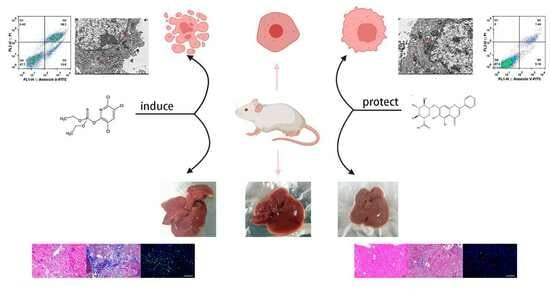
Protective Effect of Baicalin on Chlorpyrifos-Induced Liver Injury and Its Mechanism
Abstract
:1. Introduction
2. Results
2.1. LD50 of CPF
2.2. The Relationship between BA and CPF on the Survival Rate of AML12 Cells
2.3. Determination of Cellular Indicators in Cell Supernatants
2.4. The Contents of Inflammatory Factors in the Supernatant of Each Experimental Group
2.5. Protective Effect of BA against Oxidative Stress in CPF-Induced AML12 Cells
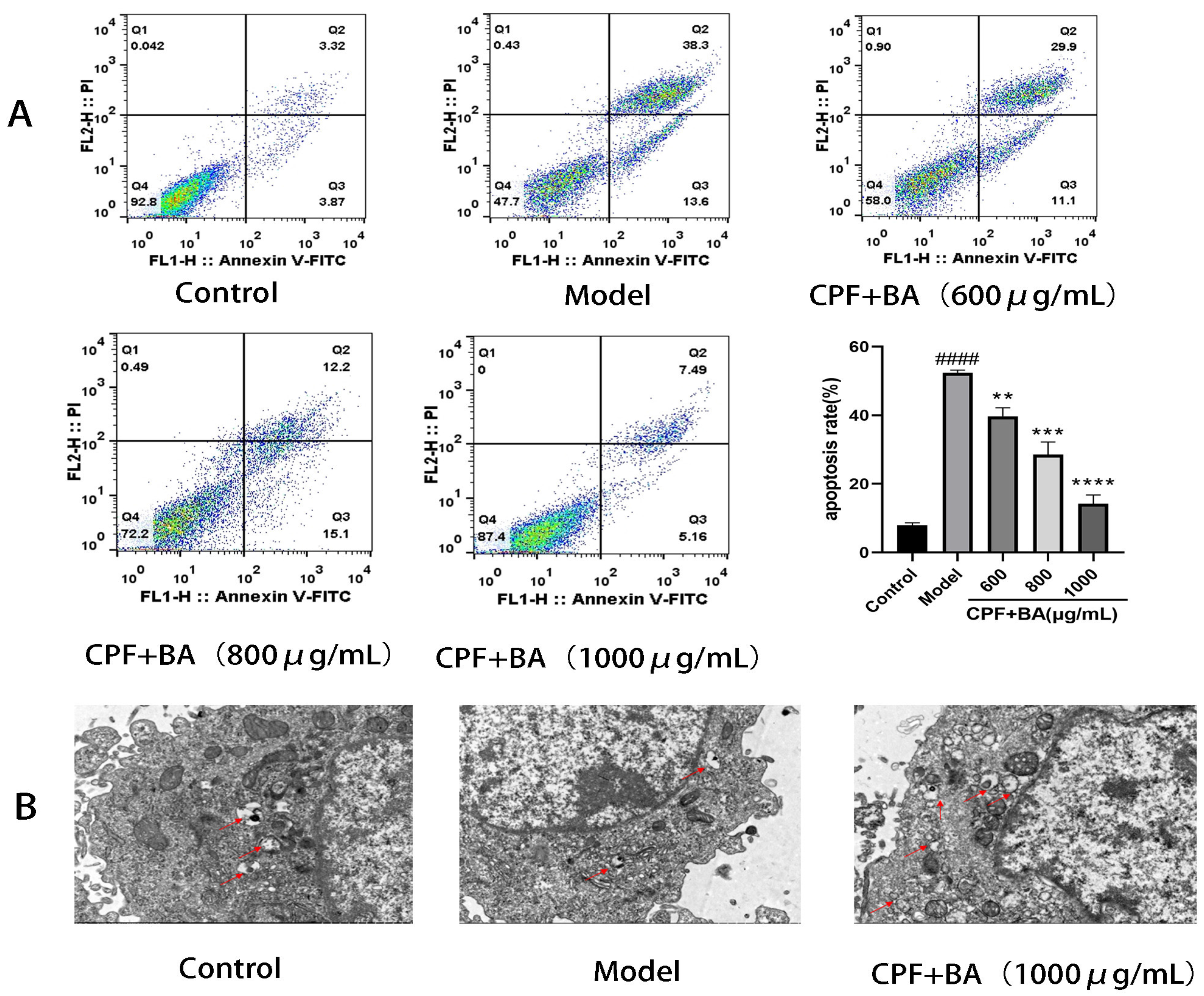
2.6. Annexin V-FITC/PI Staining
2.7. BA Improves CPF-Induced AML12 Cells Injury by Enhancing Autophagy
2.8. Study on Mechanism of Apoptosis of CPF-Induced AML12 Cells Induced by BA
2.9. BA Improved CPF-Induced Kunming Female Mouse Liver Injury
2.10. BA Improved the Inflammatory Response of CPF-Induced Kunming Female Mouse Liver Injury
2.11. BA Improved Oxidative Stress of CPF-Induced Kunming Female Mouse Liver Injury
2.12. Liver Histopathology Testing
2.13. TUNEL Staining Analysis
2.14. BA Exerts Anti-CPF-Induced Kunming Female Mouse Liver Injury by Enhancing Autophagy
2.15. BA Improved CPF-Induced Kunming Female Mouse Liver Injury by Regulating Apoptosis Pathway
3. Discussion
4. Materials and Methods
4.1. Experimental Articles and Detection Reagents
4.2. Cell Culture
4.3. CCK-8 Measures the LD50 of CPF on AML12
4.4. Screening of Baicalin Therapeutic Concentrations and AML12 Cells Treatment
4.5. ELISA
4.6. Measurement of Cellular Oxidative Stress Indicators
4.7. Annexin V-FITC/PI Staining
4.8. TEM
4.9. Animal Experimental Program
4.10. Measurement of Liver Function and Inflammatory Factors
4.11. Measurement of Oxidative Stress Indicators
4.12. Liver Histopathology Testing
4.13. TUNEL Staining
4.14. Western Blot
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, L.; Shi, X.; Xu, S. Chlorpyrifos induces the apoptosis and necroptosis of L8824 cells through the ROS/PTEN/PI3K/AKT axis. J. Hazard. Mater. 2020, 398, 122905. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.; Hu, C.; Han, M.; Guo, Q.; Li, S.; Bo, C.; Zhang, Y.; Qi, X.; Sai, L.; et al. Effects of chlorpyrifos exposure on liver inflammation and intestinal flora structure in mice. Toxicol. Res. 2021, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Joo, H.; Rose, R.L.; Hodgson, E. Metabolism of chlorpyrifos and chlorpyrifos oxon by human hepatocytes. J. Biochem. Mol. Toxicol. 2006, 20, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Nikbin, S.; Tajik, A.; Allahyari, P.; Matin, G.; Hoseini Roote, S.S.; Barati, E.; Ayazi, M.; Karimi, L.; Dayani Yazdi, F.; Javadinejad, N.; et al. Aerobic exercise and eugenol supplementation ameliorated liver injury induced by chlorpyrifos via modulation acetylcholinesterase activation and antioxidant defense. Environ. Toxicol. 2020, 35, 783–793. [Google Scholar] [CrossRef]
- Dai, C.; Li, H.; Wang, Y.; Tang, S.; Velkov, T.; Shen, J. Inhibition of oxidative stress and ALOX12 and NF-κB pathways contribute to the protective effect of baicalein on carbon tetrachloride-induced acute liver injury. Antioxidants 2021, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Qian, J.; Wu, G.; Ma, Z. Baicalin attenuates liver hypoxia/reoxygenation injury by inducing autophagy. Exp. Ther. Med. 2018, 16, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Long, Y.; Ren, G.; Zhang, Y.; Chen, J.; Huang, R.; Yang, L. Punicalagin reversed the hepatic injury of tetrachloromethane by antioxidation and enhancement of autophagy. J. Med. Food 2019, 22, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Ding, W.-X. Targeting Pink1-Parkin-mediated mitophagy for treating liver injury. Pharmacol. Res. 2015, 102, 264–269. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Zhang, J.A.; Luan, C.; Huang, D.; Ju, M.; Chen, K.; Gu, H. Induction of autophagy by baicalin through the AMPK-mTOR pathway protects human skin fibroblasts from ultraviolet B radiation-induced apoptosis. Drug Des. Devel. Ther. 2020, 14, 417–428. [Google Scholar] [CrossRef]
- Lin, C.; Tsai, S.C.; Tseng, M.T.; Peng, S.F.; Kuo, S.C.; Lin, M.W.; Hsu, Y.M.; Lee, M.R.; Amagaya, S.; Huang, W.W.; et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int. J. Oncol. 2013, 42, 993–1000. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Feng, Y.; Tong, Y.; Jia, Y.; Wang, C.; Cui, R.; Qu, K.; Liu, C.; Zhang, J. PPARγ alleviates sepsis-induced liver injury by inhibiting hepatocyte pyroptosis via inhibition of the ROS/TXNIP/NLRP3 signaling pathway. Oxid. Med. Cell Longev. 2022, 2022, 1269747. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Tang, W.; Geng, P.; Zhu, H.; Zhou, W.; Huang, H.; Zhou, P.; Shi, X. Baicalin down-regulating hepatitis B virus transcription depends on the liver-specific HNF4α-HNF1α axis. Toxicol. Appl. Pharmacol. 2020, 403, 115131. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, R.; Qiu, T.; Wang, J.; Meng, J.; Zhu, J.; Wang, D.; Wu, Y.; Liu, J. The protective effect of baicalin on duck hepatitis A virus type 1-induced duck hepatic mitochondria dysfunction by activating nuclear erythroid 2-related factor 2/antioxidant responsive element signaling pathway. Poult. Sci. 2021, 100, 101032. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Y.; Ke, X.; Zhang, R.; Zuo, L.; Wang, M.; Chen, Z.; Luo, X.; Wang, J. Baicalin ameliorates alcohol-induced hepatic steatosis by suppressing SREBP1c elicited PNPLA3 competitive binding to ATGL. Arch. Biochem. Biophys. 2022, 722, 109236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018, 72, 655–666. [Google Scholar] [CrossRef]
- Shen, K.; Feng, X.; Pan, H.; Zhang, F.; Xie, H.; Zheng, S. Baicalin Ameliorates Experimental Liver Cholestasis in Mice by Modulation of Oxidative Stress, Inflammation, and NRF2 Transcription Factor. Oxidative Med. Cell Longev. 2017, 2017, 6169128. [Google Scholar] [CrossRef]
- Sun, J.; Yang, X.; Sun, H.; Huang, S.; An, H.; Xu, W.; Chen, W.; Zhao, W.; He, C.; Zhong, X.; et al. Baicalin inhibits hepatocellular carcinoma cell growth and metastasis by suppressing ROCK1 signaling. Phytother. Res. 2023, 37, 4117–4132. [Google Scholar] [CrossRef]
- Louis, H.; Le Moine, O.; Goldman, M.; Devière, J. Modulation of liver injury by interleukin-10. Acta Gastro Enterol. Belg. 2003, 66, 7–14. [Google Scholar]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Wang, R.; Song, F.; Li, S.; Wu, B.; Gu, Y.; Yuan, Y. Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des. Devel. Ther. 2019, 13, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J. Recept. Signal Transduct. Res. 2021, 41, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef]
- Albers, J.W.; Cole, P.; Greenberg, R.S.; Mandel, J.S.; Monson, R.R.; Ross, J.H.; Snodgrass, W.R.; Spurgeon, A.; van Gemert, M. Analysis of chlorpyrifos exposure and human health: Expert panel report. J. Toxicol. Environ. Health Part B Crit. Rev. 1999, 2, 301–324. [Google Scholar]
- Meuling, W.J.A.; Ravensberg, L.C.; Roza, L.; van Hemmen, J.J. Dermal absorption of chlorpyrifos in human volunteers. Int. Arch. Occup. Environ. Health 2005, 78, 44–50. [Google Scholar] [CrossRef]
- Fenske, R.A.; Farahat, F.M.; Galvin, K.; Fenske, E.K.; Olson, J.R. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int. J. Occup. Environ. Health 2012, 18, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Zurich, M.G.; Honegger, P.; Schilter, B.; Costa, L.G.; Monnet-Tschudi, F. Involvement of glial cells in the neurotoxicity of parathion and chlorpyrifos. Toxicol. Appl. Pharmacol. 2004, 201, 97–104. [Google Scholar] [CrossRef]
- Alaa-Eldin, E.A.; El-Shafei, D.A.; Abouhashem, N.S. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. Environ. Sci. Pollut. Res. 2017, 24, 1532–1543. [Google Scholar] [CrossRef]
- Medithi, S.; Kasa, Y.D.; Jee, B.; Venkaiah, K.; Jonnalagadda, P.R. Alterations in reproductive hormone levels among farm women and their children occupationally exposed to organophosphate pesticides. Women Health 2022, 62, 454–464. [Google Scholar] [CrossRef]
- Dutta, A.L.; Sahu, C.R. Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. Springerplus 2013, 2, 541. [Google Scholar] [CrossRef]
- De Anna, J.S.; Castro, J.M.; Darraz, L.A.; Elías, F.D.; Cárcamo, J.G.; Luquet, C.M. Exposure to hydrocarbons and chlorpyrifos alters the expression of nuclear receptors and antioxidant, detoxifying, and immune response proteins in the liver of the rainbow trout, Oncorhynchus mykiss. Ecotox. Environ. Safe. 2021, 208, 111394. [Google Scholar] [CrossRef] [PubMed]
- Puchner, A.; Blüml, S. IL-6 blockade in chronic inflammatory diseases. Wien. Med. Wochenschr. 2015, 165, 14–22. [Google Scholar] [CrossRef]
- Klein, C.; Wüstefeld, T.; Assmus, U.; Roskams, T.; Rose-John, S.; Müller, M.; Manns, M.P.; Ernst, M.; Trautwein, C. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J. Clin. Investig. 2005, 115, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Rutz, S.; Ouyang, W. Regulation of Interleukin-10 Expression. Adv. Exp. Med. Biol. 2016, 941, 89–116. [Google Scholar] [PubMed]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Guo, X.Z.; Shao, X.D.; Liu, M.P.; Xu, J.H.; Ren, L.N.; Zhao, J.J.; Li, H.Y.; Wang, D. Effect of bax, bcl-2 and bcl-xL on regulating apoptosis in tissues of normal liver and hepatocellular carcinoma. World J. Gastroenterol. 2002, 8, 1059–1062. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Malhi, H.; Mott, J.L.; Gores, G.J. Apoptosis and necrosis in the liver. Compr. Physiol. 2013, 3, 977–1010. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhang, K.; Liu, K.; Pei, H.; Shi, K.; He, Z.; Zong, Y.; Du, R. Protective Effect of Baicalin on Chlorpyrifos-Induced Liver Injury and Its Mechanism. Molecules 2023, 28, 7771. https://doi.org/10.3390/molecules28237771
Wang R, Zhang K, Liu K, Pei H, Shi K, He Z, Zong Y, Du R. Protective Effect of Baicalin on Chlorpyrifos-Induced Liver Injury and Its Mechanism. Molecules. 2023; 28(23):7771. https://doi.org/10.3390/molecules28237771
Chicago/Turabian StyleWang, Ruibing, Ke Zhang, Kaiyue Liu, Hongyan Pei, Kun Shi, Zhongmei He, Ying Zong, and Rui Du. 2023. "Protective Effect of Baicalin on Chlorpyrifos-Induced Liver Injury and Its Mechanism" Molecules 28, no. 23: 7771. https://doi.org/10.3390/molecules28237771