Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases
Abstract
:1. Introduction
1.1. Immunomodulatory Effects of Innate and Adaptive Immune Systems
1.2. Examples of Therapeutically Important Medicinal Herbs
1.2.1. Ashwagandha (Withania somnifera)
1.2.2. Astragalus (Astragalus membranaceus)
1.2.3. Echinacea (Echinacea purpurea)
1.2.4. Garlic (Allium sativum)
1.2.5. Ginger (Zingiber officinale)
1.2.6. Ginseng (Panax ginseng)
1.2.7. Licorice (Glycyrrhiza glabra)
1.2.8. Shatavari (Asparagus racemosus)
1.2.9. Tulsi (Ocimum sanctum)
2. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Andres, C.M.C.; Perez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Perez-Lebena, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, M.F.; Masten, B.J. Dendritic cells: Immune regulators in health and disease. Physiol. Rev. 2002, 82, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Nevinsky, G.A.; Buneva, V.N. Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. Int. J. Mol. Sci. 2020, 21, 5392. [Google Scholar] [CrossRef] [PubMed]
- Pirofski, L.A.; Casadevall, A. Immunomodulators as an antimicrobial tool. Curr. Opin. Microbiol. 2006, 9, 489–495. [Google Scholar] [CrossRef]
- Petroni, G.; Buqué, A.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Immunomodulation by targeted anticancer agents. Cancer Cell 2021, 39, 310–345. [Google Scholar] [CrossRef]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory role of the antimicrobial LL-37 peptide in autoimmune diseases and viral infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef]
- Fung, J.J. Tacrolimus and transplantation: A decade in review. Transplantation 2004, 77, S41–S43. [Google Scholar] [CrossRef]
- Cassone, G.; Manfredi, A.; Vacchi, C.; Luppi, F.; Coppi, F.; Salvarani, C.; Sebastiani, M. Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease: Lights and Shadows. J. Clin. Med. 2020, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The amazing and mighty ginger. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tharakan, A.; Shukla, H.; Benny, I.R.; Tharakan, M.; George, L.; Koshy, S. Immunomodulatory Effect of Withania somnifera (Ashwagandha) Extract—A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. J. Clin. Med. 2021, 10, 3644. [Google Scholar] [CrossRef] [PubMed]
- Sikandan, A.; Shinomiya, T.; Nagahara, Y. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-kappaB pathways and by regulating cytokines. Int. J. Mol. Med. 2018, 42, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Denzler, K.; Moore, J.; Harrington, H.; Morrill, K.; Huynh, T.; Jacobs, B.; Waters, R.; Langland, J. Characterization of the Physiological Response following In Vivo Administration of Astragalus membranaceus. Evid. Based Complement. Altern. Med. 2016, 2016, 6861078. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, X.; Gao, J.; Yang, H.; Duan, Y.; Feng, Y.; He, X.; Gong, X.; Wang, H.; Wu, X.; et al. Astragaloside III Enhances Anti-Tumor Response of NK Cells by Elevating NKG2D and IFN-gamma. Front. Pharmacol. 2019, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Yang, W.; Zhang, L.; Wu, S.; Yan, C.; Li, Q. Astragalus membranaceus Injection Suppresses Production of Interleukin-6 by Activating Autophagy through the AMPK-mTOR Pathway in Lipopolysaccharide-Stimulated Macrophages. Oxid. Med. Cell. Longev. 2020, 2020, 1364147. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Han, Q.J.; Wang, K.L.; Xu, Y.L.; Lan, J.H.; Cao, G.T. Astragalus and Ginseng Polysaccharides Improve Developmental, Intestinal Morphological, and Immune Functional Characters of Weaned Piglets. Front. Physiol. 2019, 10, 418. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, Y.; Wu, L.; Senchina, D.S.; Wurtele, E.S.; Murphy, P.A.; Kohut, M.L.; Cunnick, J.E. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J. Med. Food 2007, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabian, S.; Lopez-Roa, R.I.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef] [PubMed]
- Lestari, S.R.; Atho’illah, M.F.; Christina, Y.I.; Rifa’i, M. Single garlic oil modulates T cells activation and proinflammatory cytokine in mice with high fat diet. J. Ayurveda Integr. Med. 2020, 11, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Padiyappa, S.D.; Avalappa, H.; Somegowda, M.; Sridhara, S.; Venkatesh, Y.P.; Prabhakar, B.T.; Pramod, S.N.; Almujaydil, M.S.; Shokralla, S.; Abdelbacki, A.M.M.; et al. Immunoadjuvant and Humoral Immune Responses of Garlic (Allium sativum L.) Lectins upon Systemic and Mucosal Administration in BALB/c Mice. Molecules 2022, 27, 1375. [Google Scholar] [CrossRef] [PubMed]
- Washiya, Y.; Nishikawa, T.; Fujino, T. Enhancement of intestinal IgA production by Ajoene in mice. Biosci. Biotechnol. Biochem. 2013, 77, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Su, H.M.; Lii, C.K.; Sheen, L.Y. Effect of supplementation with garlic oil on activity of Th1 and Th2 lymphocytes from rats. Planta Medica 2009, 75, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136, 816S–820S. [Google Scholar] [CrossRef]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T.; Karegar, S.J. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; You, S. Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination. Nutrients 2022, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, M.; Rapak, A. Effect of Natural Compounds on NK Cell Activation. J. Immunol. Res. 2018, 2018, 4868417. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, M.; Feng, Y.; Zheng, H.; Lei, P.; Ma, X.; Han, X.; Guan, H.; Hou, D. Effect of ginseng polysaccharides on NK cell cytotoxicity in immunosuppressed mice. Exp. Ther. Med. 2016, 12, 3773–3777. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Min, H. Ginseng, the ‘Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, J.; Sakwiwatkul, K.; Li, R.; Hu, S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int. Immunopharmacol. 2010, 10, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.M.; Hoffman, C.; Pascual, D.W.; Hardy, M.E. 18beta-glycyrrhetinic acid delivered orally induces isolated lymphoid follicle maturation at the intestinal mucosa and attenuates rotavirus shedding. PLoS ONE 2012, 7, e49491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, R.; Yang, R.; Xiao, Y.; Yan, J.; Zheng, C.; Xiao, W.; Huang, C.; Wang, Y. Licorice extract inhibits growth of non-small cell lung cancer by down-regulating CDK4-Cyclin D1 complex and increasing CD8+ T cell infiltration. Cancer Cell Int. 2021, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yu, L.; Zhang, Y.; Liu, Z.; Zhang, H.; Zhang, Y.; Liu, P.; Du, P. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. Int. Immunopharmacol. 2020, 84, 106578. [Google Scholar] [CrossRef]
- Ting, Y.; Yong, T.J.; Gang, J.; Mang, L.G.; Gang, T.; Ling, C.X.; Yi, C.J.; Bo, K.; Hua, Z. Effects of dietary licorice extract on serum Biochemical index, tissues antioxidant capacity and immunity function of weaned piglets. Phytother. Res. 2020, 24, 129–135. [Google Scholar]
- Yu, J.-Y.; Ha, J.Y.; Kim, K.-M.; Jung, Y.-S.; Jung, J.-C.; Oh, S. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef]
- Bhat, J.; Damle, A.; Vaishnav, P.P.; Albers, R.; Joshi, M.; Banerjee, G. In vivo enhancement of natural killer cell activity through tea fortified with Ayurvedic herbs. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Varma, S.; Bamola, V.D.; Naik, S.N.; Mirdha, B.R.; Padhi, M.M.; Mehta, N.; Mahapatra, S.C. Double-blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. J. Ethnopharmacol. 2011, 136, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Singh, D.K.; Kumar, S.; Bhatia, A.K. Immunomodulating property of Ocimum sanctum by regulating the IL-2 production and its mRNA expression using rat’s splenocytes. Asian Pac. J. Trop. Med. 2010, 3, 8–12. [Google Scholar] [CrossRef]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szelag, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, H.H.; Elfaki, E. The immunomodulatory role of Withania somnifera (L.) dunal in inflammatory diseases. Front. Pharmacol. 2023, 14, 1084757. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef] [PubMed]
- Henley, A.B.; Yang, L.; Chuang, K.L.; Sahuri-Arisoylu, M.; Wu, L.H.; Bligh, S.W.; Bell, J.D. Withania somnifera Root Extract Enhances Chemotherapy through ‘Priming’. PLoS ONE 2017, 12, e0170917. [Google Scholar] [CrossRef]
- Udayakumar, R.; Kasthurirengan, S.; Mariashibu, T.S.; Rajesh, M.; Anbazhagan, V.R.; Kim, S.C.; Ganapathi, A.; Choi, C.W. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int. J. Mol. Sci. 2009, 10, 2367–2382. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmed, R.S.; Chandra, N.; Arora, V.K.; Ali, A. In vivo, Extract from Withania somnifera Root Ameliorates Arthritis via Regulation of Key Immune Mediators of Inflammation in Experimental Model of Arthritis. Antiinflamm. Antiallergy Agents Med. Chem. 2019, 18, 55–70. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Luo, Y. Anti-Aging Implications of Astragalus membranaceus (Huangqi): A Well-Known Chinese Tonic. Aging Dis. 2017, 8, 868–886. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Wu, J.M.; Wang, M.Y.; Wu, M.H.; Chen, K.Y.; Yeh, S.L.; Lin, M.T. Modulatory Effects of Astragalus Polysaccharides on T-Cell Polarization in Mice with Polymicrobial Sepsis. Mediat. Inflamm. 2015, 2015, 826319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Xu, H. Effect of Astragalus polysaccharide in treatment of diabetes mellitus: A narrative review. J. Tradit. Chin. Med. 2019, 39, 133–138. [Google Scholar] [PubMed]
- Sun, J.; Wei, S.; Zhang, Y.; Li, J. Protective Effects of Astragalus Polysaccharide on Sepsis-Induced Acute Kidney Injury. Anal. Cell. Pathol. 2021, 2021, 7178253. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rui, S.; Chen, C.; Zhang, G.; Li, Z.; Wang, J.; Luo, Y.; Zhu, H.; Ma, X. Protective effects of astragalus polysaccharide nanoparticles on septic cardiac dysfunction through inhibition of TLR4/NF-kappaB signaling pathway. Int. J. Biol. Macromol. 2020, 153, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.P.; Gao, L.X.; Hou, L.F.; Yang, X.Q.; He, P.L.; Yang, Y.F.; Tang, W.; Yue, J.M.; Li, J.; Zuo, J.P. Astragaloside II triggers T cell activation through regulation of CD45 protein tyrosine phosphatase activity. Acta Pharmacol. Sin. 2013, 34, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, X.; Zhu, R.; Song, P. Effect of astragaloside IV on the immunoregulatory function of adipose-derived mesenchymal stem cells from patients with psoriasis vulgaris. J. Tradit. Chin. Med. 2022, 42, 513–519. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Wang, Y.; Yang, H.; Cao, L.; Gan, S.; Ma, J.; Liu, H. Immuno-stimulatory activity of Astragalus polysaccharides in cyclophosphamide-induced immunosuppressed mice by regulating gut microbiota. Int. J. Biol. Macromol. 2023, 242, 124789. [Google Scholar] [CrossRef]
- Wei, D.; Xu, H.; Gai, X.; Jiang, Y. Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3beta signaling pathways. Acta Cir. Bras. 2019, 34, e201900708. [Google Scholar] [CrossRef]
- Kim, J.; Moon, E.; Kwon, S. Effect of Astragalus membranaceus extract on diabetic nephropathy. Endocrinol. Diabetes Metab. Case Rep. 2014, 2014, 140063. [Google Scholar] [CrossRef]
- Goto, S.; Fujii, H.; Watanabe, K.; Shimizu, M.; Okamoto, H.; Sakamoto, K.; Kono, K.; Nishi, S. Renal protective effects of astragalus root in rat models of chronic kidney disease. Clin. Exp. Nephrol. 2023, 27, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Kuo, Y.H.; Wu, L.H.; Chang, C.M.; Cheng, K.J.; Tyan, Y.C.; Lee, C.H. The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int. J. Med. Sci. 2020, 17, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Abbas, A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.K.; Khan, J.A. Immunomodulatory activity of Pinus radiata extract against coccidiosis in broiler chicken. Pak. Vet. J. 2017, 37, 145–149. [Google Scholar]
- Park, S.J.; Lee, M.; Kim, D.; Oh, D.H.; Prasad, K.S.; Eun, S.; Lee, J. Echinacea purpurea Extract Enhances Natural Killer Cell Activity In Vivo by Upregulating MHC II and Th1-type CD4(+) T Cell Responses. J. Med. Food 2021, 24, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.F.; Goncalves, S.M.; Goncalves, V.M.F.; Llaguno, C.P.; Macias, F.; Tiritan, M.E.; Cunha, C.; Carvalho, A.; Reis, R.L.; Ferreira, H.; et al. Echinacea purpurea Fractions Represent Promising Plant-Based Anti-Inflammatory Formulations. Antioxidants 2023, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Pacilio, M.; Di Carlo, G.; Esposito, E.; Pinto, L.; Meli, R. In-vivo and in-vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum. J. Pharm. Pharmacol. 2002, 54, 1379–1383. [Google Scholar] [CrossRef]
- Ciganovic, P.; Jakupovic, L.; Momchev, P.; Nizic Nodilo, L.; Hafner, A.; Zovko Koncic, M. Extraction Optimization, Antioxidant, Cosmeceutical and Wound Healing Potential of Echinacea purpurea Glycerolic Extracts. Molecules 2023, 28, 1177. [Google Scholar] [CrossRef]
- Mao, C.F.; Sudirman, S.; Lee, C.C.; Tsou, D.; Kong, Z.L. Echinacea purpurea Ethanol Extract Improves Male Reproductive Dysfunction with Streptozotocin-Nicotinamide-Induced Diabetic Rats. Front. Vet. Sci. 2021, 8, 651286. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2020, 19, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, M.M.; Dodamani, A.S.; Karibasappa, G.N.; Vishwakarma, P.K.; Vathar, J.B.; Sonawane, K.R.; Jadhav, H.C.; Khobragade, V.R. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. Ayu 2018, 39, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Sarkar, P.K. Inhibitory effect of garlic on bacterial pathogens from spices. World J. Microbiol. Biotechnol. 2003, 19, 565–569. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Alshammari, N.; Saeed, A.; Aqil, F.; Saeed, M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front. Pharmacol. 2023, 14, 1154034. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T. Ginger and its health claims: Molecular aspects. Crit. Rev. Food Sci. Nutr. 2011, 51, 383–393. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Ahmed, N.; Karobari, M.I.; Yousaf, A.; Mohamed, R.N.; Arshad, S.; Basheer, S.N.; Peeran, S.W.; Noorani, T.Y.; Assiry, A.A.; Alharbi, A.S.; et al. The antimicrobial efficacy against selective oral microbes, antioxidant activity and preliminary phytochemical screening of Zingiber officinale. Infect. Drug Resist. 2022, 15, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- Ike, K.; Uchida, Y.; Nakamura, T.; Imai, S. Induction of interferon-gamma (IFN-γ) and T helper 1 (Th1) immune response by bitter gourd extract. J. Vet. Med. Sci. 2005, 67, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Qorbanpour, M.; Fahim, T.; Javandel, F.; Nosrati, M.; Paz, E.; Seidavi, A.; Ragni, M.; Laudadio, V.; Tufarelli, V. Effect of dietary ginger (Zingiber officinale Roscoe) and multi-strain probiotic on growth and carcass traits, blood biochemistry, immune responses and intestinal microflora in broiler chickens. Animals 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Mahmoudi, M.; Shahram, F.; Poursani, S.; Jamshidi, F.; Tavakoli, H. The effect of ginger supplementation on IL2, TNFalpha, and IL1beta cytokines gene expression levels in patients with active rheumatoid arthritis: A randomized controlled trial. Med. J. Islam. Repub. Iran 2019, 33, 154. [Google Scholar] [PubMed]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Mir Taheri, M. The effects of ginger on fasting blood sugar, hemoglobin a1c, apolipoprotein B, apolipoprotein a-I and malondialdehyde in type 2 diabetic patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar] [PubMed]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, X.; Hu, Y.; Fan, X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Hong, M.; Sung, H.; Kim, S.; Suk, K.T. Effect of Korean Red Ginseng in chronic liver disease. J. Ginseng Res. 2017, 41, 450–455. [Google Scholar] [CrossRef]
- Yan, M.; Diao, M.; Zhang, C.; Shen, X.; Zhan, X.; Xi, C.; Zhao, C.; Zhang, T. Lactoferrin-ginsenoside Rg3 complex ingredients: Study of interaction mechanism and preparation of oil-in-water emulsion. Food Chem. 2021, 363, 130239. [Google Scholar] [CrossRef]
- Nakaya, T.-A.; Kita, M.; Kuriyama, H.; Iwakura, Y.; Imanishi, J. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J. Interferon Cytokine Res. 2004, 24, 93–100. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, C.T. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology 2019, 27, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, M.-H.; Byon, Y.-Y.; Park, J.W.; Jee, Y.; Joo, H.-G. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J. Vet. Sci. 2007, 8, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, A.R.; Yoo, E.S.; Baik, K.U.; Park, M.H. Ginsenosides from Panax ginseng differentially regulate lymphocyte proliferation. Planta Medica 2002, 68, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kikuzaki, H.; Fukuda, S.; Nakatani, N. Antibacterial compounds of licorice against upper airway respiratory tract pathogens. J. Nutr. Sci. Vitaminol. 2001, 47, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Morteza-Semnani, K.; Ghoreishi, M.R. The treatment of atopic dermatitis with licorice gel. J. Dermatol. Treat. 2003, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, L.-Q.; Yuan, B.-C.; Liu, Y. The pharmacological activities of licorice. Planta Medica 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Wei, J.-H.; Zheng, Y.-F.; Li, C.-Y.; Tang, Y.-P.; Peng, G.-P. Bioactive constituents of oleanane-type triterpene saponins from the roots of Glycyrrhiza glabra. J. Asian Nat. Prod. Res. 2014, 16, 1044–1053. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Marappan, G.; et al. Use of licorice (Glycyrrhiza glabra) herb as a feed additive in poultry: Current knowledge and prospects. Animals 2019, 9, 536. [Google Scholar] [CrossRef]
- Guo, A.; He, D.; Xu, H.-B.; Geng, C.-A.; Zhao, J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci. Rep. 2015, 5, 14046. [Google Scholar] [CrossRef]
- Bordbar, N.; Karimi, M.H.; Amirghofran, Z. The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell. Immunol. 2012, 280, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Dobrean, V.; Zăhan, M.; Virag, P. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Veena, N.; Arora, S.; Kapila, S.; Singh, R.R.B.; Katara, A.; Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Immunomodulatory and antioxidative potential of milk fortified with Asparagus racemosus (Shatavari). J. Mech. Phys. Solids 2014, 2, 13–19. [Google Scholar]
- Gautam, M.; Saha, S.; Bani, S.; Kaul, A.; Mishra, S.; Patil, D.; Satti, N.; Suri, K.; Gairola, S.; Suresh, K. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: Implications for immunoadjuvant potential. J. Ethnopharmacol. 2009, 121, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.N.; Verma, N.K. Asparagus racemosus: Chemical constituents and pharmacological activities—A review. Eur. J. Biomed. Pharm. Sci. 2017, 4, 207–213. [Google Scholar]
- Shaha, P.; Bellankimath, A. Pharmacological profile of Asparagus racemosus: A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1215–1223. [Google Scholar] [CrossRef]
- Kanwar, A.S.; Bhutani, K.K. Effects of Chlorophytum arundinaceum, Asparagus adscendens and Asparagus racemosus on pro-inflammatory cytokine and corticosterone levels produced by stress. Phytother. Res. 2010, 24, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Garg, M.; Prajapati, P.; Singh, P.K.; Chopra, R.; Kumari, A.; Mittal, A. Adaptogenic property of Asparagus racemosus: Future trends and prospects. Heliyon 2023, 9, e14932. [Google Scholar] [CrossRef]
- Yamani, H.A.; Pang, E.C.; Mantri, N.; Deighton, M.A. Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Their Major Constituents against Three Species of Bacteria. Front. Microbiol. 2016, 7, 681. [Google Scholar] [CrossRef]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sharma, S.; Mittal, A.; Gupta, S.; Dua, A. Phytochemical and antioxidant profiling of Ocimum sanctum. J. Food Sci. Technol. 2020, 57, 3852–3863. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, H.; Yadav, D.; Maurya, A.; Yadav, H.; Yadav, R.; Shukla, A.C.; Sharma, M.; Gupta, V.K.; Palazon, J. Biodiversity, Biochemical Profiling, and Pharmaco-Commercial Applications of Withania somnifera: A Review. Molecules 2023, 28, 1208. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Mahmood, Z.; Shahid, M.; Afzal, M.N.; Jahangir, M.; Ali Shah, S.M.; Tahir, I.M.; Riaz, M.; Hussain, S.; Akram, M.; et al. Withania somnifera Chemical Constituents’ In Vitro Antioxidant Potential and Their Response on Spermatozoa Parameters. Dose Response 2022, 20, 15593258221074936. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, T.; Oniszczuk, A.; Gondek, E.; Guz, L.; Puk, K.; Kocira, A.; Kusz, A.; Kasprzak, K.; Wójtowicz, A. Active polyphenolic compounds, nutrient contents and antioxidant capacity of extruded fish feed containing purple coneflower (Echinacea purpurea (L.) Moench.). Saudi J. Biol. Sci. 2019, 26, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.-O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Murray, M.T. Glycyrrhiza glabra (Licorice). In Textbook of Natural Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 641–647.e3. [Google Scholar]
- Parihar, S.; Sharma, D. A brief overview on Asparagus racemous. IJRAR 2021, 8, 96–108. [Google Scholar]
- Kaushal, S. Ocimum sanctum (Holy Basil)—A Herb for All Reasons; CORVETTE Press: Chittorgarh, India, 2022; p. 31. [Google Scholar]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze-drying and low-temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef] [PubMed]
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-Ard, P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Perez, A.F.; Herrera-Calderon, O.; Quintero-Saumeth, J. Uncaria tomentosa (cat’s claw): A promising herbal medicine against SARS-CoV-2/ACE-2 junction and SARS-CoV-2 spike protein based on molecular modeling. J. Biomol. Struct. Dyn. 2022, 40, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Devi, P. A review on Tinospora cordifolia: As an Immunomodulating agent. Himal. J. Health Sci. 2021, 6, 6–14. [Google Scholar] [CrossRef]
- Thokchom, S.D.; Gupta, S.; Kapoor, R. Arbuscular mycorrhiza augments essential oil composition and antioxidant properties of Ocimum tenuiflorum L.—A popular green tea additive. Ind. Crops Prod. 2020, 153, 112418. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Bilčíková, J.; Habán, M. Milk thistle (Silybum marianum): A valuable medicinal plant with several therapeutic purposes. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 836–843. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Antioxidant effects of Schisandra chinensis fruits and their active constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef]
- Mullaicharam, A.; Halligudi, N. St John’s wort (Hypericum perforatum L.): A Review of its Chemistry, Pharmacology and Clinical properties. Int. J. Res. Phytochem. Pharmacol. Sci. 2019, 1, 5–11. [Google Scholar] [CrossRef]
- Gahatraj, S.; Bhusal, B.; Sapkota, K.; Dhami, B.; Gautam, D. Common medicinal plants of Nepal: A review of Triphala: Harro (Terminalia chebula), Barro (Terminalia bellirica), and Amala (Emblica officinalis). Asian J. Pharmacogn. 2020, 4, 5–13. [Google Scholar]
- Kaviya, M.; Balamuralikrishnan, B.; Sangeetha, T.; Senthilkumar, N.; Malaisamy, A.; Sivasamy, M.; Poorni, L.; Pushparaj, K.; Arun, M.; Anand, A.V. Evaluation of phytoconstituents of Triticum aestivum grass extracts on nutritional attributes, antioxidant, and antimicrobial activities against food pathogens with molecular in silico investigation. Food Front. 2023, 4, 831–848. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A. Commiphora myrrh: A phytochemical and pharmacological update. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Sukhikh, A.; Larichev, T.; Ivanova, S.; Prosekov, A.; Dmitrieva, A. Isolation of the Main Biologically Active Substances and Phytochemical Analysis of Ginkgo biloba Callus Culture Extracts. Molecules 2023, 28, 1560. [Google Scholar] [CrossRef]
- Al-Attraqchi, O.H.; Deb, P.K.; Al-Attraqchi, N.H.A. Review of the Phytochemistry and Pharmacological Properties of Valeriana officinalis. Curr. Tradit. Med. 2020, 6, 260–277. [Google Scholar] [CrossRef]
- Soleimani, M.; Arzani, A.; Arzani, V.; Roberts, T.H. Phenolic compounds and antimicrobial properties of mint and thyme. J. Herb. Med. 2022, 36, 100604. [Google Scholar] [CrossRef]
- Kwon, Y. Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci. Biotechnol. 2019, 28, 1599–1606. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.-J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef]
- Mohapatra, S.; Iqubal, A.; Ansari, M.J.; Jan, B.; Zahiruddin, S.; Mirza, M.A.; Ahmad, S.; Iqbal, Z. Benefits of black cohosh (Cimicifuga racemosa) for women health: An up-close and in-depth review. Pharmaceuticals 2022, 15, 278. [Google Scholar] [CrossRef]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, S.; Singh, R. Phytochemical constituents of guggul and their biological qualities. Mini-Rev. Org. Chem. 2020, 17, 277–288. [Google Scholar] [CrossRef]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An updated overview on its beneficial properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Marovska, G.I.; Hambarliyska, I.P.; Petkova, N.T.; Ivanov, I.G.; Vasileva, I.N.; Slavov, A.M. Chemical Composition and Antioxidant Activity of Ethanol Extracts Obtained from Lavender (Lavandula angustifolia Mill.). Philipp. J. Sci. 2023, 152, 861–870. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef] [PubMed]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef] [PubMed]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Zydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, S.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Phytochemical Profiles, Antioxidant Activity and Antiproliferative Mechanism of Rhodiola rosea L. Phenolic Extract. Nutrients 2022, 14, 3602. [Google Scholar] [CrossRef]
- Edwards, S.E.; da Costa Rocha, I.; Williamson, E.M.; Heinrich, M. Slippery Elm. In Phytopharmacy: An Evidence-Based Guide to Herbal Medicinal Products; John Wiley & Son: Hoboken, NJ, USA, 2015; p. 360. [Google Scholar]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid. Med. Cell. Longev. 2021, 2021, 6643827. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef] [PubMed]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a Source of Biologically Active Compounds Supporting the Therapy of Co-Existing Diseases in Metabolic Syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Corral, P.; Wang, Y.; Botello, J.; Kingston, R.; Daniels, T.; Salloum, R.G.; Johnston, E.; Huo, Z.; Lu, J.; et al. Kava as a Clinical Nutrient: Promises and Challenges. Nutrients 2020, 12, 3044. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Akhtar, N.; Akram, M.; Shah, P.A.; Saeed, T.; Ahmed, K.; Asif, H. Pharmacological activity of Althaea officinalis L. J. Med. Plants Res. 2011, 5, 5662–5666. [Google Scholar]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red Clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Joulia, P.; Amiel, A.; Fabre, B.; David, B.; Fabre, N.; Fiorini-Puybaret, C. Comparison of the Phytochemical Composition of Serenoa repens Extracts by a Multiplexed Metabolomic Approach. Molecules 2019, 24, 2208. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Xu, T.; Zhong, Y.; Xu, M.; Liu, Y.; Li, M.; Fan, B.; Wang, F.; Xiao, J.; et al. Chinese yam (Dioscorea): Nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 2023, 134, 29–40. [Google Scholar] [CrossRef]
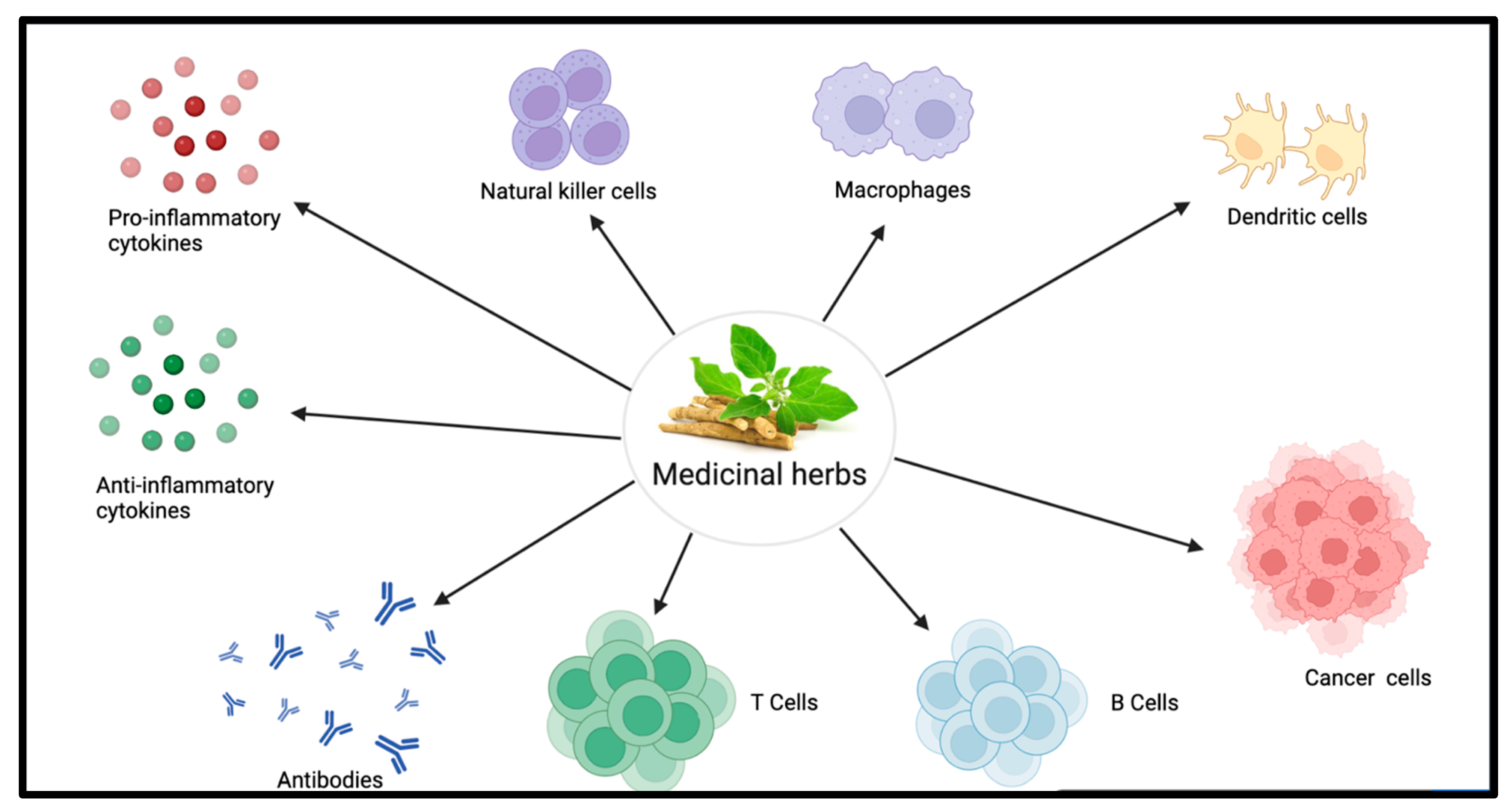
| Immunomodulatory Effects | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medicinal Herbs | Bioactive Component | T-Cells | B-Cells | NK Cells | Antibodies | Pro-Inflammatory Cytokines | Anti-Inflammatory Cytokines | References |
 Ashwagandha (Withania somnifera) Ashwagandha (Withania somnifera) | withanolides | + | + | + | IgA, IgM, IgG, IgG2, IgG3 and IgG4 | - | + | [15,16] |
 Astragalus (Astragalus membranaceus) Astragalus (Astragalus membranaceus) | polysaccharides, saponins, flavonoids, astragalosides, lipopolysaccharides | + | + | + | IgG, IgA, IgM | - | + | [17,18,19,20] |
 Echinacea (Echinacea purpurea) Echinacea (Echinacea purpurea) | alkamides, phenolic compounds | + | + | + | + | - | + | [21,22] |
 Garlic (Allium sativum) Garlic (Allium sativum) | allicin, diallyl sulfide (DAS), Z-ajoene | + | + | + | IgG, IgA | - | + | [23,24,25,26,27,28] |
 Ginger (Zingiber officinale) Ginger (Zingiber officinale) | gingerol, polyphenols, shogaols, paradols, zingerone | + | + | + | Total IgG, IgG1 | - | + | [29,30,31,32,33] |
 Ginseng (Panax ginseng) Ginseng (Panax ginseng) | ginsenosides, polysaccharides | + | + | + | IgM, IgG1, IgG2a, IgG2b | - | + | [34,35,36] |
 Licorice (Glycyrrhiza glabra) Licorice (Glycyrrhiza glabra) | triterpenoids, saponin, flavonoids, sterols | + | + | + | IgG, IgM | - | + | [37,38,39,40,41] |
 Tulsi (Ocimum sanctum) Tulsi (Ocimum sanctum) | saponins, flavonoids, terpenoids, linoleic acid | + | - | + | + | + | + | [42,43,44] |
| Medicinal Plants | Active Components | References |
|---|---|---|
| 1. Ashwagandha (Withania somnifera) | Alkaloids, flavonoids, steroids, tannins, withanolides, and glycosides | [46,113,114] |
| 2. Astragalus (Astragalus membranaceus) | Astragalus polysaccharide (APS), alkaloids, saponins, and flavonoids | [115,116] |
| 3. Echinacea (Echinacea purpurea) | Alkamides, polysaccharides, caffeic acid derivatives, and cichoric acid | [65,117] |
| 4. Garlic (Allium sativum) | Allicin, diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), alliin, S-allyl-cysteine, and Z-ajoene | [118] |
| 5. Ginger (Zingiber officinale) | polyphenols (Gingerols), shogaols, terpenes, polysaccharides, organic acids, and paradols | [119] |
| 6. Ginseng (Panax ginseng) | Ginsenosides, gintonins, and polysaccharides | [120] |
| 7. Licorice (Glycyrrhiza glabra) | Triterpenoids, saponin (glycyrrhetinic acid), flavonoids and sterols | [121] |
| 8. Shatavari (Asparagus racemosus) | Saponins, polysaccharides, flavonoids, polyphenols, and alkaloids | [122] |
| 9. Tulsi (Ocimum sanctum) | alkaloids, saponins, flavonoids, terpenoids, linoleic acid, tannins, glycosides, carbohydrates, and proteins | [123] |
| 10. Turmeric (Curcuma longa) | Curcuminoids, flavonoids, phenolic acid, amino acids, and sesquiterpenes | [124] |
| 11. Andrographis (Andrographis paniculata) | Andrographolide, flavonoids, neoandrographolide, andrographine, and panicolines | [125] |
| 12. Black elderberry (Sambucus nigra) | Polyphenols, anthocyanins, flavonols, tannins, and procyanidins | [126] |
| 13. Cat’s claw (Uncaria tomentosa) | Proanthocyanidins, spiroxindole alkaloids, quinovic acid glycosides, Indole glycosides alkaloids, and tannins | [127] |
| 14. Guduchi (Tinospora cordifolia) | Alkaloids, aliphatics, diterpenoid lactones, steroids, and glycosides | [128] |
| 15. Holy basil (Ocimum tenuiflorum) | Phenols, flavonoids, polyphenols, eugenol, and methylchavicol | [129] |
| 16. Milk thistle (Silybum marianum) | Flavonolignans, flavonoids, tocopherol, proteins, and sterols | [130] |
| 17. Schisandra (Schisandra chinensis) | Flavonoids, phenolic acids, dibenzocyclooctadiene lignans, triterpenoids, and tannins | [131] |
| 18. St. John’s wort (Hypericum perforatum) | Flavonoids, naphthodianthrones (hypericin), carbolic acids, phloroglucins (hyperforin), and xanthones | [132] |
| 19. Triphala | Tannins, flavonoids, gallic acid, glucosides, chebulic acid, and quercetin | [133] |
| 20. Wheatgrass (Triticum aestivum) | Flavonoids, chlorophyll, tannins, terpenoids, steroids, alkaloids, and glycosides | [134] |
| 21. Myrrh | Steroids, terpenoids, flavonoids, lignans, and carbohydrates | [135] |
| 22. Chamomile (Matricaria chamomilla) | Flavonoids, terpenoids, sesquiterpenes, phenolic acids, and coumarins | [136] |
| 23. Ginkgo (Ginkgo biloba) | Flavonoids, ginkgolides, glycosides, terpenoids, and sesquiterpenes | [137] |
| 24. Valerian (Valeriana officinalis) | Flavonoids, lignans, curcuminoids, tannins, phenolic acids, and quinones | [138] |
| 25. Peppermint (Mentha piperita) | Menthol, flavonoids, phenolic acids, menthone, acetaldehyde, limonene, alkaloids, saponins, and glycosides | [139] |
| 26. Saw palmetto (Serenoa repens) | Fatty acids (laurate, myristate, palmitate, linoleate), and phytosterols | [140] |
| 27. Sage (Salvia officinalis) | Alkaloids, fatty acids, flavonoids, tannins, steroids, terpenoids, saponins, and coumarins | [141] |
| 28. Aloe vera (Aloe vera) | Flavonoids, polysaccharides, saponins, vitamins, anthraquinones, fatty acids, salicylic acid, and lignins | [142] |
| 29. Bilberry (Vaccinium myrtillus) | Anthocyanins, terpenoids, flavonoids, tannins, phenolic acids, and coumarins | [143] |
| 30. Black cohosh (Actaea racemosa) | Terpenoids, phenolic acids, flavonoids, alkaloids, tannins, and aromatic acids | [144] |
| 31. Feverfew (Tanacetum parthenium) | Sesquiterpene lactones, flavonoids, polyenes, and volatile oils (camphor, camphene) | [145] |
| 32. Guggulu (Commiphora mukul) | Terpenoids, steroids, flavonoids, gluggultetrols, lignans, polysaccharides, and amino acids | [146] |
| 33. Hawthorn (Crataegus spp.) | Phenolic acids, flavonoids, pyrocatechin, terpenoids, lignans, steroids, and organic acids (fumaric, tartaric) | [147] |
| 34. Lavender (Lavandula angustifolia) | Phenolic acids, flavonoids, terpenoids (hydrocarbons, oxidated), Sesquiterpenes, amino acids, aldehydes, and coumarins (coumarin, herniarin) | [148] |
| 35. Lemon balm (Melissa officinalis) | Volatile compounds (neral, geraniol, citronellal, geranial), flavonoids, phenolic acids, triterpenes, and tannins | [149] |
| 36. Nettle (Urtica dioica) | Flavonoids, phenolic acids, amino acids, carotenoids, organic acids (Acetic acid, citric acid, formic acid), fatty acids, and tannins | [150] |
| 37. Passion flower (Passiflora incarnata) | Alkaloids, flavonoids, phenolic compounds, cyanogenic glycosides, tannins, and steroids (β-Sitosterol) | [151] |
| 38. Rhodiola (Rhodiola rosea) | Phenolic compounds, flavonoids, carotenoids, vitamin E, tannins, glycosides, organic acids, and salidroside | [152] |
| 39. Slippery elm (Ulmus rubra) | Polysaccharides (D-galactose, L-rhamnose, D-galacturonic acid), phytosterols, and oleic and palmitic acids | [153] |
| 40. Yarrow (Achillea millefolium) | Flavonoids, phenolic acids, terpenes (guaianolides, sesquiterpenes), phytosterols, organic acids, and fatty acids | [154] |
| 41. Baikal skullcap (Scutellaria baicalensis) | Flavonoids (baicalein, baicalin, wogonin), phenylethanoid glycosides, polysaccharides, steroids, phenolic compounds, amine, and organic acids | [155] |
| 42. Calendula (Calendula officinalis) | Terpenoids, steroids, flavonoids, triterpeneol esters, saponins, carotenes, carbohydrates, and tocopherols | [156] |
| 43. Dandelion (Taraxacum officinale) | Sesquiterpene lactones, triterpenes, sterols, flavonoids, inulin, vitamins (A, C, E, K, B), and polyphenols (hydroxycinnamic acid) | [157] |
| 44. Eleuthero (Eleutherococcus senticosus) | Saponins, phenylpropanoids, phenolic acids, polysaccharides, coumarins, lignans, and provitamins | [158] |
| 45. Kava (Piper methysticum) | Flavonoids (flavokavains), kavalactones, and alkaloids | [159] |
| 46. Marshmallow (Althaea officinalis) | Polysaccharides, flavonoids, phytosterols, tannins, coumarins, scopoletin, and amino acids | [160] |
| 47. Red clover (Trifolium pratense) | Flavonoids, saponins, clovamides, phenolic acids, coumarins, and pterocarpans | [161] |
| 48. Saw palmetto (Serenoa repens) | Fatty acids (free and esterified), triterpenes, flavonoids, carotenoids, tocopherols (Ve), and phytosterols | [162] |
| 49. Wild yam (Dioscorea villosa) | Steroidal saponin (Diosgenin), allantoin, polysaccharides, and alkaloids | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, H.H.; Elasbali, A.M.; Alanazi, M.K.; El Azab, E.F. Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Molecules 2023, 28, 8045. https://doi.org/10.3390/molecules28248045
Alanazi HH, Elasbali AM, Alanazi MK, El Azab EF. Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Molecules. 2023; 28(24):8045. https://doi.org/10.3390/molecules28248045
Chicago/Turabian StyleAlanazi, Hamad H., Abdelbaset Mohamed Elasbali, Maged K. Alanazi, and Eman Fawzy El Azab. 2023. "Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases" Molecules 28, no. 24: 8045. https://doi.org/10.3390/molecules28248045
APA StyleAlanazi, H. H., Elasbali, A. M., Alanazi, M. K., & El Azab, E. F. (2023). Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Molecules, 28(24), 8045. https://doi.org/10.3390/molecules28248045








