solid ratio (20 and 30 mg/L), ultrasound power (400 and 500 W), pH value (2 and 3), extraction temperature (50 and 60 °C), extraction time (20 and 30 min) | 12 | Recovering yield of bioactive compounds is dependent on experimental conditions and type of compound | [109] |
| Kaempferia parviflora Rhizomes | Methoxyflavone | Ultrasonic bath at 40 kHz of frequency and 160 W of power | L12 (+1, −1) | Single | Type of solvent (methanol and ethanol), organic solvent concentration (50 and 95%), extraction time (5 and 30 min), temperature (30 and 80 °C), solvent-to-solid ratio (10 ad 50 mL/g) | 12 | The most critical variables were ethanol concentration, solvent-to-solid ratio, and extraction time | [71] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Piper betle leaves | Total phenols and flavonoids | Ultrasonic bath at 37 kHz and 400 W of power | 33 | Multiple | Temperature (50, 60, and 70 °C), ethanol concentration (70, 80, and 90% v/v), and solid-to-liquid-ratio (1:10, 1:20, and 1:30 g/mL) | 17 | Solid-to-liquid ratio had significant effects on yield | [92] |
| Myrtle (Myrtus communis L.) | Phenolic compounds and total anthocyanins | Ultrasonic probe | 36 | Single | Solvent concentration (50–100% for phenolic and 25–75% for anthocyanins), temperature (10–60 °C), amplitude (30–70%), cycle (0.2–0.7 s), pH (2–7), and liquid-to-solid ratio (10:0.5–20:0.5 mL/g) | 54 | Interaction between solvent and temperature and interaction between cycle and liquid-to-solid ratio had significant effects on phenolics yield, where solvent and pH had significant effects on anthocyanins yield | [111] |
| Malagueta peppers | Phenolic compounds and flavonoids | NI | 33 | Multiple | Solvent volume (8, 12, and 16 mL), time (15, 30, and 45 min), temperature (40, 50, and 60 °C) | 15 | The best UAE conditions were 16 mL of solvent during 15 min at 55 °C | [98] |
| Common centaury (Centaurium erythraea Rafn) | Total phenolic compounds | Ultrasonic bath at 40 kHz and 150 W of power | 34 with three center point | Single | Time (20, 25, and 30 min), solvent concentration (30, 50, and 70% v/v), liquid-to-solid ratio (5, 10, and 15 mL/g), temperature (40, 55, and 70 °C) | 29 | All extraction factors and their interaction significantly influenced the UAE yield | [4] |
| Yellow and Red Tamarillo fruits (Solanum betacum) | Phenolic compounds and flavonoids | Ultrasonic probe at 6 mm diameter and 500 W of power, amplitude of 0–100%, and pulse cycle of 2 s | 33 with five central point | Multiple | Time (5, 10, and 15 min), amplitude (20, 40, and 60%), solvent concentration (50, 60, and 65%) | 17 | All extraction factors and their interaction significantly influenced the UAE yield | [112] |
| Muicle (Justicia spicigera) leaves | Phenolic compounds | Ultrasonic probe at 7 mm diameter, 400 W and 24 kHz | 33 | Single | Pulse cycle (0.4, 0.7, and 1 s), amplitude (40, 70 and 100%), time (2, 7, and 12 min) | 15 | Pulse cycle was the most important factor followed by amplitude for UAE process | [113] |
| Annona muricata by-products | Phenolic compounds | Ultrasonic probe at 7 mm diameter, 400 W and 24 kHz | 33 | Single | Pulse cycle (0.4, 0.7, and 1 s), amplitude (40, 70 and 100%), time (5, 10, and 15 min) | 15 | The yield recovering depended on the composition of matrix | [114] |
| Psidium cattleianum leaves | Phenolic compounds | Ultrasonic probe at 7 mm diameter, 400 W and 24 kHz | 33 | Single | Pulse cycle (0.4, 0.7, and 1 s), amplitude (60, 80 and 100%), time (2, 4, and 6 min) | 15 | Extraction time had significant effects on yield | [31] |
| Pomegranate peel | Total phenolics and flavonoids | Ultrasonic probe at 6 mm diameter, 500 W and 40 kHz | 34 | Single | Pulse cycle (0.2, 0.5, and 0.8 s), amplitude (50, 65, and 80%), time (5, 10, and 15 min), methanol concentration (30, 50, and 70%) | 29 | Methanol concentration and amplitude has significant effect on UAE process | [115] |
| jabuticaba (Myrciaria cauliflora) fruit | Phenolic compounds and anthocyanins | Ultrasonic probe at 7 mm diameter, 200 W and 24 kHz | 36 | Single | Methanol concentration (25, 50, and 75%), temperature (10, 40, and 70 °C), amplitude (30, 50, and 70%), cycle (2, 4.5, and 7 s), solvent-to-sample ratio (10:1.5, 15:1.5, and 20:1.5) | 54 | Solvent composition was the most important factor that influenced the UAE yield recovering | [57] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Spinach roots | TPC and TFC | Ultrasonic probe of 9 mm diameter at 200 W, 20 kHz of frequency | CCD with 3 independent variables at 5 levels (−α, −1, 0, +1, + α) | Multiple | Amplitude (10, 25, 40, 55, and 70%), temperature (0, 10, 20, 30, and 40 °C), time (2, 3, 4, 5, and 5 min), ethanol concentration (0, 20, 40, 60, and 80%) | 30 | TPC and flavonoids yield were influenced by independent variables during extraction process | [2] |
| Acerola residues | Carotenoids, phenolics, and flavonoids | Ultrasonic bath at 50 kHz and 250 W of power | CCRD with 8 factorial, 6 axial, and 3 central points | Multiple | Ethanol concentration (0–99.5%), ethanol: residue ratio (1–10 mL/g), and extraction time (10–60 min) | 17 | All factors significantly influenced the UAE yield recovering in a bioactive compound-response manner | [116] |
| Safflower seed | NI | NI | CCD with 3 independent variables at 5 levels (−α, −1, 0, +1, + α) | Single | Extraction time (5–55 min), temperature (26–94 °C), and ethanol concentration (0–100%) | 17 | The highest extraction yield was observed applying 80% ethanol concentration for 45 min at 40 °C | [80] |
| Ficaria kochii | TPC and TFC | Ultrasonic bath at 50–60 kHz | CCRD with 3 independent variables at 5 levels (−α, −1, 0, +1, + α) | Single | Time (30–60 min), solvent-to-solid ratio (1–13%), and temperature (30–70 °C) | 20 | All factors significantly influenced the UAE yield recovering in a bioactive compound-response manner | [51] |
| Chestnut shells | Polyphenols | Ultrasound probe at 13 mm diameter and 50% of amplitude | CCD with two independent variables at 5 levels | Multiple | Time (4, 10, 25, 40, and 46 min) and temperature (34, 40, 55, 70, and 76 °C) | 13 | The extraction time has significant effect on UAE yield recovering, while temperature did not show significant effect | [117] |
| Garlic leaves | TPC and TFC | Ultrasound probe at 16 mm diameter at 20 kHz and 700 W of power | CCRD with 3 independent variables at 5 levels (−α, −1, 0, +1, + α) | Single | Ultrasound amplitude (19, 30, 45, 60, and 70%), time (1.6, 5, 10, 15, and 18.4 min), and ethanol concentration (33, 40, 50, 60, and 66.8%) | 20 | The highest extraction yield was observed under 50% ethanol concentration for 13 min and 53% amplitude | [20] |
| Garcinia indica | TPC and TFC | NI | CCFC with 5 independent variables at 5 levels (−α, −1, 0, +1, + α) | Single | Ultrasound intensity (46, 60, 70, 80, and 93 Wcm2), methanol concentration (49, 60, 67, 75, and 85%), pulse cycle (0.05, 0.2, 0.4, 0.6, and 0.88 s), particle size (0.1, 0.25, 0.625, 1, and 1.52 mm), temperature (9.3, 30, 45, 60, 80.6 °C) | 48 | The extraction yield was dependent on experimental conditions for both bioactive compounds | [118] |
| Black locust flowers | TPC | Ultrasonic bath at 40 kHz | CCD with 3 independent variables at 5 levels (−α, −1, 0, +1, + α) | Multiple | Ethanol concentration (33–67%), temperature (33–67 °C), and time (17–33 min) | 17 | The highest extraction yield was observed under 60% ethanol concentration for 30 min | [84] |
| Sideritis raeseri | TPC | Ultrasonic bath | CCD with 4 independent variables at five levels (−α, −1, 0, +1, + α) | Multiple | Extraction time (5, 20, 35, 50, and 65 min), ethanol concentration (10, 30, 50, 70, and 90%), solid-to-liquid ration (1:10, 1:20, 1:30, 1:40, and 1:50 g/mL), temperature (20, 35, 50, 65, and 80 °C) | 30 | The highest extraction yield was observed under 65% ethanol concentration for 50 min at 63 °C using a solid-to-liquid ratio of 1:40 | [119] |
| Triticum aestivum seeds | TPC | Ultrasonic bath at 40 kHz and 150 W of power | CCD with 3 independent variables at 5 levels (−α, −1, 0, +1, + α) | Multiple | Ethanol concentration (33, 40, 50, 60, and 67% v/v), temperature (33, 40, 50, 60, and 67 °C), and time (17, 20, 25, 30, and 33 min) | 18 | The highest extraction yield was observed under 56% ethanol concentration for 28 min at 59 °C | [120] |
| Ceratonia siliqua | Polyphenols | Ultrasonic bath at 40 kHz of frequency and 160 W of power | Non-standard central composite design with α = 1.6818 for rotatability | Multiple | Solvent-to-solid ratio (0.05, 0.08, 0.2, 0.21 mL/g), ethanol concentration (0, 20, 5, 80, 100), particle size (0.3, 0.5, 1.0, and 2.0 mm) | 17 | The effect depended in the type of extracted polyphenol compound | [73] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Coffee leaves | Polyphenols | NI | L8 (26) | Multiple | Ethanol concentration (0 and 60%), temperature (30 and 80 °C), ultrasound power (0 and 210 W), time (10 and 40 min), coffee leaf age (young and mature), liquid: solid ratio (10:1 and 40:1) | 8 | Liquid: solid ratio, ethanol concentration, and extraction temperature were the most significant factor that influenced the recovery yield of bioactive compounds | [127] |
| Red cabbage | Anthocyanins | Ultrasound probe at 10 mm of diameter | L9 (34) | Single | Temperature (15, 30, and 45 °C), time (30, 60, and 90 min), power (50, 75, and 100 W), pulse mode (0.3, 0.65, and 1) | 9 | Time, temperature, and power ultrasound were the most important factors that contribute the yield extraction | [70] |
| Butterfly pea petals | Anthocyanins and total phenolic compound | Ultrasound bath at an output power of 160 W | L9 (33) | Multiple | Extraction time (30, 45, and 60 min), temperature (40, 60, and 80 °C), liquid: solid ratio (5, 7.5, and 10 mL/g) | 9 | Liquid–solid ratio showed the highest contribution for recovering anthocyanin and total phenolic content | [24] |
| Curcuma longa rhizomes | Curcumin | Ultrasound bath | L9 (34) | Single | Extraction time (20, 40, and 60 min), solvent viscosity (0.32, 0.6, and 1.2 cp), sieve number (10, 20, and 40), solvent volume (10, 20, and 30 mL) | 9 | Curcumin yield was influenced by the UAE conditions | [130] |
| Azadirachta indica | Phenolic compounds | Ultrasound probe at 2 cm of diameter and 13.5 cm height, frequency of 20 kHz and 120 W, pulse mode 5 s on/off | L16 | Single | Particle size (0.15, 0.212, 0.425, and 0.6 mm), irradiation time (15, 30, 45, and 60 min), solid-to-liquid ratio (1:20, 1:30, 1:40, and 1:1:50), temperature (25, 35, 45, and 55 °C) | 16 | Particle size significant influence the yield recovering followed by temperature | [76] |
| Hamelia patens | Polyphenols | Ultrasound bath | L9 (33) | Single | Solid: liquid ratio (1:8, 1:12, and 1:16), extraction time (10, 20, and 30 min), ethanol concentration (0, 35, and 70%) | 9 | Solid: liquid ratio was the most important factor that influenced the yield recovering of polyphenols followed by ethanol concentration | [131] |
| Clitoria ternatea petals | Anthocyanins | Ultrasound bath | L27 (33) and S/N ratio | Single | Time (30, 40, and 50 min), temperature (40, 50, and 60 °C), solvent-to-liquid ratio (10:1, 20:1, and 30:1 mL/g) | 27 | The optimum conditions for UAE of anthocyanins were 50 °C at 10:1 mL/g for 30 min | [132] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Physalis angulata | Polyphenols | Ultrasound bath at 50/60 Hz and 90 W of power for 10 min at 30 °C | SCD | Single | Water (0–100%), methanol (0–100%), ethanol (0–100%), sonication time (15 min), extractor volume (15 mL) | 7 | The best proportions of solvents were 57% water, 35% ethanol, and 8% methanol | [136] |
| Cashew apple | Carotenoids | Ultrasound bath at 40 kHz and 80 W of power | SCD | Single | Acetone (0–100%), ethanol (0–100%), petroleum ether (0–100%), methanol (0–100%) | 15 | The best proportions of solvents were 44% acetone and 56% methanol | [137] |
| Mauritia flexuosa | Carotenoids | Ultrasound bath at 40 kHz and 80 W of power | LSD | Multiple | Acetone, ethanol, methanol, acetonitrile | 25 | The best proportions of solvents were 75% acetone and ethanol 25% | [134] |
| Pineapple by-product | Polyphenols | NI | SCD | Single | Water (0–100%), ethanol (0–100%), and acid solution 1 mol L−1 HCl (0–100%) | 13 | The highest polyphenol yield was obtained using ethanol and acid solution in a proportion of 50:50 | [138] |
| Moroccan Pimpinella anisum | Polyphenols and flavonoids | Ultrasound bath at 37 kHz and 100 W of power | SCD | Multiple | Water, ethanol, methanol, dichloromethane, chloroform, acetone, ethyl acetate, hexane, butanol and acetonitrile | 12 | The best proportions of solvents were 44% water, 22% ethanol, and 34% methanol | [135] |
| Taraxacum assemanii | Polyphenols | Ultrasound bath at 35 kHz | SCD | Single | Ethanol (0–100), methanol (0–100), water (0–100) | 14 | Ethanol-water (68:32) were the best proportion of extraction solvent | [133] |
| Eugenia uniflora leaves | Polyphenols | NI | SCD | Multiple | Water, methanol, ethanol and acetone | 15 | The best proportions of solvents were 46% water, 13% methanol, 18% ethanol, and 23% acetone | [139] |
| Capsicum frutescens | Polyphenols | Ultrasound bath | SCD | Multiple | Ethanol (0–100), methanol (0–100), water (0–100) | 10 | The best mixture proportion was 95% ethanol and water 5% | [98] |
| Mango peel | Polyphenols | Ultrasound probe at 2 cm diameter | SCD | Single | Ethanol, acetone, hexane | NI | The best mixture proportion was 60% ethanol and 40% acetone | [74] |
| Source | Bioactive Compound | Ultrasonic Equipment | DOE | Single or Multiple Response | Factors and Levels | Number of Runs | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Walnut male flowers | Phenolic compounds and flavonoids | Ultrasound bath | Three factors, three levels D-optimal design | Multiple | Extraction time (10, 30, and 50 min), solvent type (methanol, ethanol, and acetone), and water in solvent (20, 40, and 60% v/v) | 21 | The higher extraction yield was performed after 30 min of extraction containing 40% water in acetone | [86] |
| Cynara sco- Lymus leaves | Phenolics and flavonoids | Ultrasound probe at 13 mm of diameter, and 500 W of power at 20 kHz of frequency | Two factors D-optimal design | Multiple | Extraction time (20–60 min), Ultrasound amplitude (30–80%) | 19 | The best extraction conditions were 20.05 min of extraction time and 65.02% of ultrasonic amplitude | [145] |
| Echinacea purpurea using | Polyphenols | Ultrasound bath at 320 W of power and 35 kHz of frequency for 30 min | Four factor D-optimal design | Multiple | Temperature (25–75 °C), sonication time (0–60 min), solvent concentration (0–100%), and solvent type (methanol and ethanol) | 34 | The optimal UAE conditions were 41.70% methanol at 75 °C for 51.8 min | [144] |
| Grapefruit leaves | Phenolic compounds | Ultrasound probe at 20 kHz of frequency and 125 W of power | Six factors D-optimal design | Multiple | Ethanol concentration (0–50%), extraction time (15–60 min), temperature (25–50 °C), solid: liquid ratio (50–100 g/L), ultrasound power density (0.25–0.5 kW/L), probe type (thin and thick) | 34 | The optimal UAE conditions were ethanol concentration of 10.80% at 30.37 °C for 58.52 min | [141] |
| Wild thyme aerial parts | Phenolic compounds | Ultrasound probe at 6 mm of diameter | Three factor D-optimal design | Single | Time (1, 3, 5, 7, and 10 min), ultrasound amplitude (20, 30, and 40%), ethanol concentration (30, 50, and 70%) | 19 | The optimal UAE conditions were time 5 min, amplitude 30%, and ethanol concentration 50% | [143] |
| Olive leaves | Oleuropin | Ultrasound probe at 13 mm of diameter and 20 kHz of frequency | Five factors D-optimal design | Multiple | Amplitude (32–89%), sonication time (1–15 min), ethanol or methanol concentration (50–80%), probe position (1.5–4 cm), duty cycle (0.3–1%), solvent: solid ratio 12.80 mg/L, temperature 30 °C | 31 | The optimum UAE conditions were amplitude 81.91%, time 14.22 min, MeOH 76.97%, probe position 3.89 cm, duty-cycle 0.93% | [142] |
| Source | Bioactive Compound | Screening DOE | Optimizing DOE | RSM | Mathematical Model | Ref. |
|---|---|---|---|---|---|---|
| Ripe carob pods | Polyphenols | Placket-Burman | Non-standard central composite | RSM | Quadratic model | [73] |
| Pistacia lentiscus Leaves | Polyphenols | Fractional factorial design | Box-Behnken | RSM | Quadratic model | [90] |
| Cecropia sp. | Polyphenols | Fractional factorial | Central composite | RSM | Quadratic model | [81] |
| Sour cherries | Polyphenols and anthocyanins | Fractional factorial | Face-centered central composite | RSM | Quadratic model | [106] |
| Haskap berries | Anthocyanins | Placket-Burman | Box-Behnken | RSM | Linear and quadratic models | [91] |
| Hawthron seed | Flavonoids | Placket-Burman | Box-Behnken | RSM | Linear and quadratic models | [107] |
| Grape pomace | Phenolic compounds | Placket-Burman | Face-centered central composite | RSM | Quadratic model | [108] |
| Kaempferia parviflora Rhizomes | Methoxyflavon | Placket-Burman | Box-Behnken | RSM | Linear and quadratic models | [71] |
| Rubia sylvatica | Anthocyanins | Placket-Burman | Box-Behnken | RSM | Linear and quadratic models | [109] |
| Coffee leaves | Phenolic compounds | Taguchi design | Box-Behnken | RSM | Quadratic model | [127] |
| Mauritia flexuosa | Carotenoids | Simplex-lattice | Central composite | RSM | Linear, quadratic, and cubic models | [134] |
| Malagueta peppers | Phenolic compounds | Full factorial | Box-Behnken | RSM | Quadratic model | [98] |
| Croton heliotropiifolius Kunth leaves | Phenolic compounds | Full factorial | Doehlert | RSM | Quadratic model | [147] |
| Design of Experiment | Advantages | Limitations |
|---|---|---|
| Full factorial | Robust DOE It is possible to evaluate the main and the interaction effects clearly | Number of factors should be 2 to 5. Substantial increase in the number of experiments as the number of factors increases. Complexity in interpreting complex interactions. Confusion issues may arise when there are interactions. Difficulty handling categorical factors. |
| Fractional factorial | It is recommended when the number of factors exceeds 4 Allows for study of interactions and quadratic effects within variables Reduced number of experimental runs compared to full factorial design | Designs with high degree of aliasing may result in high collinearity between variables. May lose important information by omitting some combinations. Not suitable for all experiments due to the design fraction. Difficulty in studying higher-order interactions. Choosing the appropriate fraction can be challenging. |
| Plackett-Burman | It is a useful tool for initiating the optimization process by screening a substantial number of factors (>4) Eliminate non-significant variables from the models | The aliasing pattern is highly complex, each main effect is aliased with every two-way interaction not involving that effect. Lack of fit is difficult to assess, and first-order effects may be confounded with interaction effects. Limited in its ability to study non-linear responses. Does not provide information on the influence of categorical factors. |
| Box-Behnken | Allows for study of interactions and quadratic effects within variables Reduced number of experimental runs compared to full factorial design | Substantial increase in the number of experiments as the number of factors increases. At least 3 factors and 3 levels are required. It does not examine borderline regions of experiment factors. Cannot handle categorical factors. The choice of central points can affect the accuracy of estimates. |
| Central composite | It no need for a three-level factorial design for building a second-order quadratic model Allows for study of interactions and quadratic effects within variables It contains the extreme factor combinations Maximum information in a minimum experimental trial | The star points are outside the hypercube. Depending upon the Design, the squared terms in the model will not be orthogonal to each other. Inability to estimate individual interaction terms Efficiency may decrease in the presence of interactions. Sensitive to the choice of axial and central points. |
| Taguchi | Robust DOE It is a screening tool for identifying the significant factors that affect the process A good amount of data can be obtained with lesser resources | It exhibited difficulty in accounting for interactions between parameters. It is not appropriate in dynamically changing processes. Limited in terms of flexibility for some types of responses. Design robustness may depend on the appropriate choice of factor levels. |
| Simplex-Centroid | It is widely used for obtaining formulations It minimizes the model error and the number of required experiments | Not suitable for experiments with many factors. Efficiency may decrease if factors are highly correlated. Does not allow the evaluation of complex interactions. Interpretation of effects can be complicated. |
| D-optimal | Significant reduction in number of experimental runs Allow the study of multiple combinations of multilevel factors, independently if the number of variable levels of factors is different in the same experimental design | May require use of extensive computational resources Requires prior knowledge of effect variances. Does not guarantee a unique design, which can lead to suboptimal solutions. Interpretation can be challenging for experimenters unfamiliar with optimal design theory. Implementation can be costly and require additional resources. |
| Doehlert | It is enables to examinate multiple variables with different levels within a single matrix, reducing the number of experiments | It does not have any of the properties of the response surface matrices that include isovariance by rotation, orthogonality, and uniform precision. Not efficient when the number of factors is large. Limited in terms of handling categorical factors. Interpretation can be complicated for complex responses. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Aurora-Vigo, E.F.; Villagrán, Z.; Rodríguez-Lafitte, E.; Ruvalcaba-Gómez, J.M.; Solano-Cornejo, M.Á.; Zamora-Gasga, V.M.; Montalvo-González, E.; Gómez-Rodríguez, H.; Aceves-Aldrete, C.E.; et al. Design of Experiments for Optimizing Ultrasound-Assisted Extraction of Bioactive Compounds from Plant-Based Sources. Molecules 2023, 28, 7752. https://doi.org/10.3390/molecules28237752
Anaya-Esparza LM, Aurora-Vigo EF, Villagrán Z, Rodríguez-Lafitte E, Ruvalcaba-Gómez JM, Solano-Cornejo MÁ, Zamora-Gasga VM, Montalvo-González E, Gómez-Rodríguez H, Aceves-Aldrete CE, et al. Design of Experiments for Optimizing Ultrasound-Assisted Extraction of Bioactive Compounds from Plant-Based Sources. Molecules. 2023; 28(23):7752. https://doi.org/10.3390/molecules28237752
Chicago/Turabian StyleAnaya-Esparza, Luis Miguel, Edward F. Aurora-Vigo, Zuamí Villagrán, Ernesto Rodríguez-Lafitte, José Martín Ruvalcaba-Gómez, Miguel Ángel Solano-Cornejo, Victor Manuel Zamora-Gasga, Efigenia Montalvo-González, Horacio Gómez-Rodríguez, César Eduardo Aceves-Aldrete, and et al. 2023. "Design of Experiments for Optimizing Ultrasound-Assisted Extraction of Bioactive Compounds from Plant-Based Sources" Molecules 28, no. 23: 7752. https://doi.org/10.3390/molecules28237752
APA StyleAnaya-Esparza, L. M., Aurora-Vigo, E. F., Villagrán, Z., Rodríguez-Lafitte, E., Ruvalcaba-Gómez, J. M., Solano-Cornejo, M. Á., Zamora-Gasga, V. M., Montalvo-González, E., Gómez-Rodríguez, H., Aceves-Aldrete, C. E., & González-Silva, N. (2023). Design of Experiments for Optimizing Ultrasound-Assisted Extraction of Bioactive Compounds from Plant-Based Sources. Molecules, 28(23), 7752. https://doi.org/10.3390/molecules28237752










