Simulating Crystal Structure, Acidity, Proton Distribution, and IR Spectra of Acid Zeolite HSAPO-34: A High Accuracy Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Unit Cell
2.2. Calculated Crystal Structure
2.3. Thermal Stability, Acidity Strength, and Vibrational Frequency of Brönsted Hydroxyl (ν(O-H))
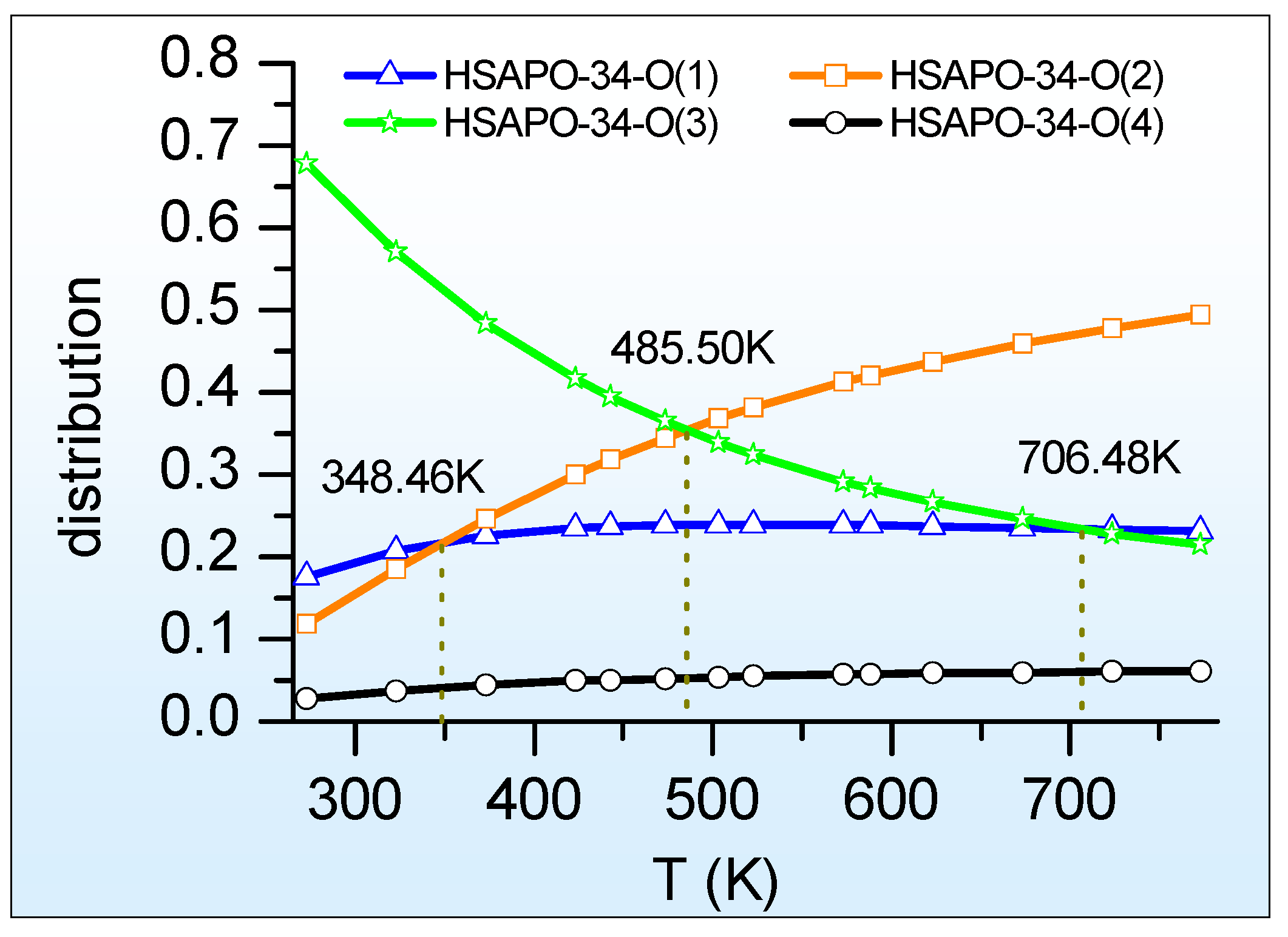
2.3.1. Thermal Stability
2.3.2. Vibrational Frequency of Brönsted Hydroxyl (ν(O-H))
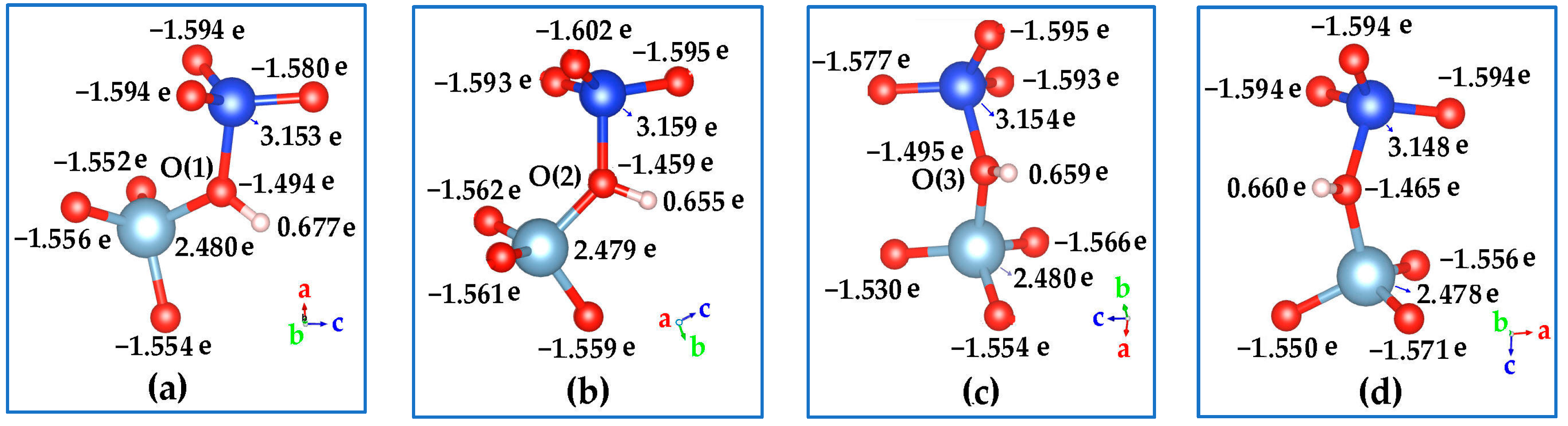
2.3.3. Proton Siting and Proton Distribution
2.3.4. Acid Strength
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Alumniophosphate Molecular Sieves: A New Class of Microporous Crystalline Inorganic Solids. J. Am. Soc. Chem. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Smith, L.; Cheetham, A.K.; Morris, R.E.; Marchese, L.; Thomas, J.M.; Wright, P.A.; Chen, J. On the Nature of Water Bound to a Solide Acid Catalyst. Science 1996, 271, 799–802. [Google Scholar] [CrossRef]
- Smith, L.; Cheetham, A.K.; Marchese, L.; Thomas, J.M.; Wright, P.A.; Chen, J.; Gianotti, E. A Quantitative Description of the Active Sites in the Dehydrated Acid Catalyst HSAPO-34 for the Conversion of Methanol to Olefins. Catal. Lett. 1996, 41, 13–16. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishio, T.; Katada, N.; Sastre, G.; Niwa, M. Ammonia IRMS-TPD measurements on Brønsted acidity of proton-formed SAPO-34. Phys. Chem. Chem. Phys. 2011, 13, 3311–3318. [Google Scholar] [CrossRef]
- Potter, M.E. Down the Microporous Rabbit Hole of Silicoaluminophosphates: Recent Developments on Synthesis, Characterization, and Catalytic Applications. ACS Catal. 2020, 10, 9758–9789. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Rostami, R.B.; Lemraski, A.S.; Ghavipour, M.; Behbahani, R.M.; Shahraki, B.H.; Hamule, T. Kinetic modelling of methanol conversion to light olefins process over silicoaluminophosphate (SAPO-34) catalyst. Chem. Eng. Res. Des. 2016, 106, 347–355. [Google Scholar] [CrossRef]
- Dai, W.; Cao, G.; Yang, L.; Wu, G.; Dyballa, M.; Hunger, M.; Guan, N.; Li, L. Insights into the catalytic cycle and activity of methanol-to-olefin conversion over low-silica AlPO-34 zeolites with controllable Brønsted acid density. Catal. Sci. Technol. 2017, 7, 607–618. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Qu, Y.; Liu, H.; Tang, C.; Miao, S.; Feng, Z.; An, H.; Li, C. Highly Selective Conversion of Carbon Dioxide to Lower Olefins. ACS Catal. 2017, 7, 8544–8548. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, D.; Bao, X. Catalysis for Selected C1 Chemistry. Chem 2020, 6, 2497–2514. [Google Scholar] [CrossRef]
- Han, J.; Yang, G.; Ding, H.; Chen, X. Revealing inherent factors of SAPO-34 zeolites etching towards the fabrication of hierarchical structure. Microporous Mesoporous Mater. 2021, 319, 111067. [Google Scholar] [CrossRef]
- Vallace, A.; Kester, G.; Casteel, W.; Lau, G.; Whitley, R.; Coe, C. A Study of Structural Defects in X- and Y-Type Zeolites and Their Effect on Their Transformation to Aluminum-Rich Chabazite. J. Phys. Chem. C 2021, 125, 12848–12856. [Google Scholar] [CrossRef]
- Usman, M.; Ghanem, A.S.; Shah, S.N.A.; Garba, M.D.; Khan, M.Y.; Khan, S.; Humayun, M.; Khan, A.L. A Review on SAPO-34 Zeolite Materials for CO2 Capture and Conversion. Chem. Rec. 2022, 22, e202200039. [Google Scholar] [CrossRef] [PubMed]
- Kheimi, M.; Salamah, S.K. Simulation of temperature swing adsorption process to purify hydrogen for fuel cell uses by SAPO34 as adsorbent. Chemosphere 2023, 338, 139454. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ran, J.; Du, X.; Wang, X.; Ran, Z.; Chen, Y.; Zhang, L.; Crittenden, J. Understanding the nature of NH3-coordinated active sites and the complete reaction schemes for NH3-SCR using Cu-SAPO-34 catalysts. Phys. Chem. Chem. Phys. 2021, 23, 4700. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, E.L. Theoretical study of nitrogen dioxide and nitric oxide co-adsorption and DeNOx reaction on Cu-SAPO−34 and Cu-SSZ-13 in presence of Brønsted acid sites. Mol. Catal. 2018, 447, 47–55. [Google Scholar] [CrossRef]
- Wilson, S.; Barger, P. The characteristics of SAPO-34 which influence the conversion of methanol to light olefins. Microporous Mesoporous Mater. 1999, 29, 117–126. [Google Scholar] [CrossRef]
- Aguayo, A.T.; Gayubo, A.G.; Vivanco, R.; Olazar, M.; Bilbao, J. Role of acidity and microporous structure in alternative catalysts for the transformation of methanol into olefins. Appl. Catal. A Gen. 2005, 283, 197–207. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Schmieg, S.J.; Weng, D. Role of Brønsted acidity in NH3 selective catalytic reduction reaction on Cu/SAPO-34 catalysts. J. Catal. 2015, 324, 98–106. [Google Scholar] [CrossRef]
- Li, X.; Shen, W.; Zheng, A. The influence of acid strength and pore size effect on propene elimination reaction over zeolites: A theoretical study. Microporous Mesoporous Mater. 2019, 278, 121–129. [Google Scholar] [CrossRef]
- Sierraalta, A.; Añez, R.; Coll, D.S.; Alejos, P. Conversion of methanol to dimethyl ether over silicoaluminophosphates: Isolated acid sites and the influence of silicon islands. A DFT-ONIOM study. Microporous Mesoporous Mater. 2020, 292, 109732. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Lok, B.M.; Patton, R.L.; Wilson, S.T. Aluminophosphate molecular sieves and the periodic table. Pure Appl. Chem. 1986, 58, 1351–1358. [Google Scholar] [CrossRef]
- Shen, W.; Li, X.; Wei, Y.; Tian, P.; Deng, F.; Han, X.; Bao, X. A study of the acidity of SAPO-34 by solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2012, 158, 19–25. [Google Scholar] [CrossRef]
- Sastre, G.; Lewis, D.W.; Catlow, C.R.A. Modeling of Silicon Substitution in SAPO-5 and SAPO-34 Molecular Sieves. J. Phys. Chem. B 1997, 101, 5249–5262. [Google Scholar] [CrossRef]
- Dent, L.S.; Smith, J.V. Crystal Structure of Chabazite, A Molecular Sieve. Nature 1958, 181, 1794–1796. [Google Scholar] [CrossRef]
- Martins, G.A.V.; Berlier, G.; Coluccia, S.; Pastore, H.O.; Superti, G.B.; Gatti, G.; Marchese, L. Revisiting the Nature of the Acidity in Chabazite-Related Silicoaluminophosphates: Combined FTIR and 29Si MAS NMR Study. J. Phys. Chem. C 2007, 111, 330–339. [Google Scholar] [CrossRef]
- ZubkoY, S.A.; Kustov, L.M.; Kazansky, V.B.; Girnus, I.; Fricke, R. Investigation of Hydroxyl Groups in Crystalline Silicoaluminophosphate SAPO-34 by Diffuse Reflectance Infrared Spectroscopy. J. Chem. Soc. Faraday Trans. 1991, 87, 897–900. [Google Scholar] [CrossRef]
- Pfeifer, H.; Freude, D.; Hunger, M. Nuclear magnetic resonance studies on the acidity of zeolites and related catalysts. Zeolites 1985, 5, 274–286. [Google Scholar] [CrossRef]
- Iwase, Y.; Motokura, K.; Koyama, T.-R.; Miyaji, A.; Baba, T. Influence of Si Distribution in Framework of SAPO-34 and its Particle Size on Propylene Selectivity and Production Rate for Conversion of Ethylene to Propylene. Phys. Chem. Chem. Phys. 2009, 11, 9268–9277. [Google Scholar] [CrossRef]
- Sena, F.C.; Souza, B.F.D.; Almeida, N.C.D.; Cardoso, J.S.; Fernandes, L.D. Influence of framework composition over SAPO-34 and MeAPSO-34 acidity. Appl. Catal. A Gen. 2011, 406, 59–62. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Wang, L.; Han, J.; Lou, C.; Xu, S.; Zhang, Y.; Wu, R.A.; Tian, P.; Liu, Z. Recognizing the Minimum Structural Units Driving the Crystallization of SAPO-34 in a Top-Down Process. Chem. Eur. J. 2023, 29, e202203886. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Y.; Wang, J.; Dong, X.; Tian, Q.; Han, Y. Recent Progress in the Direct Synthesis of Hierarchical Zeolites: Synthetic Strategies and Characterization Methods. Mater. Chem. Front. 2017, 1, 2195–2212. [Google Scholar] [CrossRef]
- Izadbakhsh, A.; Farhadi, F.; Khorasheh, F.; Sahebdelfar, S.; Asadi, M.; Feng, Y.Z. Effect of SAPO-34′s composition on its physico-chemical properties and deactivation in MTO process. Appl. Catal. A Gen. 2009, 364, 48–56. [Google Scholar] [CrossRef]
- Skylaris, C.-K. A Benchmark for Materials Simulation-Material Properties Can Now Be Predicted Reliably from First-Principles Calculations. Science 2016, 351, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Gale, J.D.; Shah, R.; Payne, M.C.; Stich, I.; Terakura, K. Methanol in microporous materials from first principles. Catal. Today 1999, 50, 525–532. [Google Scholar] [CrossRef]
- Jeanvoine, Y.; Ángyán, J.G.; Kresse, G.; Hafner, J. Brønsted Acid Sites in HSAPO-34 and Chabazite: An Ab Initio Structural Study. J. Phys. Chem. B 1998, 102, 5573–5580. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.E.; Armiento, R.; Schultz, P.A.; Mattsson, T.R. Nonequivalence of the generalized gradient approximations PBE and PW91. Phys. Rev. B 2006, 73, 195123. [Google Scholar] [CrossRef]
- Kohn, W. Nobel Lecture: Electronic Structure of Matter-Wave Functions and Density Functionals. Rev. Mod. Phys. 1999, 71, 1253–1266. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Hellmann, H. A New Approximation Method in the Problem of Many Electrons. J. Chem. Phys. 1935, 3, 61. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Lin-Chung, P.J. Limitation of the Pseudopotential Method. Phys. Rev. B 1973, 8, 4043–4045. [Google Scholar] [CrossRef]
- Ziesche, P.; Kurth, S.; Perdew, J.P. Density Functionals from LDA to GGA. Comput. Mater. Sci. 1998, 11, 122–127. [Google Scholar] [CrossRef]
- Perdew, J.P.; Schmidt, K. Jacob’s Ladder of Density Functional Approximations for the Exchange-Correlation Energy. AIP Conf. Proc. 2001, 577, 1–20. [Google Scholar]
- Mackrodt, W.C. A Note on An Aspect of Pseudopotential Theory. Theor. Chim. Acta 1973, 30, 119–126. [Google Scholar] [CrossRef]
- Sun, J.; Ruzsinszky, A.; Perdew, J.P. Strongly Constrained and Appropriately Normed Semilocal Density Functional. Phys. Rev. Lett. 2015, 115, 036402. [Google Scholar] [CrossRef]
- Sun, J.; Remsing, R.C.; Zhang, Y.; Sun, Z.; Ruzsinszky, A.; Peng, H.; Yang, Z.; Paul, A.; Waghmare, U.; Wu, X.; et al. Accurate first-principles structures and energies of diversely bonded systems from an efficient density functional. Nat. Chem. 2016, 8, 831–836. [Google Scholar] [CrossRef]
- Zhang, Y.; Kitchaev, D.A.; Yang, J.; Chen, T.; Dacek, S.T.; Samiento-Pérez, R.A.; Marques, M.A.L.; Peng, H.; Ceder, G.; Perdew, J.P.; et al. Efficient First-Principles Prediction of Solid Stability: Towards Chemical Accuracy. NPJ Comput. Mater. 2018, 4, 9. [Google Scholar] [CrossRef]
- Peng, H.; Yang, Z.-H.; Perdew, J.P.; Sun, J. Versatile van der Waals Density Functional Based on a Meta-Generalized Gradient Approximation. Phys. Rev. X 2016, 6, 041005. [Google Scholar] [CrossRef]
- Chen, X.-F. Periodic Density Functional Theory (PDFT) Simulating Crystal Structures with Microporous CHA Framework: An Accuracy and Efficiency Study. Inorganics 2023, 11, 215. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases (accessed on 5 November 2023).
- Ángyán, J.G.; Parsons, D.; Jeanvoine, Y. Ab initio simulations of zeolite reactivity. In Theoretical Aspects of Heterogeneous Catalysis; Nascimento, M.A.C., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 77–108. Available online: https://link.springer.com/chapter/10.1007/0-306-47667-3_4 (accessed on 5 November 2023).
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Smith, L.J.; Davidson, A.; Cheetham, A.K. Aneutron Diffraction and Infrared Spectroscopy Study of the Acid Form of the Aluminosilicate Zeolite, Chabazite (H-SSZ-13). Catal. Lett. 1997, 49, 143–146. [Google Scholar] [CrossRef]
- Marchese, L.; Chen, J.; Wright, P.A.; Thomas, J.M.J. Formation of hydronium at the Broensted site in SAPO-34 catalysts. J. Phys. Chem. 1993, 97, 8109–8112. [Google Scholar] [CrossRef]
- Shah, R.; Gale, J.D.; Payne, M.C. Methanol Adsorption in Zeolites: A First-Principles Study. J. Phys. Chem. 1996, 100, 11688–11697. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, K.J.; O’Malley, P.J. A density functional study of acidic hydroxyl groups in zeolites and their interaction with carbon monoxide. J. Phys. Chem. 1996, 100, 1814–1819. [Google Scholar] [CrossRef]
- Kilburn, L.; DeLuca, M.; Hoffman, A.J.; Patel, S.; Hibbitts, D. Comparing alkene-mediated and formaldehyde-mediated diene formation routes in methanol-to-olefins catalysis in MFI and CHA. J. Catal. 2021, 400, 124–139. [Google Scholar] [CrossRef]
- Feng, P.; Chen, X.-F.; Li, X.-J.; Zhao, D.; Xie, S.-J.; Xu, L.-Y.; He, G.-Z. The distribution analysis on the proton siting and the acid strength of the zeolite ferrierite: A computational study. Microporous Mesoporous Mater. 2017, 239, 354–362. [Google Scholar] [CrossRef]
- Simperler, A.; Bell, R.G.; Foster, M.D.; Gray, A.E.; Lewis, D.W.; Anderson, M.W. Probing the acid strength of Brønsted acidic zeolites with acetonitrile: An atomistic and quantum chemical study. J. Phys. Chem. B 2004, 108, 7152–7161. [Google Scholar] [CrossRef]
- Bordiga, S.; Palomino, G.T.; Pazè, C.; Zecchina, A. Vibrational spectroscopy of H2, N2, CO and NO adsorbed on H, Li, Na, K-exchanged ferrierite. Microporous Mesoporous Mater. 2000, 34, 67–80. [Google Scholar] [CrossRef]
- Maxwell, J.C. Illustrations of the Dynamical Theory of Gases. Available online: https://www.worldscientific.com/doi/epdf/10.1142/9781848161337_0011 (accessed on 5 November 2023).
- Henkelman, G.; Arnaldson, A.; Jopsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 31, 354–360. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The Strength of Brønsted Acid Sites in Microporous Aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B Condens. Matter. Mater. Phys. 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mat. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-energy Calculations Using a Plane—Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Flanigen, E.M. SMIII mechanism. Elsevier Sci. B 1986, 85, 653–685. [Google Scholar]
- Ghorbanpour, A.; Rimer, J.D.; Grabow, L.C. Computational Assessment of the Dominant Factors Governing the Mechanism of Methanol Dehydration over H-ZSM-5 with Heterogeneous Aluminum Distribution. ACS Catal. 2016, 6, 2287–2298. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Weckhuysen, B.M.; Nørskov, J.K. Guest–host interactions of arenes in H-ZSM-5 and their impact on methanol-to-hydrocarbons deactivation processes. J. Catal. 2013, 300, 235–241. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Henry, R.; Schuurman, Y.; Medford, A.J.; Moses, P.G.; Beato, P.; Svelle, S.; Nørskov, J.K.; Olsbye, U. Methanol-to-hydrocarbons conversion: The alkene methylation pathway. J. Catal. 2014, 314, 159–169. [Google Scholar] [CrossRef]
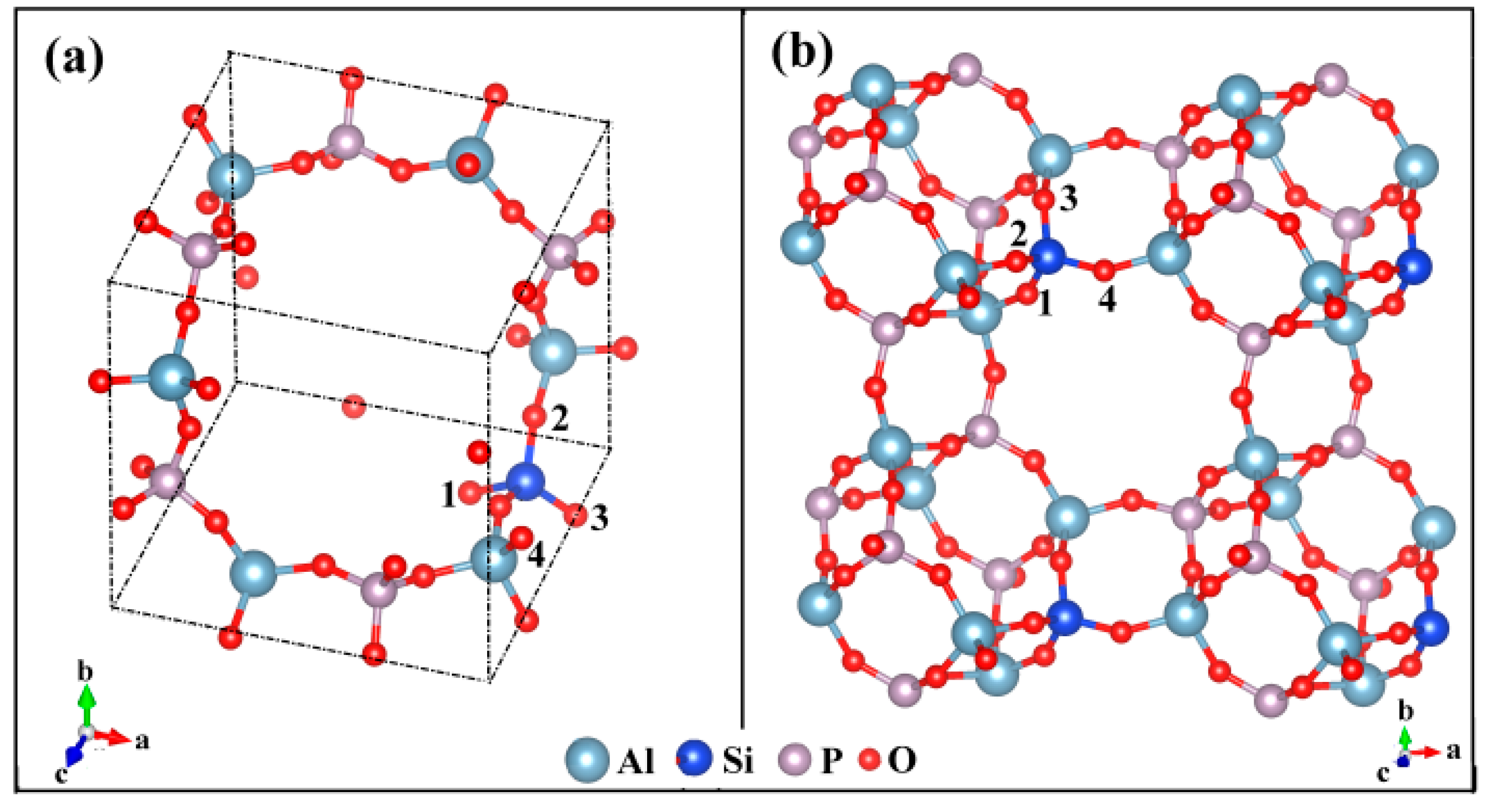



| This work | O(1) | O(2) | O(3) | O(4) |
| IZA | O(4) | O(3) | O(1) | O(2) |
| Location | 4MR(2) α 8MR(1,+) α,β | 4MR(1) α 6MR(1) α,β 8MR(1,+) | 4MR(2) α 6MR(1,+) α,β | 4MR(1) α 8MR(2,+) α,β |
| Parameters | Brönsted Acid Sites of HSAPO-34 | |||
|---|---|---|---|---|
| O(1) | O(2) | O(3) | O(4) | |
| V0/Å3 | 821.82 | 810.98 | 820.13 | 806.62 |
| E0/eV | −229.33 | −229.33 | −229.34 | −229.31 |
| B0 | 0.13 | 0.08 | 0.12 | 0.01 |
| −27.37 | −43.85 | −30.35 | −400.24 | |
| Parameters | Brönsted Acid Sites of HSAPO-34 | ||||
|---|---|---|---|---|---|
| O(1) | O(2) | O(3) | O(4) | Exp [3] | |
| a (Å) | 9.40 | 9.42 | 9.40 | 9.19 | 9.40 |
| b (Å) | 9.49 | 9.39 | 9.52 | 9.38 | 9.40 |
| c (Å) | 9.26 | 9.42 | 9.32 | 9.39 | 9.40 |
| α (°) | 94.12 | 94.79 | 93.58 | 95.77 | 94.27 |
| β (°) | 94.13 | 94.36 | 94.16 | 96.12 | 94.27 |
| γ (°) | 94.03 | 94.67 | 95.58 | 96.04 | 94.27 |
| V0 (Å3) | 819.50 | 824.46 | 807.96 | 795.26 | 822.39 |
| MAD-abc | −0.02 | 0.01 | −0.05 | −0.08 | - |
| RMSD-abc | 0.10 | 0.02 | 0.14 | 0.12 | - |
| MAD-αβγ | −0.18 | 0.34 | −0.70 | 1.71 | - |
| RMSD-αβγ | 0.18 | 0.38 | 0.85 | 1.71 | - |
| Parameters | Brönsted Acid Sites of HSAPO-34 | |||
|---|---|---|---|---|
| O(1) | O(2) | O(3) | O(4) | |
| ΔE/(kcal/mol) | 1.025 | 1.935 | 0 | 1.59 |
| DPE/(kJ/mol) | 1526 | 1522 | 1530 | 1524 |
| proton charge (e) | 0.677 | 0.655 | 0.669 | 0.66 |
| R(O-H)(Å) | 0.977 | 0.979 | 0.981 | 0.978 |
| R(Si-O(H)) | 1.766 | 1.754 | 1.774 | 1.751 |
| ν(O-H)/cm−1 | 3706 | 3684 | 3625 | 3700 |
| ν(O-H)/cm−1 [3,56] | 3625 | 3601 | - | 3630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yu, T. Simulating Crystal Structure, Acidity, Proton Distribution, and IR Spectra of Acid Zeolite HSAPO-34: A High Accuracy Study. Molecules 2023, 28, 8087. https://doi.org/10.3390/molecules28248087
Chen X, Yu T. Simulating Crystal Structure, Acidity, Proton Distribution, and IR Spectra of Acid Zeolite HSAPO-34: A High Accuracy Study. Molecules. 2023; 28(24):8087. https://doi.org/10.3390/molecules28248087
Chicago/Turabian StyleChen, Xiaofang, and Tie Yu. 2023. "Simulating Crystal Structure, Acidity, Proton Distribution, and IR Spectra of Acid Zeolite HSAPO-34: A High Accuracy Study" Molecules 28, no. 24: 8087. https://doi.org/10.3390/molecules28248087







