Poncirus trifoliata Aqueous Extract Protects Cardiomyocytes against Doxorubicin-Induced Toxicity through Upregulation of NAD(P)H Dehydrogenase Quinone Acceptor Oxidoreductase 1
Abstract
:1. Introduction
2. Results
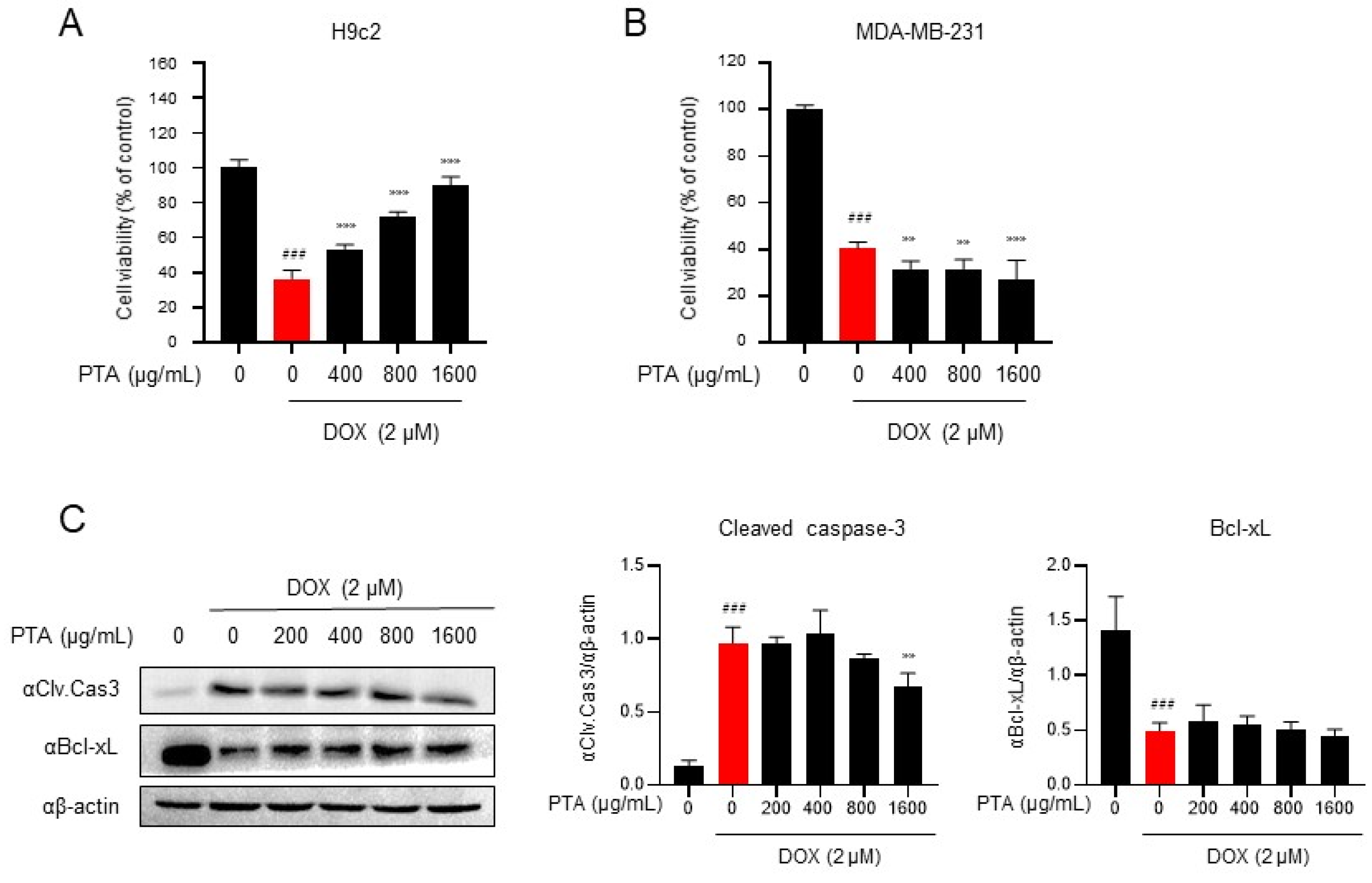
2.1. PTA Inhibits DOX-Induced Cytotoxicity in H9c2 Cells
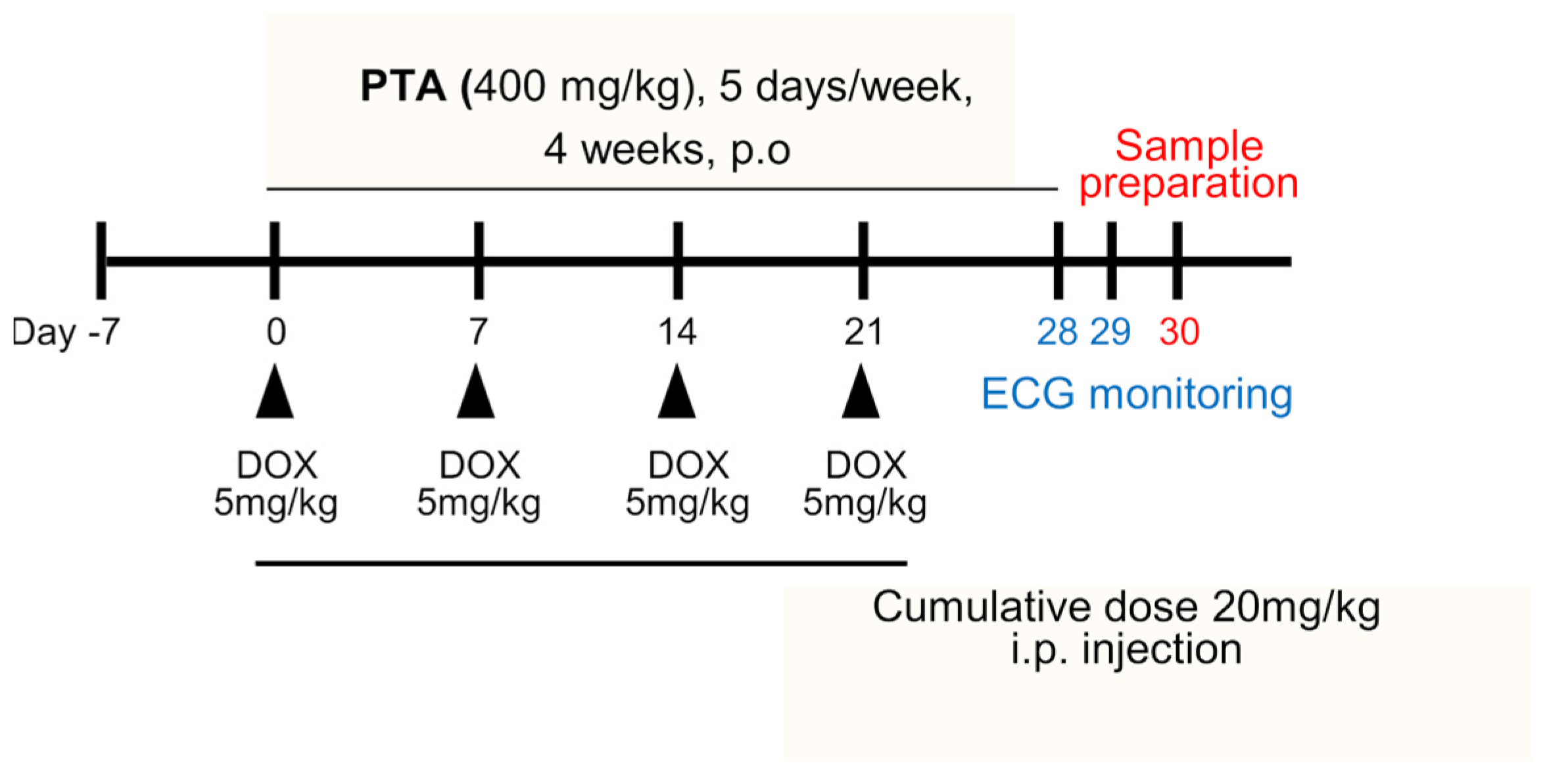
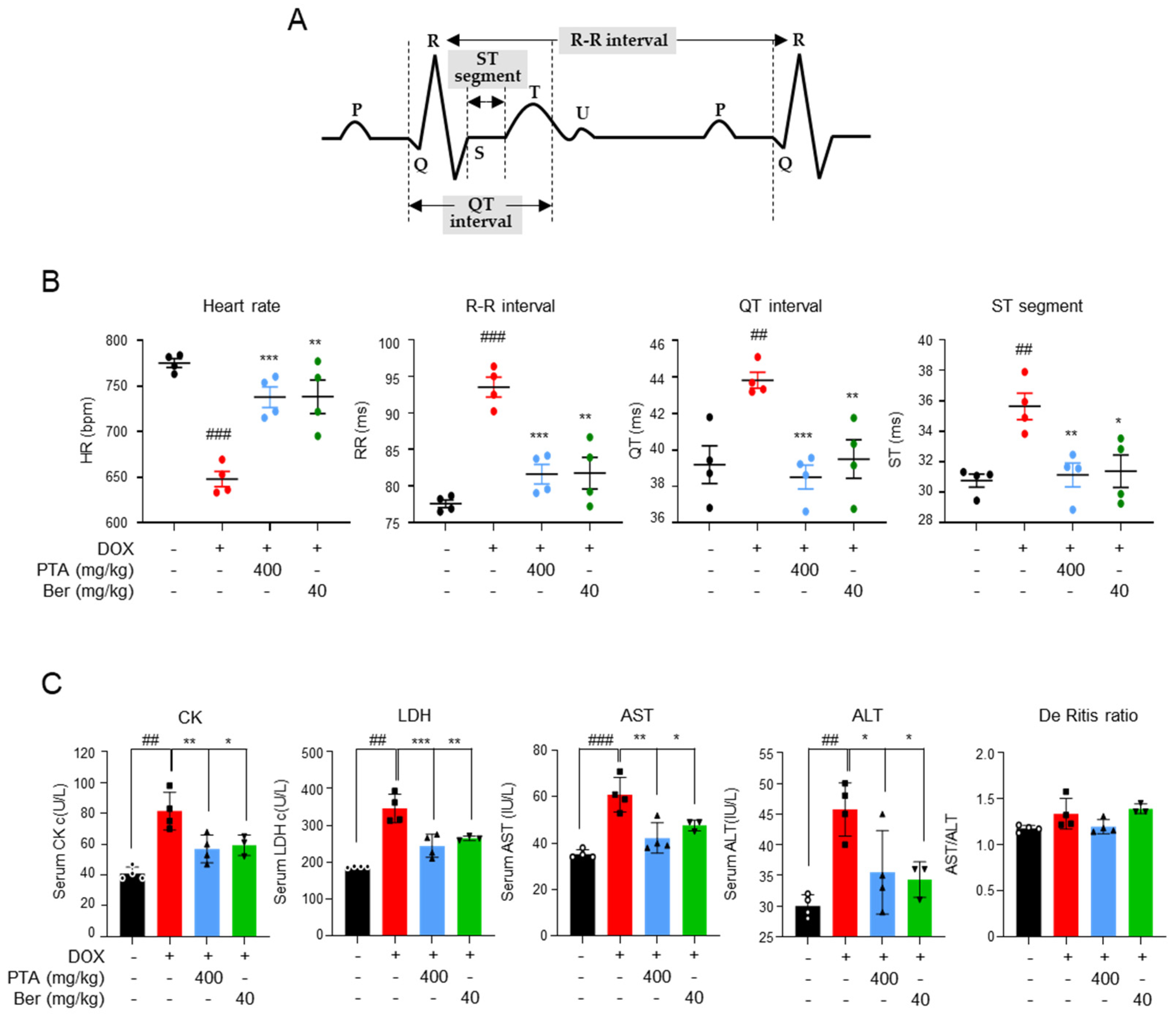
2.2. PTA Protects Cardiac Functions by Inhibiting DOX-Induced Cardiomyocytes Death In Vivo
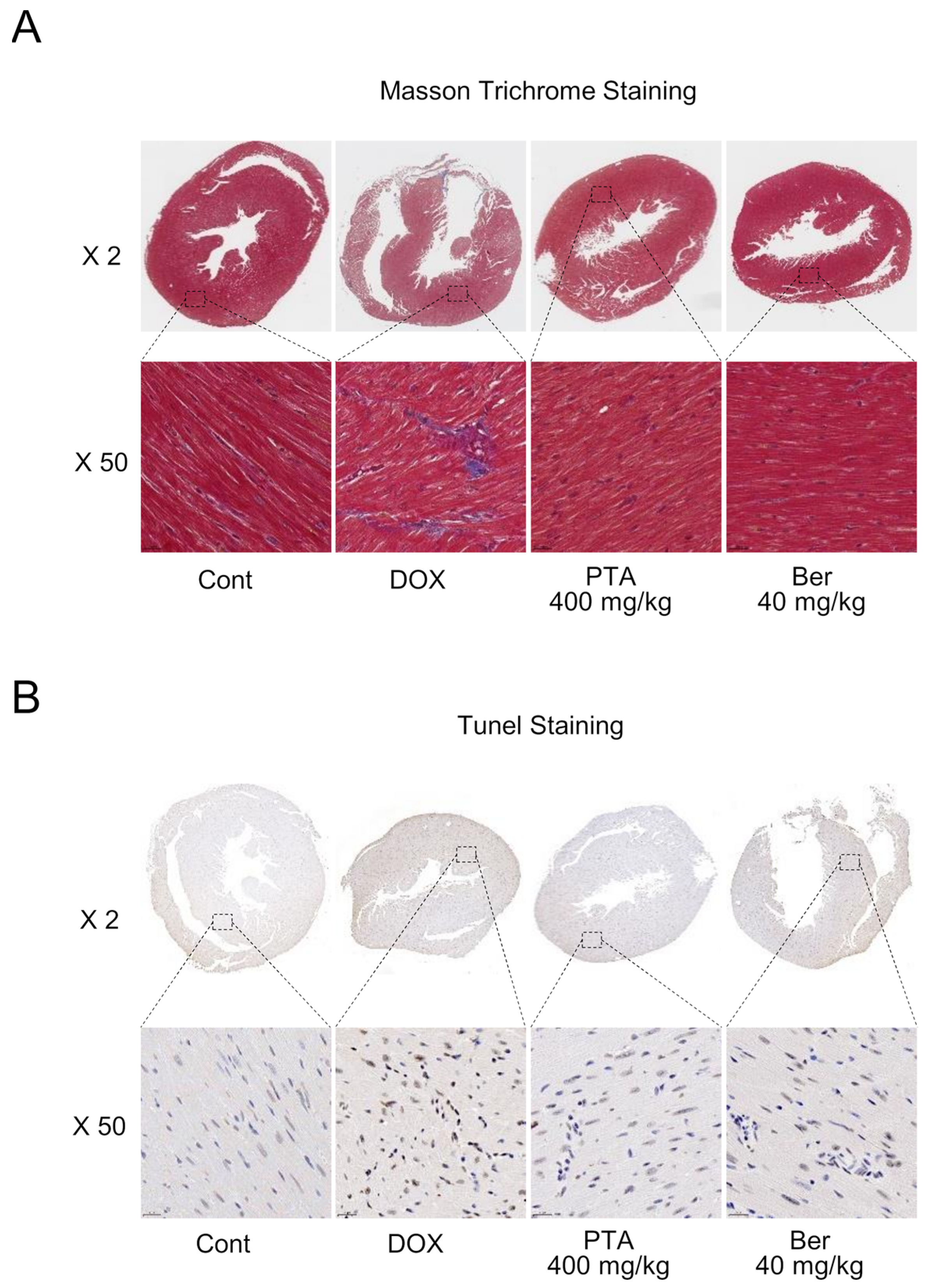
2.3. PTA Blocks DOX-Induced Cardiac Fibrosis and Death
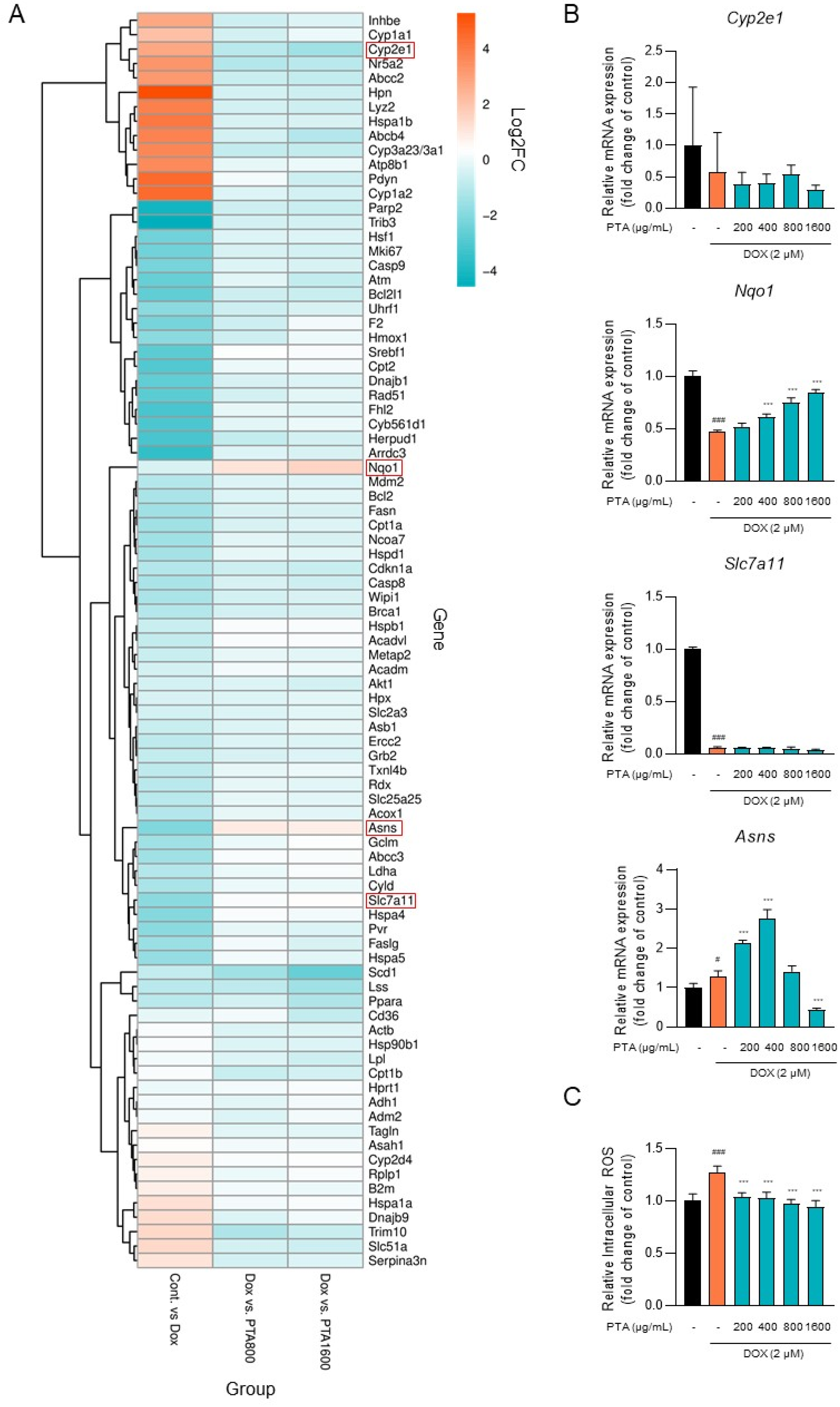
2.4. PTA Inhibits DOX-Induced ROS Generation through the Upregulation of Nqo1 Expression
2.5. Identification of the Chemical Composition of PTA
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Preparation, and Extraction
4.2. Cell Culture and Chemicals
4.3. Cell Viability Assay
4.4. Immunoblot Analysis
4.5. Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.6. Microarray Analysis of Gene Expression
4.7. Measurement of Intracellular ROS Generation
4.8. In Vivo Cardiotoxicity Model
4.9. Blood Test
4.10. Masson’s Trichrome Staining and Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
4.11. Ultra-Performance Liquid Chromatography (UPLC)-Triple Quadrupole Mass Spectrometry (TQ/MS) (UPLC-TQ/MS) Analysis
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Amadori, D.; Milandri, C.; Comella, G.; Saracchini, S.; Salvagni, S.; Barone, C.; Bordonaro, R.; Gebbia, V.; Barbato, A.; Serra, P.; et al. A phase I/II trial of non-pegylated liposomal doxorubicin, docetaxel and trastuzumab as first-line treatment in HER-2-positive locally advanced or metastatic breast cancer. Eur. J. Cancer 2011, 47, 2091–2098. [Google Scholar] [CrossRef]
- Muggia, F.M.; Hainsworth, J.D.; Jeffers, S.; Miller, P.; Groshen, S.; Tan, M.; Roman, L.; Uziely, B.; Muderspach, L.; Garcia, A.; et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: Antitumor activity and toxicity modification by liposomal encapsulation. J. Clin. Oncol. 1997, 15, 987–993. [Google Scholar] [CrossRef]
- Preusser, P.; Wilke, H.; Achterrath, W.; Fink, U.; Lenaz, L.; Heinicke, A.; Meyer, J.; Meyer, H.J.; Buente, H. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J. Clin. Oncol. 1989, 7, 1310–1317. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11, 87. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Ganan, C.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. The decrease of NAD(P)H:quinone oxidoreductase 1 activity and increase of ROS production by NADPH oxidases are early biomarkers in doxorubicin cardiotoxicity. Biomarkers 2014, 19, 142–153. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zeekpudsa, P.; Kukongviriyapan, V.; Senggunprai, L.; Sripa, B.; Prawan, A. Suppression of NAD(P)H-quinone oxidoreductase 1 enhanced the susceptibility of cholangiocarcinoma cells to chemotherapeutic agents. J. Exp. Clin. Cancer Res. 2014, 33, 11. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.J.; Hinkhouse, M.M.; Grady, M.; Gaut, A.W.; Liu, J.; Zhang, Y.P.; Weydert, C.J.; Domann, F.E.; Oberley, L.W. Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res. 2003, 63, 5513–5520. [Google Scholar] [PubMed]
- Prawan, A.; Buranrat, B.; Kukongviriyapan, U.; Sripa, B.; Kukongviriyapan, V. Inflammatory cytokines suppress NAD(P)H:quinone oxidoreductase-1 and induce oxidative stress in cholangiocarcinoma cells. J. Cancer Res. Clin. Oncol. 2009, 135, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Preethi, S.; Arthiga, K.; Patil, A.B.; Spandana, A.; Jain, V. Review on NAD(P)H dehydrogenase quinone 1 (NQO1) pathway. Mol. Biol. Rep. 2022, 49, 8907–8924. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y. NAD(P)H: Quinone oxidoreductase 1 and its potential protective role in cardiovascular diseases and related conditions. Cardiovasc. Toxicol. 2012, 12, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Ham, W.; Kim, J. Roles of NAD(P)H:quinone Oxidoreductase 1 in Diverse Diseases. Life 2021, 11, 1301. [Google Scholar] [CrossRef]
- Boroumand, M.; Pourgholi, L.; Goodarzynejad, H.; Ziaee, S.; Hajhosseini-Talasaz, A.; Sotoudeh-Anvari, M.; Mandegary, A. NQO1 C609T Polymorphism is Associated with Coronary Artery Disease in a Gender-Dependent Manner. Cardiovasc. Toxicol. 2017, 17, 35–41. [Google Scholar] [CrossRef]
- Lu, Y.; An, L.; Taylor, M.R.G.; Chen, Q.M. Nrf2 signaling in heart failure: Expression of Nrf2, Keap1, antioxidant, and detoxification genes in dilated or ischemic cardiomyopathy. Physiol. Genom. 2022, 54, 115–127. [Google Scholar] [CrossRef]
- Mikami, K.; Shirakusa, T.; Tsuruo, T. DT-diaphorase. Gan Kagaku Ryoho 1997, 24, 1606–1610. [Google Scholar]
- Lind, C.; Cadenas, E.; Hochstein, P.; Ernster, L. DT-diaphorase: Purification, properties, and function. Methods Enzym. 1990, 186, 287–301. [Google Scholar] [CrossRef]
- Bianchet, M.A.; Faig, M.; Amzel, L.M. Structure and mechanism of NAD[P]H:quinone acceptor oxidoreductases (NQO). Methods Enzym. 2004, 382, 144–174. [Google Scholar] [CrossRef]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Hussein, U.K.; Yun, B.S.; Park, H.R.; Jeong, Y.J. Anticancer effects of Poncirus fructus on hepatocellular carcinoma through regulation of apoptosis, migration, and invasion. Oncol. Rep. 2020, 44, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Mandadi, K.K.; Poulose, S.M.; Jadegoud, Y.; Nagana Gowda, G.A.; Patil, B.S. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg. Med. Chem. 2007, 15, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Loo, W.T.; Chen, J.P.; Chow, L.W.; Chou, J.W. Effects of Shugansanjie Tang on matrix metalloproteinases 1, 3 and 9 and telomerase reverse transcriptase expression in human breast cells in vitro. Biomed. Pharmacother. 2007, 61, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.G.; Kim, H.P.; Jin, D.H.; Bae, H.S.; Kim, S.H.; Park, C.H.; Lee, J.W. Saussurea lappa induces G2-growth arrest and apoptosis in AGS gastric cancer cells. Cancer Lett. 2005, 220, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.M.; Kim, M.S.; Koo, H.N.; Song, B.K.; Yoo, Y.H.; Kim, H.M. Poncirus trifoliata fruit induces apoptosis in human promyelocytic leukemia cells. Clin. Chim. Acta 2004, 340, 179–185. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxidative Med. Cell Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef]
- Zordoky, B.N.; El-Kadi, A.O. Induction of several cytochrome P450 genes by doxorubicin in H9c2 cells. Vasc. Pharmacol. 2008, 49, 166–172. [Google Scholar] [CrossRef]
- Nordgren, K.K.; Wallace, K.B. Keap1 redox-dependent regulation of doxorubicin-induced oxidative stress response in cardiac myoblasts. Toxicol. Appl. Pharmacol. 2014, 274, 107–116. [Google Scholar] [CrossRef]
- He, Y.; Xi, J.; Fang, J.; Zhang, B.; Cai, W. Aloe-emodin alleviates doxorubicin-induced cardiotoxicity via inhibition of ferroptosis. Free Radic. Biol. Med. 2023, 206, 13–21. [Google Scholar] [CrossRef]
- Park, S.-H.; Park, E.-K.; Kim, D.-H. Passive cutaneous anaphylaxis-inhibitory activity of flavanones from Citrus unshiu and Poncirus trifoliata. Planta Medica 2005, 71, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, S.-W.; Oh, J.; Hong, G.-S.; Seo, E.-K.; Oh, U.; Shim, W.-S. Ghrelin receptor is activated by naringin and naringenin, constituents of a prokinetic agent Poncirus fructus. J. Ethnopharmacol. 2013, 148, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Autschbach, R. Doxorubicin in experimental and clinical heart failure. Eur. J. Cardiothorac. Surg. 2006, 30, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Attachaipanich, T.; Chattipakorn, S.C.; Chattipakorn, N. Potential Roles of Melatonin in Doxorubicin-Induced Cardiotoxicity: From Cellular Mechanisms to Clinical Application. Pharmaceutics 2023, 15, 785. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, T.; Hu, W.; Wang, Z.; Wu, J.; Zhou, Q.; Li, P. Poncirin ameliorates cardiac ischemia-reperfusion injury by activating PI3K/AKT/PGC-1α signaling. Eur. J. Pharmacol. 2022, 917, 174759. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, L.; Liu, H.; Wang, J.; Li, J.; Yang, Y.; Wang, Y.; Cai, H.; Li, R.; Zhao, Y. Salsolinol Attenuates Doxorubicin-Induced Chronic Heart Failure in Rats and Improves Mitochondrial Function in H9c2 Cardiomyocytes. Front. Pharmacol. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2021, 12, 808480. [Google Scholar] [CrossRef]
- Martin, M.; Ramos-Medina, R.; Bernat, R.; Garcia-Saenz, J.A.; Del Monte-Millan, M.; Alvarez, E.; Cebollero, M.; Moreno, F.; Gonzalez-Haba, E.; Bueno, O.; et al. Activity of docetaxel, carboplatin, and doxorubicin in patient-derived triple-negative breast cancer xenografts. Sci. Rep. 2021, 11, 7064. [Google Scholar] [CrossRef]
- Li, K.; Sung, R.Y.; Huang, W.Z.; Yang, M.; Pong, N.H.; Lee, S.M.; Chan, W.Y.; Zhao, H.; To, M.Y.; Fok, T.F.; et al. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation 2006, 113, 2211–2220. [Google Scholar] [CrossRef]
- Neilan, T.G.; Jassal, D.S.; Perez-Sanz, T.M.; Raher, M.J.; Pradhan, A.D.; Buys, E.S.; Ichinose, F.; Bayne, D.B.; Halpern, E.F.; Weyman, A.E.; et al. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur. Heart J. 2006, 27, 1868–1875. [Google Scholar] [CrossRef]
- Pei, X.M.; Tam, B.T.; Sin, T.K.; Wang, F.F.; Yung, B.Y.; Chan, L.W.; Wong, C.S.; Ying, M.; Lai, C.W.; Siu, P.M. S100A8 and S100A9 Are Associated with Doxorubicin-Induced Cardiotoxicity in the Heart of Diabetic Mice. Front. Physiol. 2016, 7, 334. [Google Scholar] [CrossRef]
- Hullin, R.; Metrich, M.; Sarre, A.; Basquin, D.; Maillard, M.; Regamey, J.; Martin, D. Diverging effects of enalapril or eplerenone in primary prevention against doxorubicin-induced cardiotoxicity. Cardiovasc. Res. 2018, 114, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, Y.; Tokuda, K.; Guo, J.; Zhang, S.; Narahara, S.; Kawano, T.; Murata, M.; Yamaura, K.; Hoka, S.; Hashizume, M.; et al. Sodium thiosulfate prevents doxorubicin-induced DNA damage and apoptosis in cardiomyocytes in mice. Life Sci. 2020, 257, 118074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Wang, X.G.; Zhang, X.; Xie, Y.Q.; Chen, R.Z.; Chen, H.Z. C57BL/6 Mice are More Appropriate than BALB/C Mice in Inducing Dilated Cardiomyopathy with Short-Term Doxorubicin Treatment. Acta Cardiol. Sin. 2012, 28, 236–240. [Google Scholar]
- van der Vijgh, W.J.; van Velzen, D.; van der Poort, J.S.; Schluper, H.M.; Mross, K.; Feijen, J.; Pinedo, H.M. Morphometric study of myocardial changes during doxorubicin-induced cardiomyopathy in mice. Eur. J. Cancer Clin. Oncol. 1988, 24, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; De Rosa, S.; Tamme, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Abdelatty, A.; Ahmed, M.S.; Abdel-Kareem, M.A.; Dmerdash, M.; Mady, R.; Saad, A.S.; Albrakati, A.; Elmahallawy, E.K.; Elsawak, A.; Abdo, W. Acute and Delayed Doxorubicin-Induced Myocardiotoxicity Associated with Elevation of Cardiac Biomarkers, Depletion of Cellular Antioxidant Enzymes, and Several Histopathological and Ultrastructural Changes. Life 2021, 11, 880. [Google Scholar] [CrossRef]
- Younis, N.S. Doxorubicin-Induced Cardiac Abnormalities in Rats: Attenuation via Sandalwood Oil. Pharmacology 2020, 105, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, C.; Tentolouris, N.; Stefanadis, C. The ECG vertigo in diabetes and cardiac autonomic neuropathy. Exp. Diabetes Res. 2011, 2011, 687624. [Google Scholar] [CrossRef]
- Andrsova, I.; Hnatkova, K.; Sisakova, M.; Toman, O.; Smetana, P.; Huster, K.M.; Barthel, P.; Novotny, T.; Schmidt, G.; Malik, M. Heart Rate Influence on the QT Variability Risk Factors. Diagnostics 2020, 10, 1096. [Google Scholar] [CrossRef]
- Comani, S.; Mantini, D.; Alleva, G.; Di Luzio, S.; Romani, G.L. Automatic detection of cardiac waves on fetal magnetocardiographic signals. Physiol. Meas. 2005, 26, 459–475. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, Z.; Wang, F.; Yin, L.; Wu, J.; Li, D.; Song, S.; Wang, X.; Tang, Y.; Huang, C. Tubeimoside I Ameliorates Doxorubicin-Induced Cardiotoxicity by Upregulating SIRT3. Oxidative Med. Cell Longev. 2023, 2023, 9966355. [Google Scholar] [CrossRef]
- Ndrepepa, G. De Ritis ratio and cardiovascular disease: Evidence and underlying mechanisms. J. Lab. Precis. Med. 2023, 8, 6. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View From the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Melo, M.B.; Carvalho, J.L.; Melo, I.M.; Lavor, M.S.; Gomes, D.A.; de Goes, A.M.; Melo, M.M. Doxorubicin Cardiotoxicity and Cardiac Function Improvement After Stem Cell Therapy Diagnosed by Strain Echocardiography. J. Cancer Sci. Ther. 2013, 5, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, X.; Wang, Y.; Wang, F.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS ONE 2012, 7, e47351. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.R.; Martins, T.R.; Couto, R.; Deus, C.; Pereira, C.V.; Simões, R.F.; Rizvanov, A.A.; Silva, F.; Cunha-Oliveira, T.; Oliveira, P.J.; et al. Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2904–2923. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lee, S.-H.; Lee, J.; Kim, M.-S.; Lee, Y.-G.; Hwang, J.-T.; Choi, S.-Y.; Yoon, H.-G.; Lim, T.-G.; Lee, S.-H. Water Extract of Capsella bursa-pastoris Mitigates Doxorubicin-Induced Cardiotoxicity by Upregulating Antioxidant Enzymes. Int. J. Mol. Sci. 2023, 24, 15912. [Google Scholar] [CrossRef] [PubMed]
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into Doxorubicin-induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol. Pharmacol. 2019, 96, 219–232. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxidative Med. Cell Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Linnik, O.O.; Drevytska, T.I.; Gonchar, O.O.; Chornyy, S.A.; Kovalyov, O.M.; Mankovska, I.M. Doxorubicin-Induced Alterations in Pro-and Antioxidant Balance and Their Correction by Curcumin in the Neonatal Rat Cardiomyocytes Culture. Fiziolohichnyĭ Zhurnal 2015, 61, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, J.H.; Davies, K.J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J. Biol. Chem. 1986, 261, 3068–3074. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Sahebkar, A. Reversal of Doxorubicin-induced Cardiotoxicity by Using Phytotherapy: A Review. J. Pharmacopunct. 2017, 20, 243–256. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, K.S. Bioactivity of Trifoliate Orange (Poncirus trifoliate) Seed Extracts. Prev. Nutr. Food Sci. 2012, 17, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Pandey, J.; Devkota, H.P. Bioactive Chemical Constituents and Pharmacological Activities of Ponciri Fructus. Molecules 2022, 28, 255. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, E.K.; Shim, W.S. Phytotherapeutic effects of the fruits of Poncirus trifoliata (L.) Raf. on cancer, inflammation, and digestive dysfunction. Phytother. Res. 2018, 32, 616–624. [Google Scholar] [CrossRef]
- Siegel, D.; Yan, C.; Ross, D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem. Pharmacol. 2012, 83, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, Z.; Sun, M.; Dong, D. Nrf2: A dark horse in doxorubicin-induced cardiotoxicity. Cell Death Discov. 2023, 9, 261. [Google Scholar] [CrossRef]
- Cui, T.; Lai, Y.; Janicki, J.S.; Wang, X. Nuclear factor erythroid-2 related factor 2 (Nrf2)-mediated protein quality control in cardiomyocytes. Front. Biosci. 2016, 21, 192–202. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Nie, X.; Wu, Y.; Zhang, C.; Lee, S.M.; Lv, K.; Leung, G.P.; Fu, C.; Zhang, J.; et al. Natural compound glycyrrhetinic acid protects against doxorubicin-induced cardiotoxicity by activating the Nrf2/HO-1 signaling pathway. Phytomedicine 2022, 106, 154407. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, Z. Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ. Toxicol. 2019, 34, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Kuncha, M.; Borkar, R.M.; Srinivas, R.; Sistla, R. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci. 2016, 144, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Patra, A.R.; Basu, A.; Bhattacharya, S. Prevention of doxorubicin (DOX)-induced genotoxicity and cardiotoxicity: Effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed. Pharmacother. 2018, 101, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-L.; Seo, C.-S.; Kim, J.-H.; Shin, H.-K. Contents of poncirin and naringin in fruit of Poncirus trifoliata according to different harvesting times and locations for two years. Korean J. Pharmacogn. 2011, 42, 138–143. [Google Scholar]
- Kussauer, S.; David, R.; Lemcke, H. hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro- Electrode-Array Analyses Can Tell Us. Cells 2019, 8, 1331. [Google Scholar] [CrossRef]
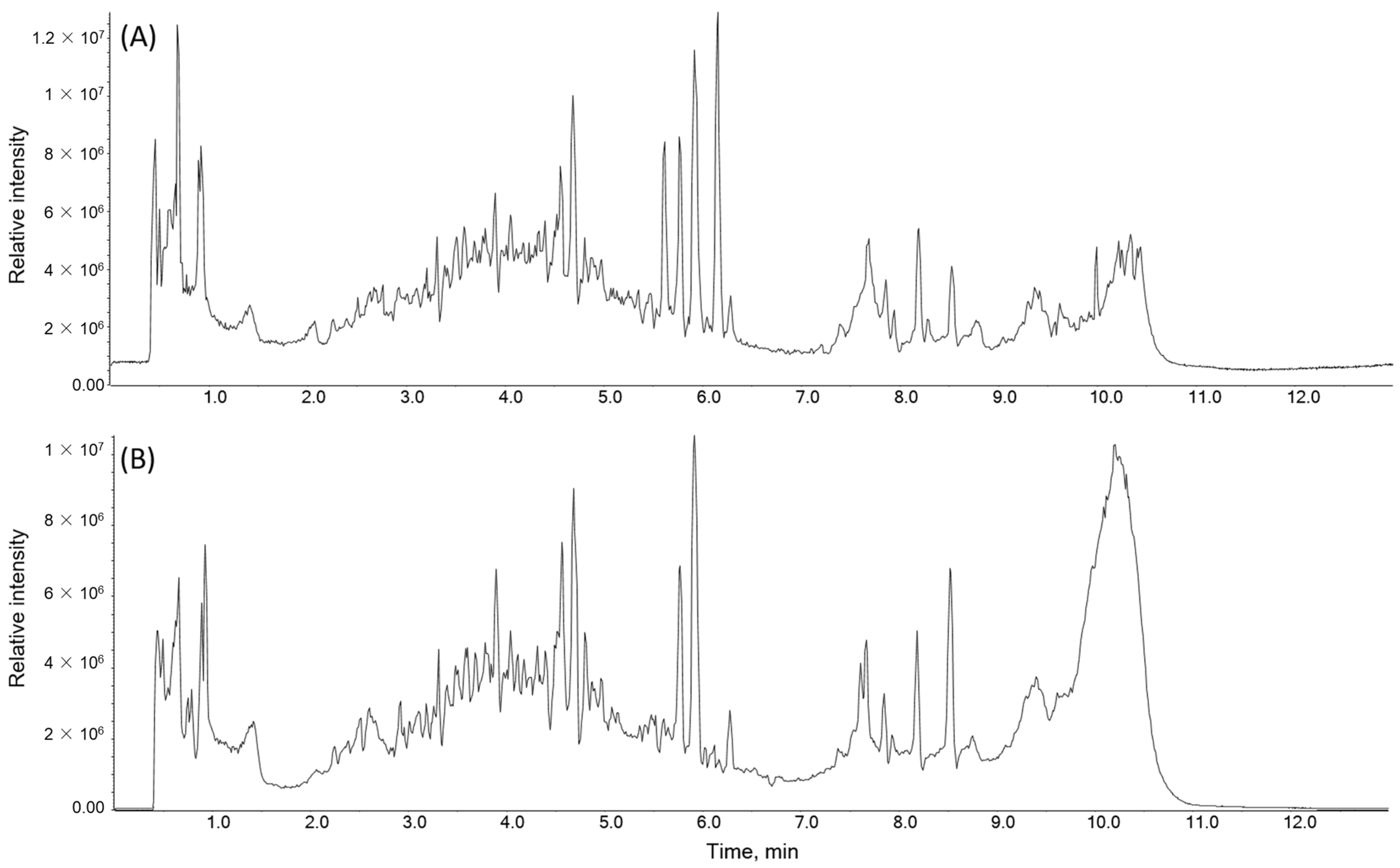
| No | Compound | Class | Rt (min) | Ionization Mode | Molecular Formula | Observed Precursor Ions (m/z) | Difference (ppm) a | Relative Intensity |
|---|---|---|---|---|---|---|---|---|
| 1 | Quercetin dihexose | Flavonoids | 3.46 | [M + H]+ | C27H30O17 | 627.1566 | 0.13 | 42,440 |
| 2 | Kaempferol dihexose | 3.64 | [M + H]+ | C27H30O16 | 611.1623 | 0.15 | 56,436 | |
| 3 | Isorhamnetin sophorose or dihexose | 3.69 | [M + H]+ | C28H32O17 | 641.1728 | 0.14 | 56,243 | |
| 4 | Quercetin pentose glucuronide | 4.26 | [M − H]− | C26H26O17 | 609.1462 | 0.48 | 77,546 | |
| 5 | Quercetin hexose | 4.36 | [M − H]− | C21H20O12 | 463.0889 | −0.14 | 14,069 | |
| 6 | Kaempferol rutinoside | 4.52 | [M − H]− | C27H30O15 | 593.1529 | −0.09 | 35,315 | |
| 7 | Poncirin | 4.53 | [M − H]− | C28H33O14 | 593.1843 | 4.55 | 1300 | |
| 8 | Naringin | 4.68 | [M − H]− | C27H32O14 | 579.1719 | −0.13 | 701,538 | |
| 9 | Naringenin | 4.68 | [M + H]+ | C15H12O5 | 273.0756 | 0.26 | 261,691 | |
| 10 | Naringenin glucoside | 4.83 | [M − H]− | C21H22O10 | 433.1144 | −0.16 | 110,209 | |
| 11 | Eriodictyol | 5.17 | [M − H]− | C15H12O6 | 287.0562 | −0.25 | 18,712 | |
| 12 | Catechin | 6.71 | [M − H]− | C15H14O6 | 289.0715 | −0.26 | 3088 | |
| 13 | Kaempferol malonylhexose | 6.80 | [M + H]+ | C24H22O14 | 535.1451 | 0.82 | 3087 | |
| 14 | Quercetin | 6.92 | [M − H]− | C15H10O7 | 301.0716 | 0.96 | 2067 | |
| 15 | Kaemprerol | 6.95 | [M + H]+ | C15H10O6 | 287.0548 | 0.25 | 3304 | |
| 16 | Isorhamnetin | 7.22 | [M − H]− | C16H12O7 | 315.0515 | −0.22 | 5558 | |
| 17 | Vanillic acid | Benzoic acid | 3.02 | [M − H]− | C8H8O4 | 167.0346 | −0.46 | 7575 |
| 18 | Protocatechuic acid | 3.08 | [M − H]− | C7H6O4 | 153.0190 | −0.50 | 4152 | |
| 19 | Cinnamic acid | Cinnamic acid | 2.77 | [M − H]− | C9H8O2 | 147.0448 | −0.52 | 132 |
| 20 | Coumaric acid | 4.32 | [M − H]− | C9H8O3 | 163.0395 | −0.48 | 107,500 | |
| 21 | Ferulic acid | 4.55 | [M − H]− | C10H10O4 | 193.0502 | −0.40 | 8523 | |
| 22 | Quinic acid | Quinic acid | 0.66 | [M + H]+ | C7H12O6 | 193.0706 | −4.97 | 76,818 |
| 23 | Coumaroyl quinic acid | 3.48 | [M − H]− | C16H18O8 | 337.0929 | −0.22 | 168,809 | |
| 24 | Feruloyl quinic acid | 3.66 | [M − H]− | C17H20O9 | 367.1038 | −0.19 | 17,189 | |
| 25 | Caffeic acid hexose | Phenolic glycosides | 3.30 | [M − H]− | C15H18O9 | 341.0883 | −0.20 | 2500 |
| 26 | Coumaric acid hexose | 3.61 | [M − H]− | C15H18O8 | 325.0937 | −0.20 | 47,655 | |
| 27 | Asparagine | Amino acid | 0.53 | [M + H]+ | C4H8N2O3 | 133.0603 | 0.52 | 45,090 |
| 28 | Valine | 0.87 | [M + H]+ | C5H11NO2 | 118.0859 | 0.58 | 85,271 | |
| 29 | Leucine | 2.03 | [M + H]+ | C6H13NO2 | 132.1014 | 0.51 | 9718 | |
| 30 | Tyrosine | 2.04 | [M + H]+ | C9H11NO3 | 182.0812 | 0.40 | 21,774 | |
| 31 | Phenylalanine | 2.77 | [M − H]− | C9H11NO2 | 164.0715 | −0.46 | 3986 | |
| 32 | Malic acid | Organic acid | 0.78 | [M − H]− | C4H6O5 | 133.0137 | −0.59 | 93,348 |
| 33 | Succinic acid | 1.74 | [M − H]− | C4H6O4 | 117.0189 | −0.65 | 1182 | |
| 34 | Glucose | Sugar | 0.59 | [M − H]− | C6H12O6 | 179.0560 | −0.41 | 19,976 |
| 35 | Choline | Other | 0.54 | [M]+ | C5H14NO | 104.1062 | −0.13 | 160,124 |
| 36 | Shikimic acid | 0.86 | [M − H]− | C7H10O5 | 173.0454 | −0.43 | 5159 | |
| 37 | Ascorbic acid | 1.35 | [M + H]+ | C6H8O6 | 175.0171 | −0.85 | 120 | |
| 38 | 3-Hydroxy-3-methylglutaric acid | 2.25 | [M − H]− | C6H10O5 | 161.0456 | −0.45 | 3463 | |
| 39 | Chlorogenic acid | 3.20 | [M − H]− | C16H18O9 | 353.0882 | −0.19 | 13,847 | |
| 40 | Tryptophan | 3.27 | [M − H]− | C11H12N2O2 | 203.0824 | −0.37 | 3919 | |
| 41 | Kynurenic acid | 3.43 | [M + H]+ | C10H7NO3 | 190.0497 | 0.37 | 13,904 | |
| 42 | Caffeoyl shikimic acid | 3.93 | [M − H]− | C16H16O8 | 335.0783 | −0.19 | 4748 | |
| 43 | Scopoletin | 4.59 | [M − H]− | C10H8O4 | 191.0347 | −0.40 | 45,206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Choi, H.-K.; Park, S.-H.; Lee, J.-I.; Lee, J. Poncirus trifoliata Aqueous Extract Protects Cardiomyocytes against Doxorubicin-Induced Toxicity through Upregulation of NAD(P)H Dehydrogenase Quinone Acceptor Oxidoreductase 1. Molecules 2023, 28, 8090. https://doi.org/10.3390/molecules28248090
Kim M-S, Choi H-K, Park S-H, Lee J-I, Lee J. Poncirus trifoliata Aqueous Extract Protects Cardiomyocytes against Doxorubicin-Induced Toxicity through Upregulation of NAD(P)H Dehydrogenase Quinone Acceptor Oxidoreductase 1. Molecules. 2023; 28(24):8090. https://doi.org/10.3390/molecules28248090
Chicago/Turabian StyleKim, Min-Sun, Hyo-Kyoung Choi, Soo-Hyun Park, Jae-In Lee, and Jangho Lee. 2023. "Poncirus trifoliata Aqueous Extract Protects Cardiomyocytes against Doxorubicin-Induced Toxicity through Upregulation of NAD(P)H Dehydrogenase Quinone Acceptor Oxidoreductase 1" Molecules 28, no. 24: 8090. https://doi.org/10.3390/molecules28248090
APA StyleKim, M.-S., Choi, H.-K., Park, S.-H., Lee, J.-I., & Lee, J. (2023). Poncirus trifoliata Aqueous Extract Protects Cardiomyocytes against Doxorubicin-Induced Toxicity through Upregulation of NAD(P)H Dehydrogenase Quinone Acceptor Oxidoreductase 1. Molecules, 28(24), 8090. https://doi.org/10.3390/molecules28248090







