Synthesis and Application of a Novel Multi-Branched Block Polyether Low-Temperature Demulsifier
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of PR-D2
2.2. Analysis of Factors Affecting Emulsion Breakage in Oilfield Samples
2.2.1. Analysis of Emulsification Properties of Crude Oil Components
2.2.2. Study of Interfacial Properties of Crude Oil Components
2.2.3. Analysis of Wax Content of Crude Oil
2.2.4. Charge Potential Analysis of Emulsions
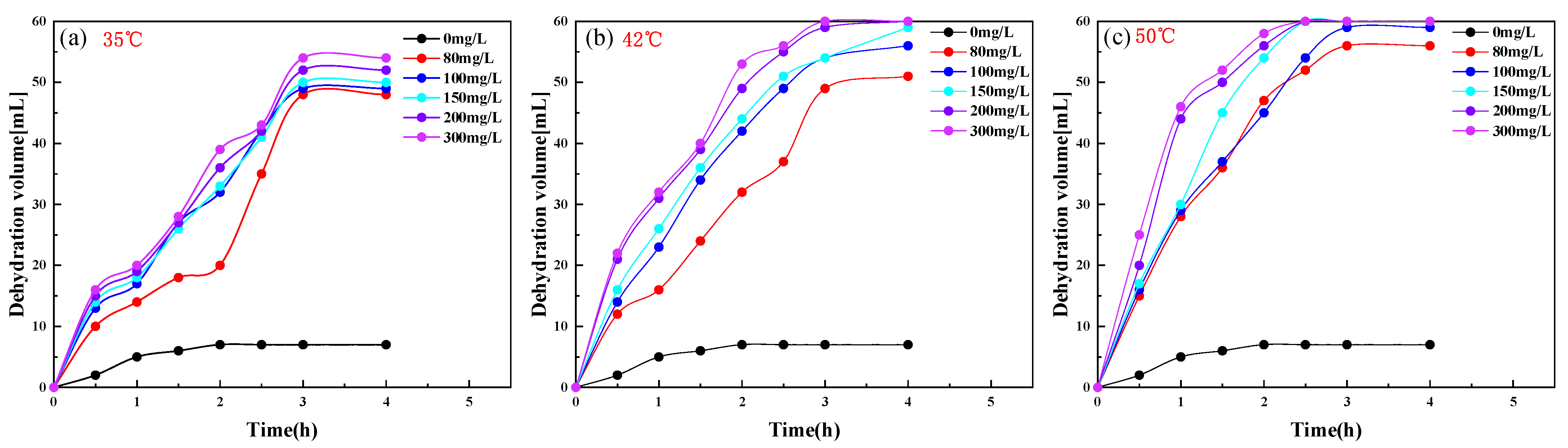
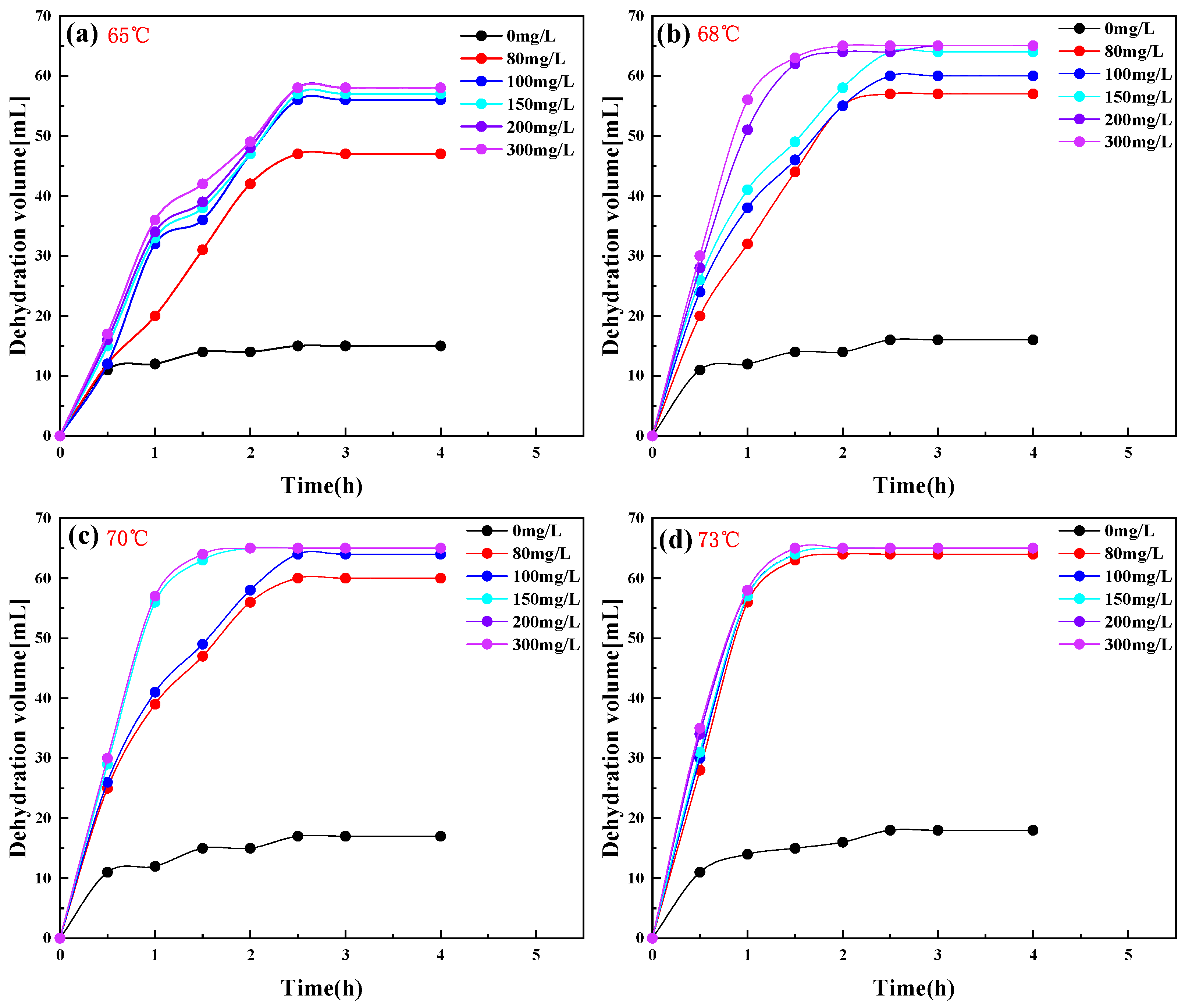
2.3. PR-D2 Dehydration Performance of Different Reaction Conditions
2.4. PR-D2 Demulsification Performance
2.4.1. Demulsification and Dehydration Performance of PR-D2 on Chun Oilfield Crude Oil
2.4.2. Demulsification and Dehydration Performance of PR-D2 on Gao Oilfield Crude Oil
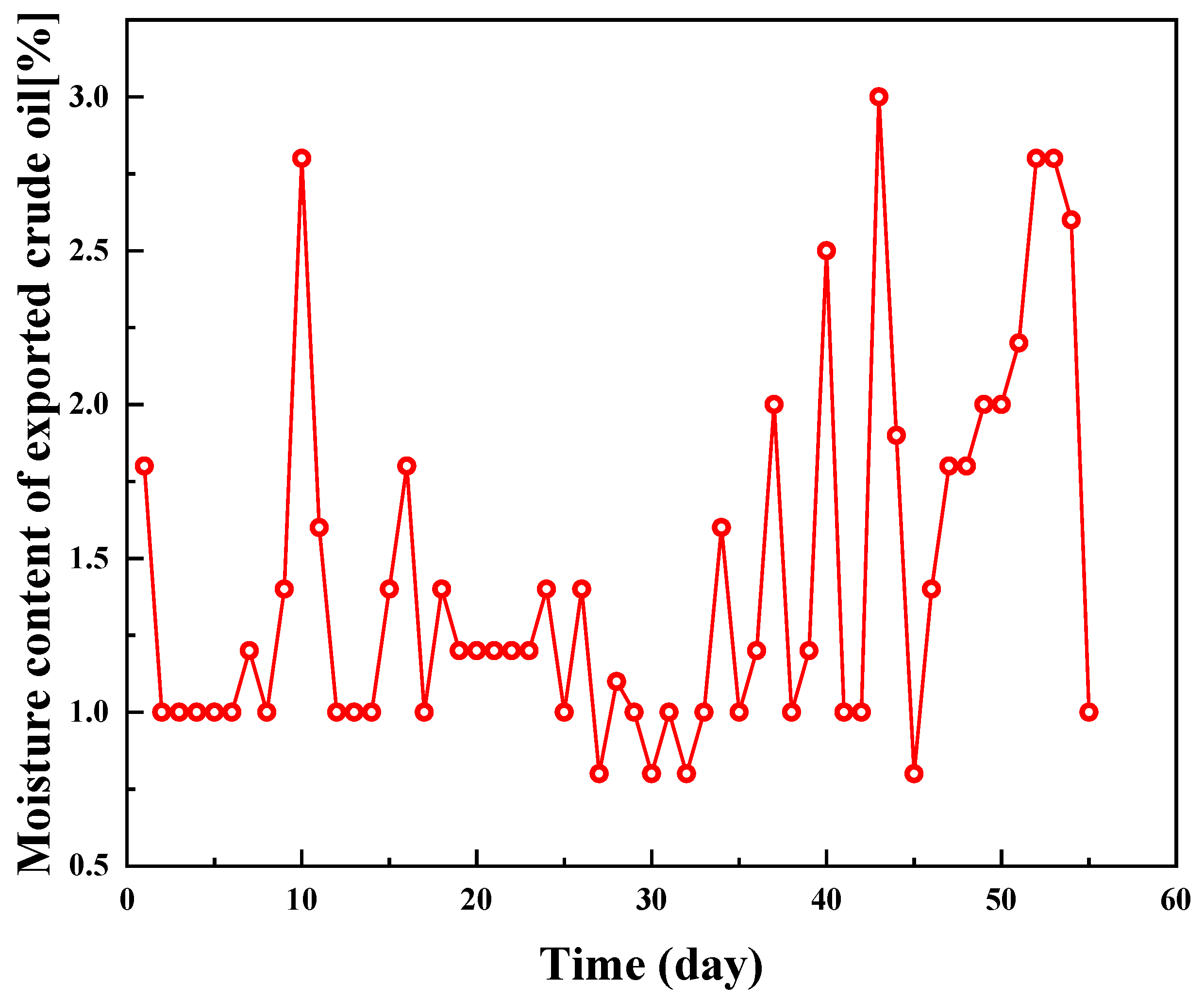
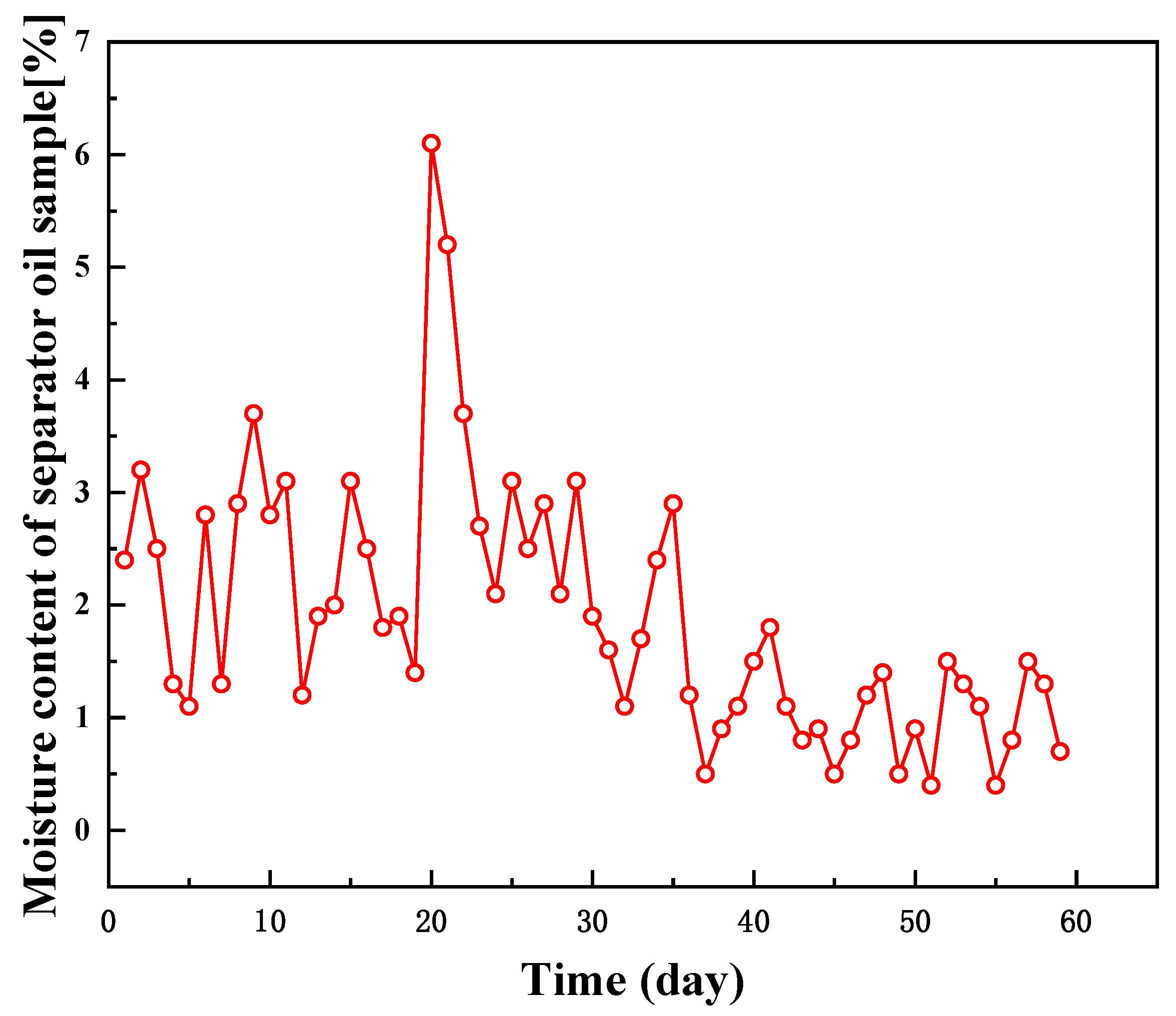
2.5. Field Experiment
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Demulsifiers
3.3. Characteristics of PR-D2
3.4. Interfacial Tension
3.5. Four-Component Measurement and Crude Oil Wax Content Measurement
3.6. Zeta Electric Potential
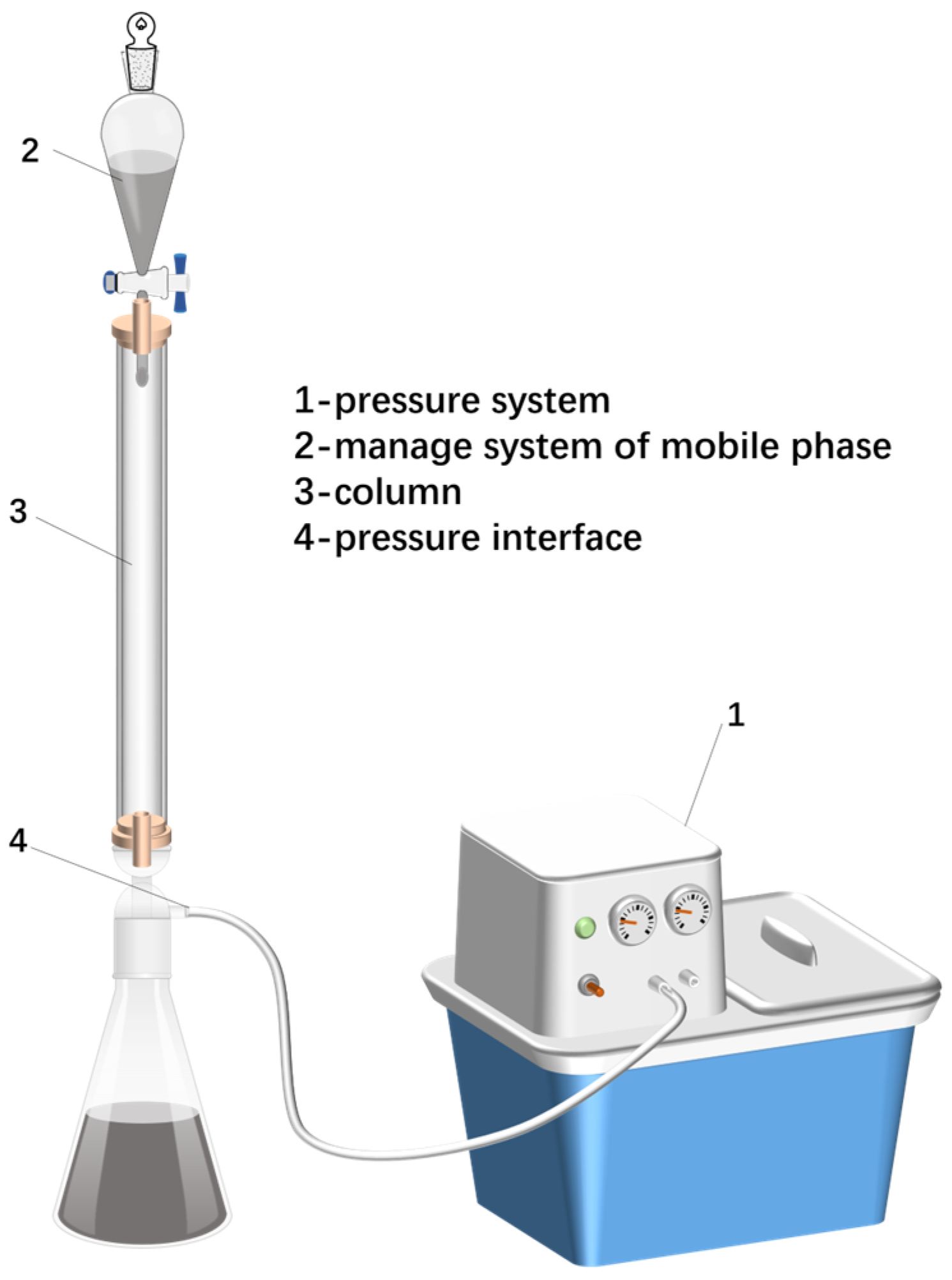
3.7. Demulsification Performance Measurable
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raya, S.A.; Mohd Saaid, I.; Abbas Ahmed, A.; Abubakar Umar, A. A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef]
- Bourrel, M.; Passade-Boupat, N. Crude Oil Surface Active Species: Consequences for Enhanced Oil Recovery and Emulsion Stability. Energy Fuels 2017, 32, 2642–2652. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, G.; Chen, Y.; Zeng, H.; Mao, Z.; Liu, L.; Wang, X.; Xu, S. Thixotropy research of laponite-hydrogel composites for water shutoff in horizontal wells. J. Pet. Sci. Eng. 2022, 208, 109600. [Google Scholar] [CrossRef]
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Li, B.; Sun, Z.; Wang, Z.; Wang, J.; Wang, Z.; Dou, X.; Fan, Y.; Li, X.; Liu, H. Effects of the particle concentration on the electro-dehydration of simulated SAGD produced ultra-heavy oil. Chem. Eng. Res. Des. 2019, 151, 157–167. [Google Scholar] [CrossRef]
- Faizullayev, S.; Adilbekova, A.; Kujawski, W.; Mirzaeian, M. Recent demulsification methods of crude oil emulsions-Brief review. J. Pet. Sci. Eng. 2022, 215, 110643. [Google Scholar] [CrossRef]
- Hu, C.; Liu, S.; Fang, S.W.; Xiang, W.J.; Duan, M. Dissipative particle dynamics investigation of demulsification process and mechanism of comb-like block polyether. Polym. Adv. Technol. 2018, 29, 3171–3180. [Google Scholar] [CrossRef]
- Ye, F.; Shen, L.W.; Liu, S.; Liu, H.Y.; Zhang, X.Y.; Zhang, Z.J.; Yang, Y.; Feng, X.N.; Tang, Y.Q.; Xiang, D.; et al. Demulsification of amphiphilic gemini ionic liquids and its demulsification mechanism. Chemosphere 2022, 309, 136650. [Google Scholar] [CrossRef]
- Wu, J.D.; Wei, W.; Li, S.H.; Zhong, Q.; Liu, F.; Zheng, J.H.; Wang, J.P. The effect of membrane surface charges on demulsification and fouling resistance during emulsion separation. J. Membr. Sci. 2018, 563, 126–133. [Google Scholar] [CrossRef]
- Buddin, M.; Ahmad, A.L.; Abd Khalil, A.T.; Puasa, S.W. A review of demulsification technique and mechanism for emulsion liquid membrane applications. J. Dispers. Sci. Technol. 2022, 43, 910–927. [Google Scholar] [CrossRef]
- Kang, W.L.; Yin, X.; Yang, H.B.; Zhao, Y.L.; Huang, Z.T.; Hou, X.Y.; Sarsenbekuly, B.; Zhu, Z.; Wang, P.X.; Zhang, X.F.; et al. Demulsification performance, behavior and mechanism of different demulsifiers on the light crude oil emulsions. Colloids Surf. A-Physicochem. Eng. Asp. 2018, 545, 197–204. [Google Scholar] [CrossRef]
- Sun, G.; Li, C.X.; Yang, S.; Yang, F.; Chen, Y.Q. Experimental Investigation of the Rheological Properties of a Typical Waxy Crude Oil Treated with Supercritical CO2 and the Stability Change in Its Emulsion. Energy Fuels 2019, 33, 4731–4739. [Google Scholar] [CrossRef]
- Wang, Z.H.; Lin, X.Y.; Rui, Z.H.; Xu, M.M.; Zhan, S.Y. The Role of Shearing Energy and Interfacial Gibbs Free Energy in the Emulsification Mechanism of Waxy Crude Oil. Energies 2017, 10, 721. [Google Scholar] [CrossRef]
- Fan, S.G.; Liu, H.; Biney, B.W.; Wang, J.; Al-shiaani, N.H.A.; Xu, G.J.; Guo, A.J.; Chen, K.; Wang, Z.X. Effect of Colloidal Stability and Miscibility of Polar/Aromatic Phases on Heavy Oil Demetallization. Energy Fuels 2022, 36, 6109–6118. [Google Scholar] [CrossRef]
- Xia, L.; Lu, S.; Cao, G. Stability and demulsification of emulsions stabilized by asphaltenes or resins. J. Colloid Interface Sci. 2004, 271, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Mwakasala, B.T.; Kang, W.L.; Yin, X.; Geng, J.; Zhao, Y.L.; Yang, H.B. Demulsifier performance at low temperature in a low permeability reservoir. Pet. Sci. Technol. 2016, 34, 1905–1912. [Google Scholar] [CrossRef]
- Ma, J.; Yao, M.Q.; Yang, Y.L.; Zhang, X.Y. Comprehensive review on stability and demulsification of unconventional heavy oil-water emulsions. J. Mol. Liq. 2022, 350, 118510. [Google Scholar] [CrossRef]
- Wang, C.J.; Fang, S.W.; Duan, M.; Xiong, Y.; Ma, Y.Z.; Chen, W.J. Synthesis and evaluation of demulsifiers with polyethyleneimine as accepter for treating crude oil emulsions. Polym. Adv. Technol. 2015, 26, 442–448. [Google Scholar] [CrossRef]
- Liu, C.; Wei, L.X.; Song, Y.; Jia, X.L.; Hu, Y.Y.; Geng, X.H.; Chao, M. Synthesis, modification, and demulsification properties of multi-branched block polyether demulsifiers. MRS Commun. 2021, 11, 603–609. [Google Scholar] [CrossRef]
- Duan, M.; He, J.; Li, D.J.; Wang, X.J.; Jing, B.; Xiong, Y.; Fang, S.W. Synthesis of a novel copolymer of block polyether macromonomer and diallyldimethylammonium chloride and its reverse demulsification performance. J. Pet. Sci. Eng. 2019, 175, 317–323. [Google Scholar] [CrossRef]
- Liu, M.; Cao, X.L.; Zhu, Y.W.; Guo, Z.Y.; Zhang, L.; Zhang, L.; Zhao, S. The effect of demulsifier on the stability of liquid droplets: A study of micro-force balance. J. Mol. Liq. 2019, 275, 157–162. [Google Scholar] [CrossRef]
- Fernandez-Tarrio, M.; Yanez, F.; Immesoete, K.; Alvarez-Lorenzo, C.; Concheiro, A. Pluronic and tetronic copolymers with polyglycolyzed oils as self-emulsifying drug delivery systems. Aaps Pharmscitech 2008, 9, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, F.L.; Li, C.Q.; Li, J.; Yang, Y. Synthesis of dendritic polyether surfactants for demulsification. Sep. Purif. Technol. 2010, 73, 349–354. [Google Scholar] [CrossRef]
- Bi, Y.G.; Li, W.S.; Liu, C.C.; Tan, Z.; Wang, Z.T.; Liu, X.C.; Wang, G.F.; Jia, X.R. Star-shaped quaternary ammonium compounds with terminal amino groups for rapidly breaking oil-in-water emulsions. Fuel 2021, 304, 121366. [Google Scholar] [CrossRef]
- Li, Z.W.; Geng, H.K.; Wang, X.J.; Jing, B.; Liu, Y.F.; Tan, Y.B. Noval tannic acid-based polyether as an effective demulsifier for water-in-aging crude oil emulsions. Chem. Eng. J. 2018, 354, 1110–1119. [Google Scholar] [CrossRef]
- Huang, W.G.; Su, L.J.; Bo, Z.S. Hyperbranched Polymers with a Degree of Branching of 100% Prepared by Catalyst Transfer Suzuki-Miyaura Polycondensation. J. Am. Chem. Soc. 2009, 131, 10348–10349. [Google Scholar] [CrossRef]
- Zhou, Z.P.; Yan, D.Y. Degree of branching of the hyperbranched polymers resulted from ab2 polycondensation with substitution effect. Chin. J. Polym. Sci. 2011, 29, 569–574. [Google Scholar] [CrossRef]
- Gentekos, D.T.; Dupuis, L.N.; Fors, B.P. Beyond Dispersity: Deterministic Control of Polymer Molecular Weight Distribution. J. Am. Chem. Soc. 2016, 138, 1848–1851. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Harding, D.R.K.; Kandile, N.G.; Badawi, A.M.; El-Tabey, A.E. Synthesis of Some Novel Nonionic Ethoxylated Surfactants Based on α-Amino Acids and Investigation of Their Surface Active Properties. J. Dispers. Sci. Technol. 2009, 30, 427–438. [Google Scholar] [CrossRef]
- Grenoble, Z.; Trabelsi, S. Mechanisms, performance optimization and new developments in demulsification processes for oil and gas applications. Adv. Colloid Interface Sci. 2018, 260, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xie, Y.P.; Cai, Z.X.; Guo, Y.L.; Zhang, H.B. Interfacial rheology, emulsifying property and emulsion stability of glyceryl monooleate-modified corn fiber gum. Food Chem. 2021, 343, 128416. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.; Frampton, H.; Huang, S.S.; Collins, I.R.; Striolo, A.; Michaelides, A. Water/oil interfacial tension reduction—An interfacial entropy driven process. Phys. Chem. Chem. Phys. 2021, 23, 25075–25085. [Google Scholar] [CrossRef] [PubMed]

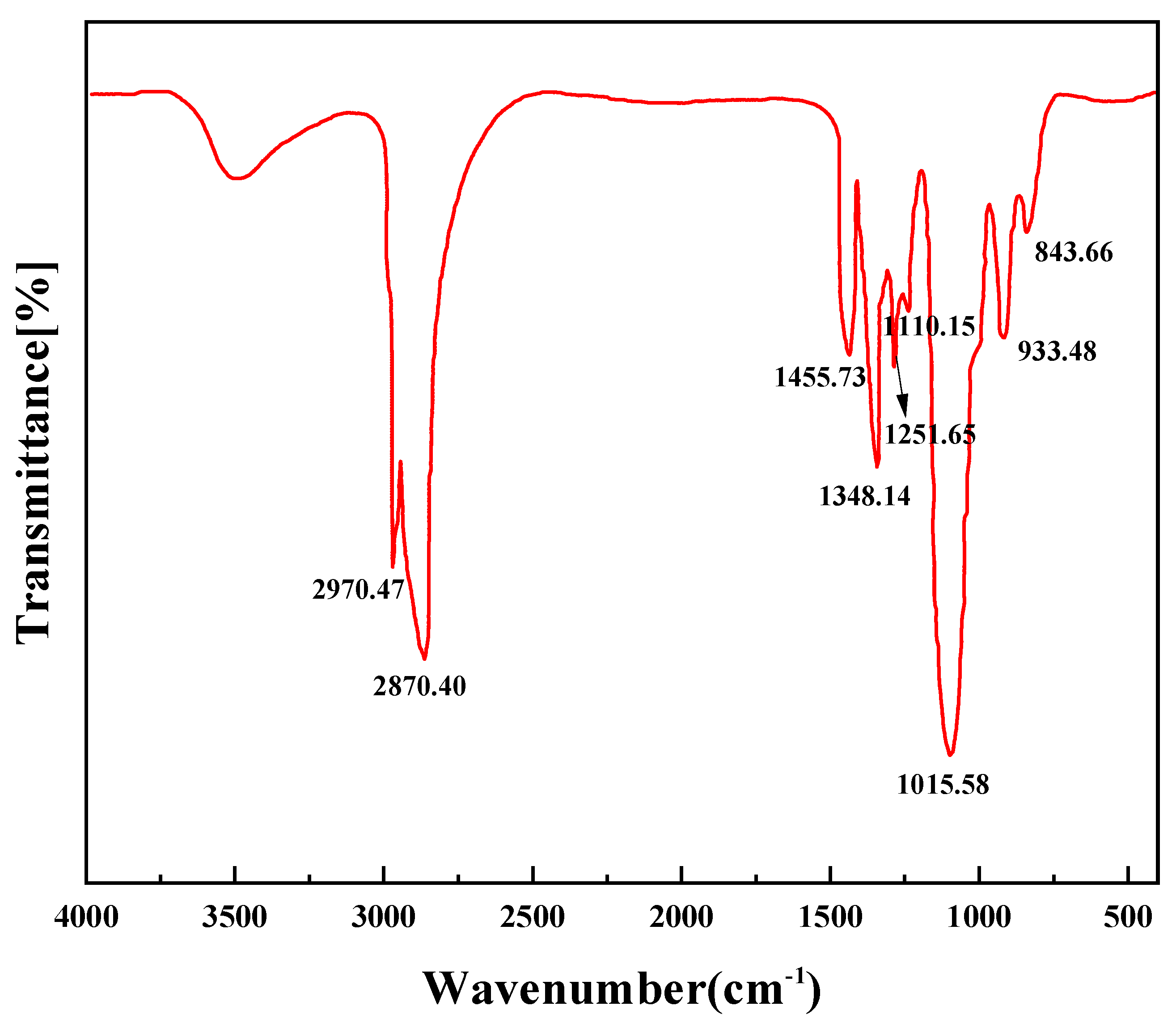
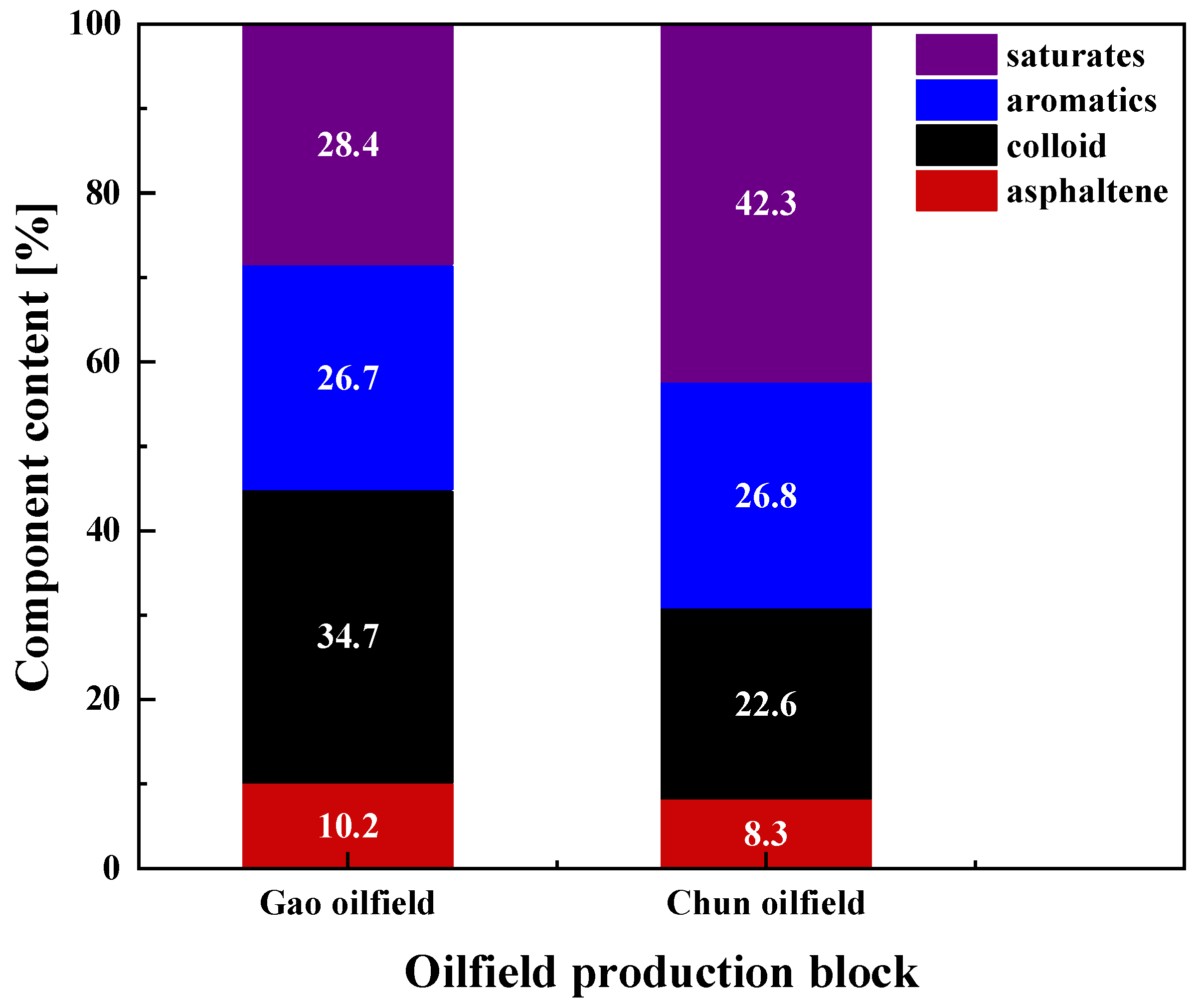
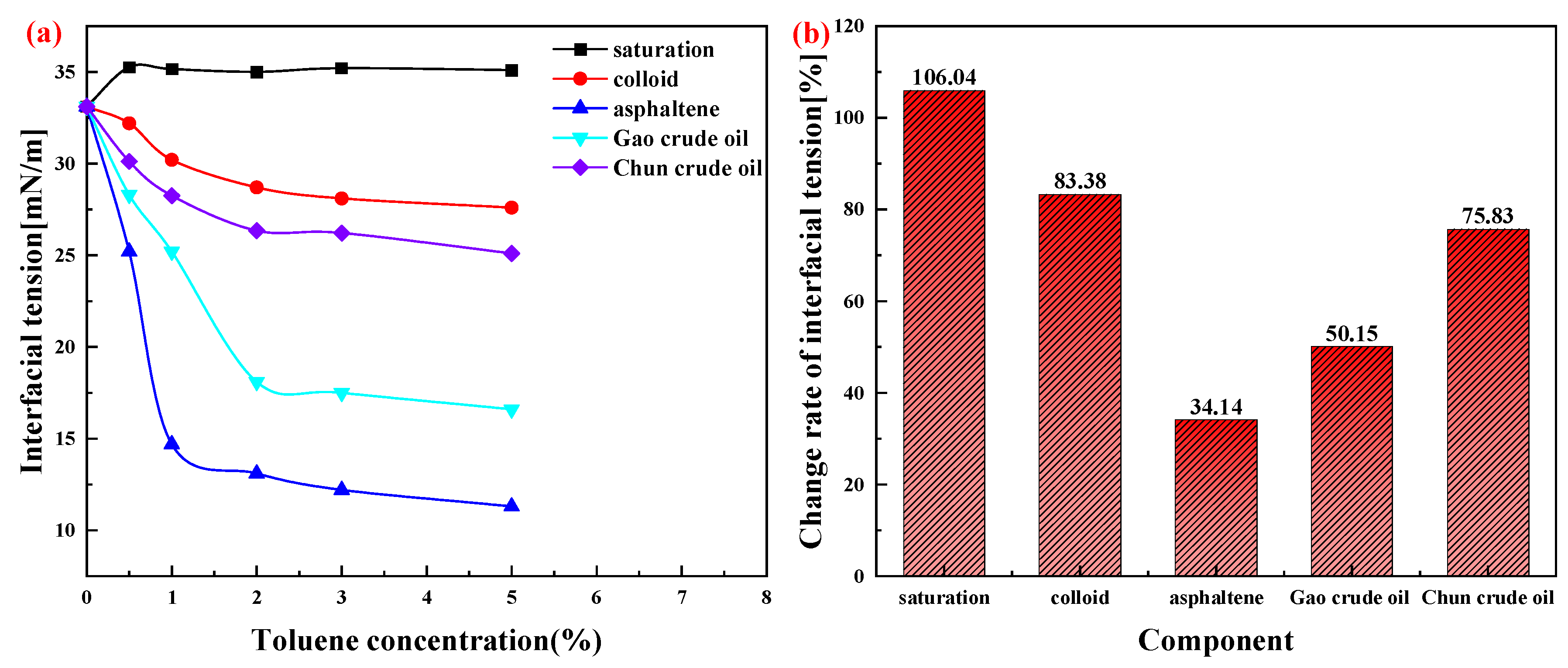
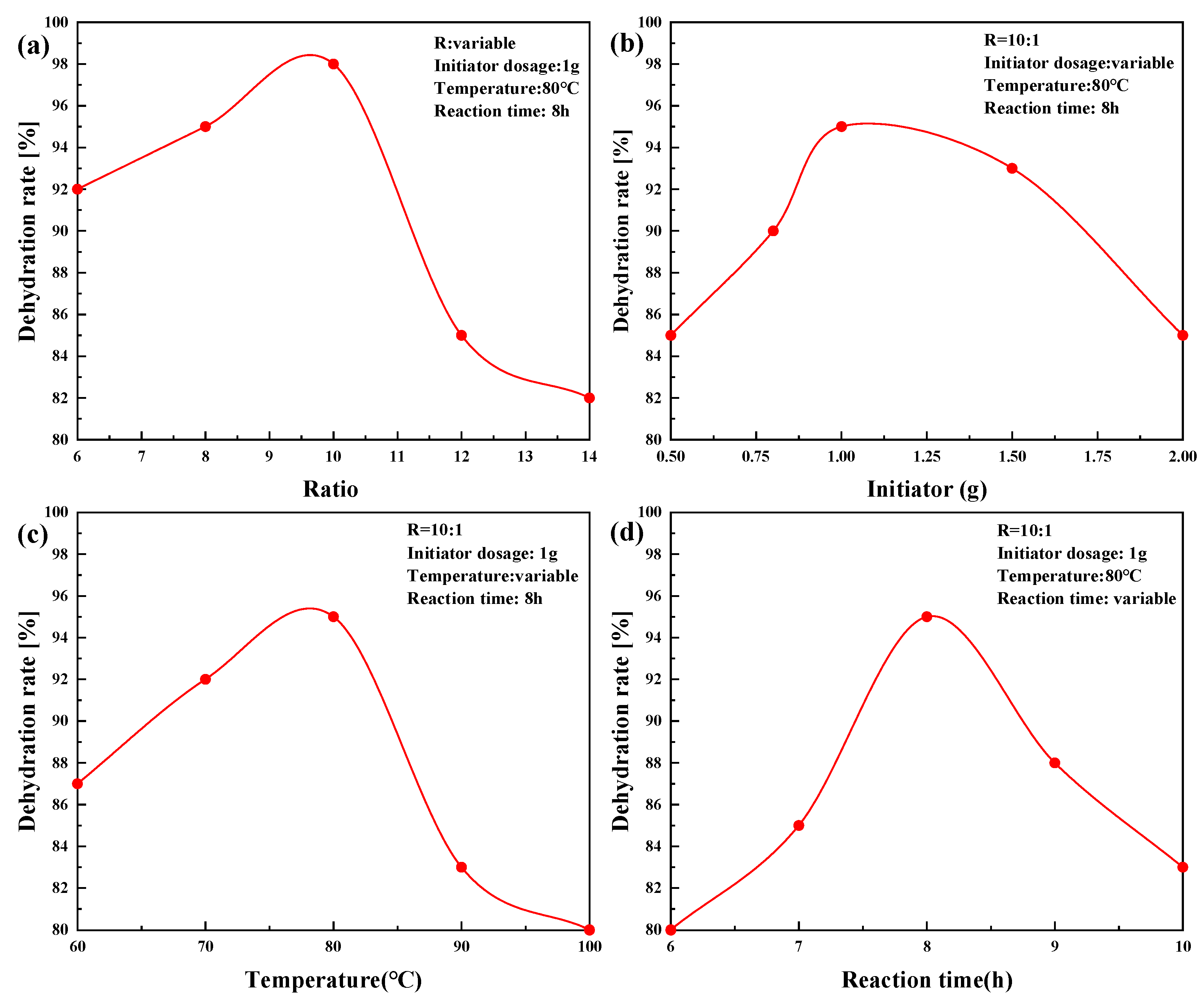
| Time (d) | Sample Source | Test Temperature (°C) | Zeta Electric Potential (mV) | Crude Oil Wax Content (%) |
|---|---|---|---|---|
| 1 | Gao oilfield | 30 | −17.3 | 13.2 |
| Chun oilfield | 30 | −8.6 | 15.6 | |
| 27 | Gao oilfield | 30 | −18.2 | 13.7 |
| Chun oilfield | 30 | −9.4 | 16.1 | |
| 75 | Gao oilfield | 30 | −17.5 | 13.5 |
| Chun oilfield | 30 | −9.1 | 15.5 |
| Code | Temperature (°C) | Dehydration Rate (%) | Cleanliness | |||||
|---|---|---|---|---|---|---|---|---|
| 0 mg/L | 80 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | 300 mg/L | |||
| a | 35 | 11.6 | 80.0 | 81.6 | 83 | 86.7 | 90.0 | Ⅱ |
| b | 42 | 11.6 | 80.0 | 93.3 | 98.3 | 100.0 | 100.0 | Ⅰ |
| c | 50 | 11.6 | 93.3 | 98.3 | 100.0 | 100.0 | 100.0 | Ⅰ |
| Code | Temperature (°C) | Dehydration Rate (%) | Cleanliness | |||||
|---|---|---|---|---|---|---|---|---|
| 0 mg/L | 80 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | 300 mg/L | |||
| c | 65 | 23.1 | 72.3 | 86.1 | 87.7 | 89.2 | 89.2 | Ⅰ |
| b | 68 | 24.6 | 87.7 | 92.3 | 98.5 | 100.0 | 100.0 | Ⅰ |
| c | 70 | 26.2 | 92.3 | 98.5 | 100.0 | 100.0 | 100.0 | Ⅰ |
| d | 73 | 27.7 | 98.5 | 100.0 | 100.0 | 100.0 | 100.0 | Ⅰ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Li, Q.; Xu, B.; Zou, T.; Zhang, Y.; Ping, W.; Ma, Q. Synthesis and Application of a Novel Multi-Branched Block Polyether Low-Temperature Demulsifier. Molecules 2023, 28, 8109. https://doi.org/10.3390/molecules28248109
Jiang S, Li Q, Xu B, Zou T, Zhang Y, Ping W, Ma Q. Synthesis and Application of a Novel Multi-Branched Block Polyether Low-Temperature Demulsifier. Molecules. 2023; 28(24):8109. https://doi.org/10.3390/molecules28248109
Chicago/Turabian StyleJiang, Shaohui, Qingsong Li, Botao Xu, Tao Zou, Yan Zhang, Wei Ping, and Qiang Ma. 2023. "Synthesis and Application of a Novel Multi-Branched Block Polyether Low-Temperature Demulsifier" Molecules 28, no. 24: 8109. https://doi.org/10.3390/molecules28248109
APA StyleJiang, S., Li, Q., Xu, B., Zou, T., Zhang, Y., Ping, W., & Ma, Q. (2023). Synthesis and Application of a Novel Multi-Branched Block Polyether Low-Temperature Demulsifier. Molecules, 28(24), 8109. https://doi.org/10.3390/molecules28248109




