Synthesis and Antibacterial Activity of Novel Triazolo[4,3-a]pyrazine Derivatives
Abstract
:1. Introduction
2. Results and Discussion
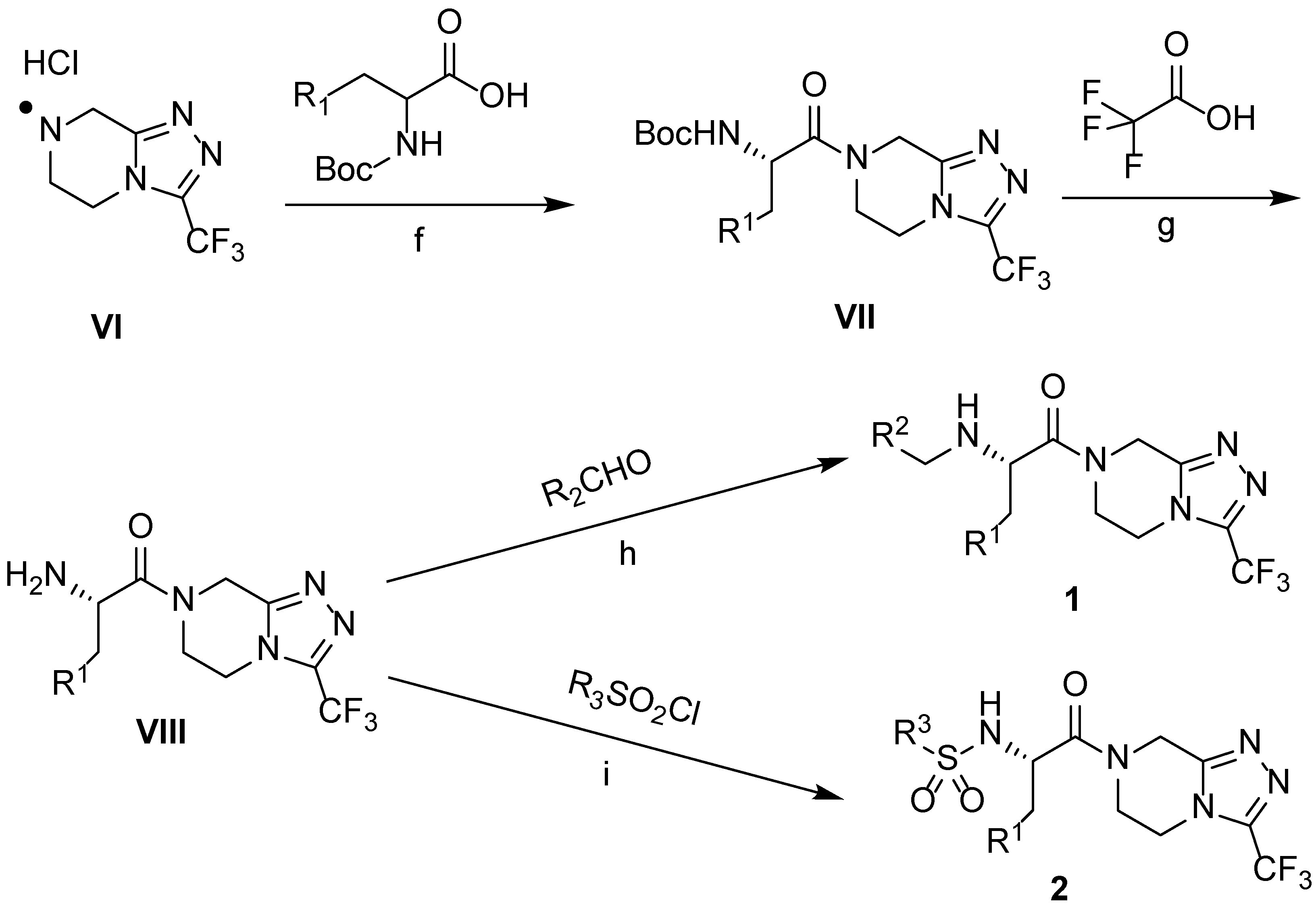
2.1. Chemistry
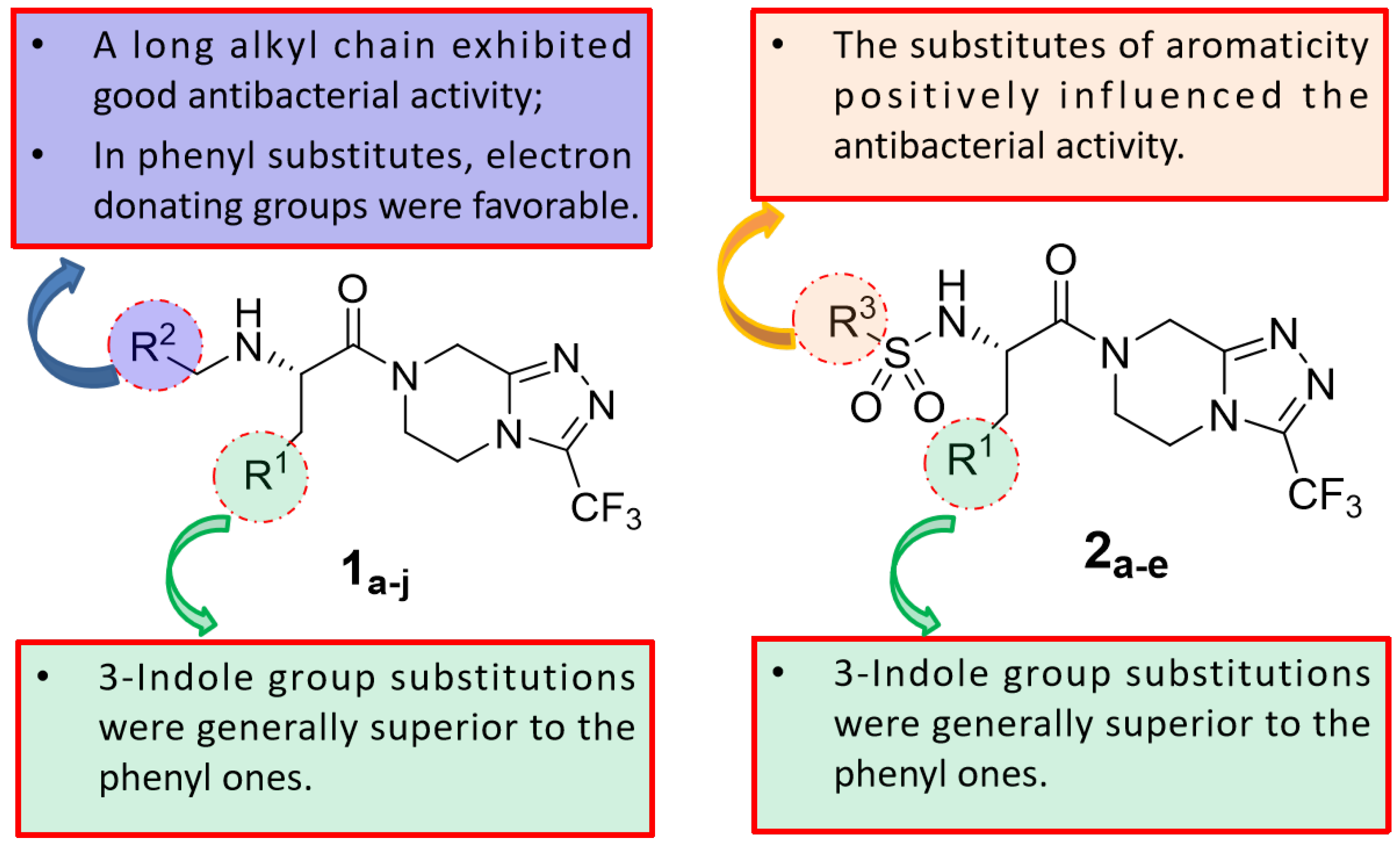
2.2. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. General Characterization
3.3. Synthesis of N′-(2-chloroacetyl)-2,2,2-trifluoroacetohydrazide (III)
3.4. Synthesis of 2-(chloromethyl)-5-(trifluoromethyl)-1,3,4-oxadiazole (IV)
3.5. Synthesis of 2,2,2-trifluoro-N′-(piperazin-2-ylidene)acetohydrazide (V)
3.6. Synthesis of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine Hydrochloride Salt (VI)
3.7. Synthesis of Tert-Butyl (S)-(1-oxo-3-phenyl-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)propan-2-yl)carbamate (VIIa)
3.8. Synthesis of Tert-Butyl (S)-(3-(1H-indol-3-yl)-1-oxo-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)propan-2-yl)carbamate (VIIb)
3.9. (S)-2-amino-3-phenyl-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)propan-1-one (VIIIa) or (S)-2-amino-3-(1H-indol-3-yl)-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)propan-1-one (VIIIb)
3.10. Generalized Procedure for the Synthesis of Compound 1
3.11. Generalized Procedure for the Synthesis of Compound 2
3.12. Antibacterial Activity Evaluation In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendapara, J.V.; Vaghasiya, M.D.; Rajani, D.P.; Ahmad, I.; Patel, H.; Kumari, P. Benzimidazole and piperidine containing novel 1,2,3-triazole hybrids as anti-infective agents: Design, synthesis, in silico and in vitro antimicrobial efficacy. J. Biochem. Mol. Toxicol. 2023, 37, e23526. [Google Scholar] [CrossRef]
- Patil, B.S.; Krishnamurthy, G.; Shashikumar, N.D.; Lokesh, M.R.; Naik, H.S.B. Synthesis and antimicrobial activity of some [1,2,4]-triazole derivatives. J. Chem. 2013, 2013, 462594. [Google Scholar] [CrossRef]
- Bao, H.; Bayeh, L.; Tambar, U.K. Catalytic enantioselective allylic amination of olefins for the synthesis of ent-sitagliptin. Synlett 2013, 24, 2459–2463. [Google Scholar] [PubMed]
- Akter, M.; Rupa, K.; Anbarasan, P. 1,2,3-Triazole and its analogues: New surrogates for diazo compounds. Chem. Rev. 2022, 122, 13108–13205. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and their derivatives: Chemistry, synthesis, and therapeutic applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Tian, G.; Song, Q.; Liu, Z.; Guo, J.; Cao, S.; Long, S. Recent advances in 1,2,3- and 1,2,4-triazole hybrids as antimicrobials and their SAR: A critical review. Eur. J. Med. Chem. 2023, 259, 115603. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef]
- Merugu, S.R.; Cherukupalli, S.; Karpoormath, R. An overview on synthetic and medicinal perspectives of [1,2,4]triazolo[1,5-a]pyrimidine scaffold. Chem. Biodivers. 2022, 19, e202200291. [Google Scholar] [CrossRef]
- Karypidou, K.; Ribone, S.R.; Quevedo, M.A.; Persoons, L.; Pannecouque, C.; Helsen, C.; Claessens, F.; Dehaen, W. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorg. Med. Chem. Lett. 2018, 28, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, W.T.; Wang, Y.; Song, S.S.; Xi, Y.; Yang, X.Y.; Shen, Y.Y.; Su, Y.; Sun, Y.M.; Gao, Y.L.; et al. Identification of [1,2,4]triazolo[4,3-a]pyrazine PARP1 inhibitors with overcome acquired resistance activities. Eur. J. Med. Chem. 2023, 259, 115709. [Google Scholar] [CrossRef]
- Reniers, F.; Anthonissen, S.; Van Meervelt, L.; Dehaen, W. Three-step synthetic pathway toward fully decorated [1,2,3]triazolo[4,5-d]pyrimidine (8-azapurine) derivatives. Org. Lett. 2023, 25, 2820–2824. [Google Scholar] [CrossRef] [PubMed]
- Gazzillo, E.; Pierri, M.; Colarusso, E.; Chini, M.G.; Ferraro, M.G.; Piccolo, M.; Irace, C.; Bruno, I.; Bifulco, G.; Terracciano, S.; et al. Exploring the chemical space of functionalized [1,2,4]triazolo[4,3-a] quinoxaline-based compounds targeting the bromodomain of BRD9. Bioorg. Chem. 2023, 139, 106677. [Google Scholar] [CrossRef] [PubMed]
- Borra, S.; Kim, H.Y.; Oh, K. One-pot tandem nickel-catalyzed α-vinyl aldol reaction and cycloaddition approach to [1,2,3]triazolo[1,5-a]quinolines. Org. Lett. 2023, 25, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Li, Z.; Tang, H.; He, L.; Zhou, W. Tandem c–n coupling/boulton–katritzky rearrangement reactions of 3-aminoisoxazoles or 1,2,4-oxadiazol-3-amines with 2-pyridyl trifluoromethanesulfonate: A rapid access to [1,2,4]triazolo[1,5-a]pyridines. Org. Chem. Front. 2022, 9, 3527–3531. [Google Scholar] [CrossRef]
- Khomenko, D.M.; Shokol, T.V.; Doroshchuk, R.O.; Starova, V.S.; Volovenko, Y.M. An alternative approach to the synthesis of [1,2,4]triazolo[1,5-a]pyridinearbonitriles, their crystal structure and dft calculations. J. Heterocycl. Chem. 2021, 58, 1278–1285. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as important antibacterial agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Wei, Y.; Xia, S.; He, C.; Xiong, W.; Xu, H. Highly enantioselective production of a chiral intermediate of sitagliptin by a novel isolate of pseudomonas pseudoalcaligenes. Biotechnol. Lett. 2016, 38, 841–846. [Google Scholar] [CrossRef]
- Xie, Y.; Shao, L.; Wang, Q.; Bai, Y.; Chen, Z.; Li, N.; Bian, X. Synthesis, nitric oxide release, and dipeptidyl peptidase-4 inhibition of sitagliptin derivatives as new multifunctional antidiabetic agents. Bioorg. Med. Chem. Lett. 2018, 28, 3731–3735. [Google Scholar] [CrossRef]
- Jethava, D.J.; Acharya, P.T.; Vasava, M.S.; Bhoi, M.N.; Bhavsar, Z.A.; Rathwa, S.K.; Patel, H.D. Design, synthesis, biological evaluation and computational study of novel triazolo [4,3-a]pyrazin analogues. J. Mol. Struct. 2019, 1184, 168–192. [Google Scholar] [CrossRef]
- Mannam, M.R.; Devineni, S.R.; Pavuluri, C.M.; Chamarthi, N.R.; Kottapalli, R.S.P. Urea and thiourea derivatives of 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine: Synthesis, characterization, antimicrobial activity and docking studies. Phosphorus Sulfur 2019, 194, 922–932. [Google Scholar] [CrossRef]
- Ravindar, L.; Hasbullah, S.A.; Rakesh, K.P.; Hassan, N.I. Triazole hybrid compounds: A new frontier in malaria treatment. Eur. J. Med. Chem. 2023, 259, 115694. [Google Scholar] [CrossRef]
- Patil, Y.; Shingare, R.; Choudhari, A.; Sarkar, D.; Madje, B. Microwave-assisted synthesis and antituberculosis screening of some 4-((3-(trifluoromethyl)-5, 6-dihydro-[1, 2, 4] triazolo [4, 3-a] pyrazin-7 (8H)-l) methyl) benzenamine hybrids. J. Heterocycl. Chem. 2019, 56, 434–442. [Google Scholar] [CrossRef]
- Jethava, D.J.; Borad, M.A.; Bhoi, M.N.; Acharya, P.T.; Patel, H.D. New dimensions in triazolo[4,3-a]pyrazine derivatives: The land of opportunity in organic and medicinal chemistry. Arab. J. Chem. 2020, 13, 8532–8591. [Google Scholar] [CrossRef]
- Balsells, J.; Dimichele, L.; Liu, J.; Kubryk, M.; Hansen, K.; Armstrong, J.D. Synthesis of [1,2,4]triazolo[4,3-a]piperazines via highly reactive chloromethyloxadiazoles. Org. Lett. 2005, 7, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Meltem, M.; Neslihan, D.; Arif, M.; Serpi, D.; Ahmet, D.; Faik, A.A. Novel azole-functionalited flouroquinolone hybrids: Design, conventional and microwave irradiated synthesis, evaluation as antibacterial and antioxidant agents. Lett. Drug Des. Discov. 2018, 15, 46–64. [Google Scholar]
- Bektaş, H.; Karaali, N.; Şahin, D.; Demirbaş, A.; Karaoglu, Ş.A.; Demirbaş, N. Synthesis and antimicrobial activities of some new 1,2,4-triazole derivatives. Molecules 2010, 15, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motaal, M.; Almohawes, K.; Tantawy, M.A. Antimicrobial evaluation and docking study of some new substituted benzimidazole-2yl derivatives. Bioorg. Chem. 2020, 101, 103972. [Google Scholar] [CrossRef]
- Liu, T.; Yao, X.; Zhang, R.; Wu, T.; Liu, Z.; Li, D.; Dong, Q. Design, synthesis and biological evaluation of novel indole-piperazine derivatives as antibacterial agents. Bioorg. Med. Chem. Lett. 2023, 89, 129320. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, R.; Kumar, A.; Kaur, S.; Priyadarshi, N.; Singhal, N.K.; Singh, K. 1,2,3-Triazole beta-lactam conjugates as antimicrobial agents. Heliyon 2020, 6, e04241. [Google Scholar] [CrossRef]
- Pokhodylo, N.; Manko, N.; Finiuk, N.; Klyuchivska, O.; Matiychuk, V.; Obushak, M.; Stoika, R. Primary discovery of 1-aryl-5-substituted-1H-1,2,3-triazole-4-carboxamides as promising antimicrobial agents. J. Mol. Struct. 2021, 1246, 131146–131158. [Google Scholar] [CrossRef]
- Deohate, P.P. Synthesis, structural study and biological activity of bridgehead nitrogen containing triazolo-thiadiazine derivatives. Chem. Sci. Trans. 2013, 2, 556–560. [Google Scholar] [CrossRef]
- Rishikesan, R.; Karuvalam, R.P.; Muthipeedika, N.J.; Sajith, A.M.; Eeda, K.R.; Pakkath, R.; Haridas, K.R.; Bhaskar, V.; Narasimhamurthy, K.H.; Muralidharan, A. Synthesis of some novel piperidine fused 5-thioxo-1h-1,2,4-triazoles as potential antimicrobial and antitubercular agents. J. Chem. Sci. 2021, 133, 3–14. [Google Scholar] [CrossRef]
- Thakur, A.; Gupta, P.R.S.; Pathak, P.; Kumar, A. Design, Synthesis, SAR, Docking and antibacterial evaluation: Aliphatic amide bridged 4-aminoquinoline clubbed 1,2,4-triazole derivatives. Int. J. ChemTech Res. 2016, 9, 575–588. [Google Scholar]
- Sahu, J.K.; Ganguly, S.; Yasir, M. Synthesis, SAR and molecular docking studies of certain new derivatives of 1,2,4-triazolo[3,4-b][1,3,4]thiadiazole as potent antimicrobial agents. Anti-Infect. Agents 2018, 16, 40–48. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, P.; Su, S.; Chen, M.; He, J.; Liu, L.; He, M.; Wang, H.; Xue, W. Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1,2,4-triazole Schiff base. RSC Adv. 2019, 9, 23045–23052. [Google Scholar] [CrossRef]
- Tiz, D.B.; Skok, Ž.; Durcik, M.; Tomašič, T.; Mašič, L.P.; Ilaš, J.; Zega, A.; Draskovits, G.; Révész, T.; Nyerges, Á.; et al. An optimised series of substituted N-phenylpyrrolamides as DNA gyrase B inhibitors. Eur. J. Med. Chem. 2019, 167, 269–290. [Google Scholar] [CrossRef]
- Gollapalli, V.R.; Bollikolla, H.B.; Allaka, T.R.; Vaddi, P.R.R.; Basireddy, S.; Ganivada, M.; Pindi, S.R. New Fluoroquinolone-1,2,4-triazoles as Potent Antibacterial Agents: Design, Synthesis, Docking Studies and in Silico ADME Profiles. Chem. Biodivers. 2023, 20, e202201259. [Google Scholar] [CrossRef]
- Pathak, P.; Novak, J.; Shukla, P.K.; Grishina, M.; Potemkin, V.; Verma, A. Design, synthesis, antibacterial evaluation, and computational studies of hybrid oxothiazolidin-1,2,4-triazole scaffolds. Arch Pharm. 2021, 354, e2000473. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.; Pochampally, J.; Dhoddi, B.N.; Bhookya, S.; Bitla, S.; Akkiraju, A.G. Synthesis, antimicrobial and antioxidant activity of triazole, pyrazole containing thiazole derivatives and molecular docking studies on COVID-19. BMC Chem. 2023, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Luo, J.B.; Wang, Z.Z.; Zhang, L.L.; Feng, J.; Xie, X.B.; Shi, Q.S.; Zhang, X.G. Synthesis, molecular docking, and evaluation of antibacterial activity of 1,2,4-triazole-norfloxacin hybrids. Bioorg. Chem. 2021, 115, 105270. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Wang, J.; Zhou, K.X.; Wang, S.K.; Liu, Y.H.; Jin, Z.; Tang, Y.Z. Design, synthesis and biological evaluation of novel pleuromutilin derivatives possessing 4-aminothiophenol linker as promising antibacterial agents. Bioorg. Chem. 2022, 126, 105859. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, Z.; Li, J.; Luo, X.; Zhu, W.; Liao, G.; Xia, J.; Zhang, W.; Pan, W.; Li, T.; et al. Design, synthesis and antibacterial evaluation of pleuromutilin derivatives. Bioorg. Med. Chem. 2022, 59, 116676. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | R3 | Yield (%) # |
| 1a | 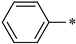 |  | _ | 69 |
| 1b |  | 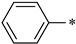 | _ | 56 |
| 1c | 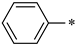 | 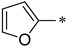 | _ | 71 |
| 1d | 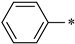 | 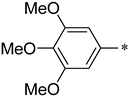 | _ | 64 |
| 1e | 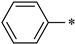 | 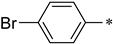 | _ | 76 |
| 1f | 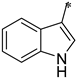 |  | _ | 53 |
| 1g | 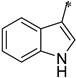 | 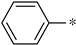 | _ | 57 |
| 1h | 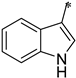 | 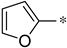 | _ | 63 |
| 1i | 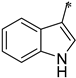 | 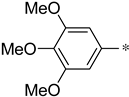 | _ | 62 |
| 1j | 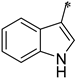 |  | _ | 65 |
| 2a | 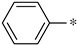 | _ |  | 73 |
| 2b | 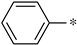 | _ | 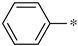 | 77 |
| 2c |  | _ | 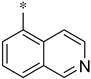 | 72 |
| 2d | 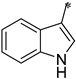 | _ | 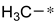 | 68 |
| 2e | 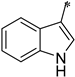 | _ |  | 65 |
| Compound | MIC (μg/mL) | |
|---|---|---|
| S. aureus | E. coli | |
| Ampicillin | 32 | 8 |
| 1a | 64 | 64 |
| 1b | >256 | 256 |
| 1c | >256 | >256 |
| 1d | 128 | 64 |
| 1e | 128 | 128 |
| 1f | 64 | 32 |
| 1g | 128 | 64 |
| 1h | 256 | 128 |
| 1i | 64 | 32 |
| 1j | 128 | 64 |
| 2a | 256 | >256 |
| 2b | 128 | 64 |
| 2c | 64 | 64 |
| 2d | 256 | 128 |
| 2e | 32 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Dong, H.; Si, Z.; Zhao, Y.; Liang, Y. Synthesis and Antibacterial Activity of Novel Triazolo[4,3-a]pyrazine Derivatives. Molecules 2023, 28, 7876. https://doi.org/10.3390/molecules28237876
Hu Z, Dong H, Si Z, Zhao Y, Liang Y. Synthesis and Antibacterial Activity of Novel Triazolo[4,3-a]pyrazine Derivatives. Molecules. 2023; 28(23):7876. https://doi.org/10.3390/molecules28237876
Chicago/Turabian StyleHu, Zhang, Hongrui Dong, Zhenyu Si, Yurong Zhao, and Yuanwei Liang. 2023. "Synthesis and Antibacterial Activity of Novel Triazolo[4,3-a]pyrazine Derivatives" Molecules 28, no. 23: 7876. https://doi.org/10.3390/molecules28237876
APA StyleHu, Z., Dong, H., Si, Z., Zhao, Y., & Liang, Y. (2023). Synthesis and Antibacterial Activity of Novel Triazolo[4,3-a]pyrazine Derivatives. Molecules, 28(23), 7876. https://doi.org/10.3390/molecules28237876






