Mediterranean Shrub Species as a Source of Biomolecules against Neurodegenerative Diseases
Abstract
1. Introduction
1.1. Brief Description of Neurodegenerative Diseases and Their Causes
1.2. Phenolic Compounds: Their Importance and Implication in Neurodegenerative Diseases
2. Description of the Study Area and Article Search Strategy
3. Description and Classification of the Phenolic Compounds Present in the Selected Species
| Species | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl | Cs | Cm | Cc | Ca | Cp | Cym | Cys | Cyst | Em | Es | Ea | Cv | Mc | Pl | Pt | Ro | Qi | Qs | Au | Ls | Tm | Tv | Ru | |
| Class: Phenolic acid. Sub Class: Hydroxybenzoic acids | ||||||||||||||||||||||||
| Acetovanillone | + | |||||||||||||||||||||||
| Anisic acid | + | + | + | |||||||||||||||||||||
| Benzoic acid | + | |||||||||||||||||||||||
| Benzyl benzoate | + | |||||||||||||||||||||||
| Methyl benzoate | + | |||||||||||||||||||||||
| Castalagin | + | |||||||||||||||||||||||
| Cornusilin | + | + | + | |||||||||||||||||||||
| 3-hydroxybenzoic acid | + | + | + | |||||||||||||||||||||
| 4-hydroxybenzoic acid | + | + | + | + | + | + | + | |||||||||||||||||
| 4-hydroxybenzoic acid glucuronide | + | |||||||||||||||||||||||
| Glucose p-hydroxy benzoate | + | |||||||||||||||||||||||
| Dihydroxy-methoxybenzoic acid | + | + | ||||||||||||||||||||||
| Dihydroxybenzoic acid di-pentoside | + | |||||||||||||||||||||||
| Dihydroxybenzoic acid hexoside | + | |||||||||||||||||||||||
| Ducheside A | + | |||||||||||||||||||||||
| 3,4′-dihydroxypropiofenone-3-glucoside | + | + | + | |||||||||||||||||||||
| 3-O-galloylquinic acid (Theogallin) | + | + | + | |||||||||||||||||||||
| 3-O-galloylshikimic acid | + | |||||||||||||||||||||||
| 3,4-Di-O-galloylquinic acid | + | + | + | + | ||||||||||||||||||||
| 5-O-galloylquinic acid | + | + | ||||||||||||||||||||||
| 5-O-galloylshikimic acid | + | |||||||||||||||||||||||
| Galloyl arbutin | + | |||||||||||||||||||||||
| Galloyl glucose | + | + | + | + | ||||||||||||||||||||
| Galloyl glucuronide | + | |||||||||||||||||||||||
| Galloyl hexoside | + | + | ||||||||||||||||||||||
| Galloyl-bis-HHDP glucose | + | |||||||||||||||||||||||
| Galloyl-HHDP-DHHDP-hexoside | + | |||||||||||||||||||||||
| Galloyl-HHDP-hexoside | + | |||||||||||||||||||||||
| Digallic acid | + | |||||||||||||||||||||||
| Digalloyl glucose | + | + | ||||||||||||||||||||||
| Digalloyl shikimic acid | + | |||||||||||||||||||||||
| Digalloyl-HHDP-hexoside | + | |||||||||||||||||||||||
| Digalloylarbutin | + | |||||||||||||||||||||||
| Digalloylquinic shikimic acid | + | |||||||||||||||||||||||
| Tetra-galloyl-hexoside | + | |||||||||||||||||||||||
| Trigalloylquinic acid | + | + | + | |||||||||||||||||||||
| Trigalloylshikimic acid | + | |||||||||||||||||||||||
| Pentagalloyl glucose | + | |||||||||||||||||||||||
| Ellagic acid | + | + | + | + | + | + | + | + | ||||||||||||||||
| Ellagic acid-7-xyloside | + | + | + | + | ||||||||||||||||||||
| Ellagic acid arabinoside | + | |||||||||||||||||||||||
| Ellagic acid diglucoside | + | |||||||||||||||||||||||
| Ellagic acid glucoside | + | |||||||||||||||||||||||
| Ellagic acid glucuronide | + | |||||||||||||||||||||||
| Ellagic acid hexoside | + | |||||||||||||||||||||||
| Ellagic acid mannopyranoside | + | |||||||||||||||||||||||
| Ellagic acid pentoside | + | + | ||||||||||||||||||||||
| Ellagic acid xylofuranoside | + | |||||||||||||||||||||||
| Ellagitannin | + | |||||||||||||||||||||||
| Methylellagic acid rhamnoside | + | |||||||||||||||||||||||
| 3,3′-di-O-Methylellagic acid 4-O-β-D-(2″-acetyl) glucoside | + | |||||||||||||||||||||||
| Gallic acid (3,4,5-trihydroxybenzoic acid) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||
| Gallic acid dihexoside | + | |||||||||||||||||||||||
| Gallic acid glucoside | + | |||||||||||||||||||||||
| Gallic acid hexoside | + | |||||||||||||||||||||||
| Gallotannin | + | |||||||||||||||||||||||
| Ethyl gallate | + | |||||||||||||||||||||||
| Methyl gallate | + | |||||||||||||||||||||||
| Gentisic acid | + | + | + | |||||||||||||||||||||
| Gentisoyl glucoside | + | + | + | |||||||||||||||||||||
| Gentisoyl hexoside | + | |||||||||||||||||||||||
| Glucogallin | + | + | + | + | ||||||||||||||||||||
| Hexahydroxydiphenoyl-glucose | + | + | + | + | + | + | ||||||||||||||||||
| Isoeugenol | + | |||||||||||||||||||||||
| Lambertianin C | + | |||||||||||||||||||||||
| Methoxysalicylic acid | + | |||||||||||||||||||||||
| Protocatechualdehyde (3,4-dihidroxy-benzaldehyde) | + | |||||||||||||||||||||||
| Protocatechuic acid (3,4-dihydroxy-benzoic acid) | + | + | + | + | + | + | + | + | + | |||||||||||||||
| Protocatechuic acid glucoside | + | + | ||||||||||||||||||||||
| Punicalagin | + | + | + | + | ||||||||||||||||||||
| Punicalin | + | + | + | + | ||||||||||||||||||||
| Punicalagin-gallate | + | + | + | |||||||||||||||||||||
| Sanguiin H-10 | + | |||||||||||||||||||||||
| Sanguiin H-10 isomer | + | |||||||||||||||||||||||
| Shikimic acid gallate | + | |||||||||||||||||||||||
| Shikimic acid dimer | + | + | + | |||||||||||||||||||||
| Strictinin ellagitannin | + | |||||||||||||||||||||||
| Syringic acid | + | + | + | + | + | + | + | + | ||||||||||||||||
| Syringyl-shikimic acid | + | + | + | |||||||||||||||||||||
| TriGG-dehydrohexahydroxydiphenoyl (DHHDP)-glucose | + | |||||||||||||||||||||||
| Uralenneoside | + | |||||||||||||||||||||||
| Vanillic acid | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| Vanillic acid sulfoquinovoside | + | |||||||||||||||||||||||
| Class: Phenolic acid. Sub Class: Hydroxycinnamic acids | ||||||||||||||||||||||||
| Caffeic acid (3,4-dihydroxycinnamic acid) | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| caffeic acid 4-O-glucoside | + | |||||||||||||||||||||||
| Caffeic acid derivate | + | + | + | + | ||||||||||||||||||||
| Caffeic acid hexoside | + | + | ||||||||||||||||||||||
| Caffeic acid trimer | + | |||||||||||||||||||||||
| Dihydrocaffeic acid | + | + | ||||||||||||||||||||||
| Caffeoyl arbutin | + | |||||||||||||||||||||||
| Caffeoyl ferulic acid | + | |||||||||||||||||||||||
| Caffeoyl feruloyl tartaric acid | + | |||||||||||||||||||||||
| Caffeoyl hexoside | + | |||||||||||||||||||||||
| Caffeoyl hexoside derivative | + | |||||||||||||||||||||||
| 4-O-Caffeoyl quinic acid | + | + | + | |||||||||||||||||||||
| Caffeoyl quinic acid glucoside | + | + | + | |||||||||||||||||||||
| Caffeoyl quinic acid derivative | + | + | ||||||||||||||||||||||
| Caffeoyl tartaric acid (caftaric acid) | + | + | + | |||||||||||||||||||||
| Dicaffeoyl shikimic acid | + | |||||||||||||||||||||||
| 1,4-dicaffeoyl quinic acid | + | + | ||||||||||||||||||||||
| 3,5-dicaffeoyl quinic acid | + | |||||||||||||||||||||||
| 6-Caffeoyl sucrose | + | |||||||||||||||||||||||
| Chlorogenic acid (3-O-caffeoylquinic acid) | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||
| Methyl caffeate | + | |||||||||||||||||||||||
| Cinnamic acid | + | + | + | + | + | + | + | |||||||||||||||||
| Cinnamic acid derivative | + | |||||||||||||||||||||||
| Methoxycinnamic acid | + | |||||||||||||||||||||||
| Cinnamic acid-O xylosyl hexoside | + | |||||||||||||||||||||||
| Hydrocinnamic acid | + | |||||||||||||||||||||||
| Hydrocinnamic acid glucoside | + | |||||||||||||||||||||||
| Hydroxycinnamoyl-quinic acid | + | |||||||||||||||||||||||
| p-Coumaric acid | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| p-Coumaricacid derivate | + | + | ||||||||||||||||||||||
| Coumaroyl quinic acid | + | + | + | + | + | |||||||||||||||||||
| Coumaroyl quinic acid derivative | + | |||||||||||||||||||||||
| Coumaric acid hexoside | + | + | ||||||||||||||||||||||
| Chicoric acid | + | |||||||||||||||||||||||
| Ferulic acid | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| Ferulic acid derivative | + | + | ||||||||||||||||||||||
| 3-O-Feruloylquinic acid | + | + | + | + | ||||||||||||||||||||
| Feruloylquinic glucoside | + | + | + | |||||||||||||||||||||
| Feruloyl-glucoside | + | |||||||||||||||||||||||
| Hydroxy-ferulic acid hexoside | + | |||||||||||||||||||||||
| Hydroxy-ferulic acid rhamnoside | + | |||||||||||||||||||||||
| Feruloyl tartaric acid (fertaric acid) | + | + | + | + | ||||||||||||||||||||
| 6′-O-Sinapoylsucrose | + | + | ||||||||||||||||||||||
| 3,4-Dihydroxyphenyllactic acid hexoside | + | |||||||||||||||||||||||
| 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoic acid | + | |||||||||||||||||||||||
| Rosmarinic acid | + | + | + | + | + | |||||||||||||||||||
| Rosmarinic acid hexoside | + | + | ||||||||||||||||||||||
| Rosmarinic acid-3-O-glucoside | + | + | ||||||||||||||||||||||
| Dihydroxy-dihydro feruloyl methyl rosmarinic acid | + | + | + | |||||||||||||||||||||
| Methylrosmaric acid | + | + | + | + | + | + | ||||||||||||||||||
| p-Hydroxybenzylrosmarinic acid | + | + | ||||||||||||||||||||||
| Isosalvianolic acid A | + | |||||||||||||||||||||||
| Methyl melitrate | + | |||||||||||||||||||||||
| Salvianolic acid A | ||||||||||||||||||||||||
| Salvianolic acid B | + | |||||||||||||||||||||||
| Salvianolic acid C | + | |||||||||||||||||||||||
| Sinapaldehyde | + | + | ||||||||||||||||||||||
| 3-Sinapoylquinic acid | + | |||||||||||||||||||||||
| Sinapic acid | + | + | + | |||||||||||||||||||||
| Yunnaneic acid F | + | |||||||||||||||||||||||
| Verbascoside | + | |||||||||||||||||||||||
| Class: Phenolic acid. Sub Class: Hydroxyphenylacetic acids | ||||||||||||||||||||||||
| p-Hydroxyphenylacetic acid | + | + | ||||||||||||||||||||||
| 3,4-Dihydroxyphenylacetic acid | + | + | ||||||||||||||||||||||
| 3,4-dihydroxyphenyllactic acid hexoside | + | |||||||||||||||||||||||
| Class: Flavonoid. Sub Class: Flavanones | ||||||||||||||||||||||||
| Eriodictyol | + | + | ||||||||||||||||||||||
| Eriodictyol 7-O-rutinoside | + | + | ||||||||||||||||||||||
| Eriodictyol-7-O-glucuronide | + | |||||||||||||||||||||||
| Eriodictyol-O-di-hexoside | + | + | ||||||||||||||||||||||
| Eriodictyol-O-hexoside | + | + | ||||||||||||||||||||||
| Eriodictyol-O-hexuronide | + | |||||||||||||||||||||||
| Glucodistylin | + | |||||||||||||||||||||||
| Glucodistylin isomer | + | |||||||||||||||||||||||
| Hesperetin 7-O-rutinoside (Hesperidin) | + | + | + | + | + | |||||||||||||||||||
| Methyleriodictyol-O-pentosylhexoside | + | |||||||||||||||||||||||
| Naringenin | + | + | + | + | + | + | + | + | ||||||||||||||||
| Naringenin-di-hexoside | + | |||||||||||||||||||||||
| Naringenin 7-O-glucoside (Naringin-Prunina) | + | + | + | + | + | + | + | + | + | |||||||||||||||
| Pinocembrin | + | |||||||||||||||||||||||
| Class: Flavonoid. Sub Class: Flavones | ||||||||||||||||||||||||
| Apigenin | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||
| Apigenin 7-O-glucuronide | + | + | ||||||||||||||||||||||
| Apigenin glucuronide hexoside | + | + | + | |||||||||||||||||||||
| Apigenin 6,8-di-C-glucoside | + | + | + | + | ||||||||||||||||||||
| Apigenin 7-O-glucoside | + | + | + | + | + | + | ||||||||||||||||||
| Apigenin C-hexoside | + | + | ||||||||||||||||||||||
| Apigenin pentoside | + | + | ||||||||||||||||||||||
| Apigenin-7-O-rutinoside | + | |||||||||||||||||||||||
| Apigenin-O-hexoside | + | + | ||||||||||||||||||||||
| Apigenin-O-hexoside derivative | + | |||||||||||||||||||||||
| Isovitexin 7-O-glucoside | + | |||||||||||||||||||||||
| Apigenin 8-C-glucoside (Vitexin) | + | + | ||||||||||||||||||||||
| 2″-O-pentosyl-6-C-hexosyl-apigenin | + | |||||||||||||||||||||||
| 2″-O-Pentosyl-8-C-hexoside apigenin isomer I | + | |||||||||||||||||||||||
| 2″-O-Pentosyl-8-C-hexoside apigenin isomer II | + | |||||||||||||||||||||||
| 2″-O-pentosyl-8-C-hexosyl-apigenin | + | |||||||||||||||||||||||
| 2″-O-Pentoxide-8-C-hexoside apigenin | + | |||||||||||||||||||||||
| 4′-O-Rutinoside of 7-O-methylated apigenin | + | |||||||||||||||||||||||
| 6″-O-(3-hydroxy-3-methylglutaroyl)-2″-O-pentosyl-C-hexosyl-apigenin | + | |||||||||||||||||||||||
| Apigenin 4′-methyl ether | + | + | + | |||||||||||||||||||||
| Apigenin 7-methyl ether (Genkwanin) | + | + | + | |||||||||||||||||||||
| Apigenin 4′,7-dimethyl ether | + | + | + | + | ||||||||||||||||||||
| Chalcone | + | |||||||||||||||||||||||
| Chrysin derivative | + | |||||||||||||||||||||||
| Chrysin-7-O-glucoside | + | |||||||||||||||||||||||
| Circimaritin | ||||||||||||||||||||||||
| Hispidulin (Scutellarein 6-methyl ether) | + | + | ||||||||||||||||||||||
| Hispidulin 7-O-glucose (homoplantaginin) | + | |||||||||||||||||||||||
| 6″-O-(E)-feruloylhomoplantaginin | + | |||||||||||||||||||||||
| Hispidulin-rutinoside | + | |||||||||||||||||||||||
| Hypolaetin di-glucuronide | + | |||||||||||||||||||||||
| Hypolaetin 8-O-glucuronide | + | |||||||||||||||||||||||
| Isoscutellarein 7-O-glucoside | + | |||||||||||||||||||||||
| Isoscutellarein 8-O-glucuronide | + | |||||||||||||||||||||||
| Ladanein (5,6-dihydroxy-7,4′-dimethoxyflavone) | + | |||||||||||||||||||||||
| Luteolin (3′.4′.5.7-Tetrahydroxyflavone) | + | + | + | + | + | + | + | + | + | |||||||||||||||
| Chrysoeriol-O-hexoside (Luteolin 3′-methyl ether) | + | |||||||||||||||||||||||
| Diosmetin (Luteolin 4′-methyl ether) | + | |||||||||||||||||||||||
| Cirsilineol (6-Methoxyluteolin 3′,7-dimethyl ether) | + | |||||||||||||||||||||||
| Luteolin 7,3-dimethyl ether | + | |||||||||||||||||||||||
| Luteolin 3′-O-glucuronide | + | |||||||||||||||||||||||
| Luteolin-7-O-glucuronide | + | + | + | + | ||||||||||||||||||||
| Luteolin 7,4′-di-glucuronide | + | + | ||||||||||||||||||||||
| Luteolin 4-O-glucoside | + | |||||||||||||||||||||||
| Luteolin 7-O-glucoside | + | + | + | + | + | |||||||||||||||||||
| Luteolin-5-O-glucoside | + | |||||||||||||||||||||||
| Luteolin 8-C-glucoside (Orientin) | + | + | ||||||||||||||||||||||
| Luteolin 6-C-glucoside (Isoorientin) | + | |||||||||||||||||||||||
| Luteolin-O-hexorunide | + | |||||||||||||||||||||||
| Luteolin-7-O-rutinoside | + | + | + | + | + | + | ||||||||||||||||||
| Luteolin-hexoside | + | + | ||||||||||||||||||||||
| Luteolin 6-hydroxy-7-O-glucoside | + | |||||||||||||||||||||||
| Luteolin-O-malonyl-hexoside) | + | + | + | |||||||||||||||||||||
| 2″-O-Pentosyl-8-C-hexoside luteolin | + | |||||||||||||||||||||||
| 2″-O-pentosyl-6-C-hexosyl-luteolin | + | |||||||||||||||||||||||
| 2″-O-pentosyl-8-C-hexosyl-luteolin | + | |||||||||||||||||||||||
| 6″-O-(3-hydroxy-3-methylglutaroyl)-2″-O-pentosyl-C-hexosyl-luteolin | + | |||||||||||||||||||||||
| Nepitrin (Nepetin 7-O-glucoside) | + | |||||||||||||||||||||||
| 6″-O-(E)-feruloylnepitrin | + | |||||||||||||||||||||||
| Salvigenin (5-Hydroxy-6,7,4′-trimethoxyflavone) | ||||||||||||||||||||||||
| Techtochrysin | + | |||||||||||||||||||||||
| Class: Flavonoid. Sub Class: Flavonols | ||||||||||||||||||||||||
| Isorhamnetin | + | + | ||||||||||||||||||||||
| Isorhamnetin 3-O-glucoside | + | + | + | + | ||||||||||||||||||||
| Isorhamnetin 3-O-rutinoside | + | + | + | |||||||||||||||||||||
| Isorhamnetin-3-O-hexoside | + | + | + | |||||||||||||||||||||
| Isorhamnetin-O-(6″-caffeoyl)hexoside | + | |||||||||||||||||||||||
| Isorhamnetin-O-deoxyhexosyl-hexoside | + | |||||||||||||||||||||||
| Isorhamnetin-O-hexoside-O-rhamnoside | + | + | ||||||||||||||||||||||
| Galangin (3,5,7-Trihydroxyflavone) | + | |||||||||||||||||||||||
| Kaempferol | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||||
| 6-Hydroxykaempferol | + | |||||||||||||||||||||||
| Dihydrokaempferol 3-O-glucoside | + | |||||||||||||||||||||||
| Kaempferol 3-methyl ether | + | + | + | |||||||||||||||||||||
| Kaempferol 3 4′-dimethyl ether | + | + | ||||||||||||||||||||||
| Kaempferol 3 7-dimethyl ether | + | + | ||||||||||||||||||||||
| kaempferol 3,7,4′-trimmethyl ether | + | + | ||||||||||||||||||||||
| kaempferol methylether O-rutinoside | + | |||||||||||||||||||||||
| Kaempferol dimethylether hexoside | + | |||||||||||||||||||||||
| Kaempferol 3-O-(6″-galloyl) glucoside | + | + | + | |||||||||||||||||||||
| kaempferol-3-O-(6″-feruloyl)-β-D-glucopyranoside | + | |||||||||||||||||||||||
| Kaempferol 3-O-(6″-p-coumaroyl) glucoside | + | + | ||||||||||||||||||||||
| kaempferol-3-O-(2′′,6′′-di-p-coumaroyl)glucoside isomers | + | |||||||||||||||||||||||
| kaempferol-3-O-(2″,6″-di-E-p-coumaroyl)-glucopyranoside | + | |||||||||||||||||||||||
| kaempferol-3-O-(3′′-acetyl-2″,6′′-di-p-coumaroyl)glucoside | + | |||||||||||||||||||||||
| kaempferol-3-O-(3′′,4′′-diacetyl-2′′,6′′-di-p-coumaroyl)glucoside isomers | + | |||||||||||||||||||||||
| kaempferol-3-O-(6″-p-coumaroyl) glucopyranoside(Tiliroside) | + | + | + | |||||||||||||||||||||
| Kaempferol-3-galactoside-6″-rhamnoside-3′″- rhamnoside | + | |||||||||||||||||||||||
| kaempferol malonyl glucoside | + | |||||||||||||||||||||||
| kaempferol 3-O-ramnopyranoside | + | |||||||||||||||||||||||
| Kaempferol-O-rhamnoside | + | + | + | + | ||||||||||||||||||||
| Kaempferol 7-O-(6″-rhamnosyl) glucoside | + | + | + | |||||||||||||||||||||
| kaempferol 3-O-arabinofuranoside | + | |||||||||||||||||||||||
| kaempferol-3-O-arabinopyranoside | + | |||||||||||||||||||||||
| Kaempferol 3-O-glucoside (Astragalin) | + | + | + | + | ||||||||||||||||||||
| Kaempferol 3-O-rutinoside | + | + | + | + | ||||||||||||||||||||
| Kaempferol-acetyl-O-rutinoside | + | |||||||||||||||||||||||
| kaempferol-acetyl-O-rahmnoside | + | |||||||||||||||||||||||
| Kaempferol-acetyl-O-hexoside | + | + | + | + | ||||||||||||||||||||
| Kaempferol O-glucuronide | + | + | ||||||||||||||||||||||
| Kaempferol 7-O-hexuronide | + | |||||||||||||||||||||||
| Kaempferol 3-O-pentoside | + | + | ||||||||||||||||||||||
| Kaempferol O-hexoside | + | + | + | + | + | |||||||||||||||||||
| Kaempferol O-pentosyl hexoside | + | + | ||||||||||||||||||||||
| Kaempferol-3-O-hydroxybenzoyl glucoside | + | |||||||||||||||||||||||
| kaempferol-3-O-galactoside | + | |||||||||||||||||||||||
| Kaempferol xyloside | + | |||||||||||||||||||||||
| Kaempferol-O-di-hexoside | + | + | ||||||||||||||||||||||
| Morin | + | |||||||||||||||||||||||
| Myricetin (3.3′.4′.5.5′.7-Hexahydroxyflavone) | + | + | + | + | + | + | + | + | + | |||||||||||||||
| Myricetin 3-O-(6″-rhamnosyl) glucoside | + | |||||||||||||||||||||||
| Myricetin-O-(6″-benzoyl) hexoside | + | |||||||||||||||||||||||
| Myricetin-O-(6″-cinnamoyl) hexoside | + | |||||||||||||||||||||||
| Myricetin-O-(6″-p-coumaroyl) hexoside | + | |||||||||||||||||||||||
| Myricetin-O-(galloyl) hexoside | + | + | + | + | ||||||||||||||||||||
| Methoxy-myricetin-O-rhamnoside | + | |||||||||||||||||||||||
| Myricetin 3,7,4′,5′-tetramethyl ether | + | |||||||||||||||||||||||
| Myricetin-3-arabinoside | + | |||||||||||||||||||||||
| myricetin 3-O-arabinofuranoside | + | |||||||||||||||||||||||
| Myricetin-3-O-galactoside | + | |||||||||||||||||||||||
| Myricetin-3-O-glucoside | + | + | + | + | ||||||||||||||||||||
| Myricetin-3-O-glucuronide | + | + | + | |||||||||||||||||||||
| Myricetin 3-O-hexoside | + | + | + | + | + | + | ||||||||||||||||||
| Myricetin 7-O-hexuronide | + | |||||||||||||||||||||||
| Myricetin 3-O-pentoside | + | |||||||||||||||||||||||
| Myricetin 7-O-pentoside | + | + | + | |||||||||||||||||||||
| Myricetin-O-rhamnoside (Myricitrin) | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||
| Myricitrin-2′′-O-gallate (Desmanthin) | + | |||||||||||||||||||||||
| Myricetin-O-rutinoside | + | + | + | + | + | |||||||||||||||||||
| Myricetin 3-O-xyloside | + | + | ||||||||||||||||||||||
| Pinobanksin (bioflavonoide) | + | |||||||||||||||||||||||
| Quercetin (3.3′.4′.5.7-Pentahydroxyflavone) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||||||
| Quercetin-(acetyl) rutinoside | + | |||||||||||||||||||||||
| Quercetin-(acetyl) hexoside | + | + | ||||||||||||||||||||||
| Quercetin-(acetyl)-O-rhamnoside | + | |||||||||||||||||||||||
| Quercetin-O-(6″-cinnamoyl) hexoside | + | + | ||||||||||||||||||||||
| quercetin 3-O-(2′-coumaroyl) rutinoside | + | + | + | |||||||||||||||||||||
| Quercetin 3-O-(6″-p-coumaroyl) hexoside | + | |||||||||||||||||||||||
| Quercetin 3-O-(6″-galloyl) hexoside | + | |||||||||||||||||||||||
| Quercetin-O-(6″-p-hydroxybenzoyl) hexoside | + | + | ||||||||||||||||||||||
| Quercetin-O-(malonyl) hexoside | + | |||||||||||||||||||||||
| Quercetrin-O-gallate | + | + | ||||||||||||||||||||||
| Quercertin methyl ether-3-O-galactoside | + | |||||||||||||||||||||||
| Quercetin 4′,5′-dimethyl ether | + | |||||||||||||||||||||||
| Quercetin 3,7,4′,5′-tetramethyl ether | + | |||||||||||||||||||||||
| Quercetin 3-O-arabinoside | + | |||||||||||||||||||||||
| quercetin 3-O-arabinofuranoside | + | + | ||||||||||||||||||||||
| Quercetin 3-O-galactoside (Hyperoside) | + | + | + | + | ||||||||||||||||||||
| Quercetin 3-O-glucoside (Isoquercetin) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Quercetin 3-O-glucuronide | + | + | + | + | ||||||||||||||||||||
| Quercetin-O-hexoside | + | + | + | + | + | + | + | + | ||||||||||||||||
| Quercetin 3-O-hexuronide | + | + | ||||||||||||||||||||||
| Quercetin hexose protocatechuic acid | + | |||||||||||||||||||||||
| Quercetin-O-rhamnoside (Quercetrin) | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||
| Quercetin 3-O-rutinoside (Rutin) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Quercetin 3-O-pentoside | + | + | + | + | ||||||||||||||||||||
| Quercetin 3-O-xyloside | + | |||||||||||||||||||||||
| Quercetin 3-O-rhamnoside-7-O-glucoside | + | |||||||||||||||||||||||
| Quercetin 3,4-diglucoside | + | + | + | + | ||||||||||||||||||||
| Quercetin-O-deoxyhexosyl-hexoside | + | |||||||||||||||||||||||
| Quercetin-O-dihexoside | + | + | ||||||||||||||||||||||
| Quercetin-pentosyl-hexoside | + | + | ||||||||||||||||||||||
| Taxifolin (dihydroquercetin) | + | + | + | |||||||||||||||||||||
| Taxifolin-3-O-glucoside | + | + | ||||||||||||||||||||||
| Taxifolin 3-O-rhamnoside | + | |||||||||||||||||||||||
| Taxifolin-O-hexoside | + | + | ||||||||||||||||||||||
| Taxifolin pentoside | + | |||||||||||||||||||||||
| Class: Flavonoid. Sub Class: Flavanols | ||||||||||||||||||||||||
| Catechin | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||
| Catechin 3-gallate | + | |||||||||||||||||||||||
| 4,3′,4′-Trimethylcatechin | + | + | ||||||||||||||||||||||
| Catechin derivates | + | |||||||||||||||||||||||
| Catechin glucose | + | |||||||||||||||||||||||
| Catechin-( 4α→8)-Catechin (Procyanidin B3) | + | + | ||||||||||||||||||||||
| Dehydrodicatechin A | + | |||||||||||||||||||||||
| Epicatechin | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| Epicatechin derivatives | + | |||||||||||||||||||||||
| Epicatechin methyl gallate | + | |||||||||||||||||||||||
| Epicatechin gallate | + | + | + | + | + | |||||||||||||||||||
| epicatechin-4,6-catechin | + | |||||||||||||||||||||||
| epicatechin-4,8-catechin | + | |||||||||||||||||||||||
| epicatechin-4,8-epicatechin-4,8-catechin | + | |||||||||||||||||||||||
| Epicatechin-4,8-epicatechin-4,8-Epicatechin | + | |||||||||||||||||||||||
| Epicatechin-A-epicatechin | + | + | + | + | ||||||||||||||||||||
| Epicatechin-B-epicatechin-A-epicatechin | + | |||||||||||||||||||||||
| Epicatechin-epicatechin 3-O gallate | + | |||||||||||||||||||||||
| Epicatechin-epigallocatechin | + | + | + | |||||||||||||||||||||
| Epigallocatechin | + | + | + | + | ||||||||||||||||||||
| Epigallocatechin gallate(Teatannin II) | + | + | + | + | ||||||||||||||||||||
| Epigallocatechin–catechin | + | |||||||||||||||||||||||
| Epigallocatechin–epicatechin | + | |||||||||||||||||||||||
| Epigallocatechin–epigallocatechin | + | + | + | |||||||||||||||||||||
| Fzelechin-catechin-3-O-rhamnoside (proanthocyanidin) | + | |||||||||||||||||||||||
| Gallocatechin | + | + | + | |||||||||||||||||||||
| Gallocatechin-4,8-catechin | + | |||||||||||||||||||||||
| Procyanidin B2 | + | |||||||||||||||||||||||
| Tannic acid | + | |||||||||||||||||||||||
| Class: Flavonoid. Sub Class: Anthocyanins | ||||||||||||||||||||||||
| Cyanidin 3-O-arabinoside | + | + | ||||||||||||||||||||||
| Cyanidin-3-galactoside | + | |||||||||||||||||||||||
| Cyanidin 3-O-glucoside | + | + | + | + | + | + | ||||||||||||||||||
| Cyanidin-3-O-rutinoside | + | |||||||||||||||||||||||
| Cyanidin 3-O-xyloside | + | |||||||||||||||||||||||
| Cyanidin dihexoside | + | |||||||||||||||||||||||
| Cyanidin-3,5-diglucoside | + | |||||||||||||||||||||||
| Delphinidin 3-O-galactoside | + | |||||||||||||||||||||||
| Delphinidin 3-O-glucoside | + | + | + | + | ||||||||||||||||||||
| Malvidin-3-O-glucoside/Oenin | + | + | + | + | ||||||||||||||||||||
| Pelargonidin 3-O-(6″-malonyl) glucoside | + | + | ||||||||||||||||||||||
| Pelargonidin-3-O-glucoside | + | |||||||||||||||||||||||
| Pelargonidin 3-rutinoside | + | |||||||||||||||||||||||
| Peonidin 3-O-(6″-p-coumaroyl) glucoside | + | + | ||||||||||||||||||||||
| Peonidin-3-O-glucoside | + | |||||||||||||||||||||||
| Petunidin | + | + | ||||||||||||||||||||||
| Petunidin-3-O-glucoside | + | + | ||||||||||||||||||||||
| Class: Flavonoid. Sub Class: Isoflavonoids | ||||||||||||||||||||||||
| Daidzein | ||||||||||||||||||||||||
| 3′-Hydroxydaidzein | + | |||||||||||||||||||||||
| Genistein | + | + | ||||||||||||||||||||||
| 2′-Hydroxygenistein | + | |||||||||||||||||||||||
| Glycitin 6″-O-malonate | + | + | ||||||||||||||||||||||
| Class: Other polyphenols. Sub Class: Hydroxybenzaldehydes | ||||||||||||||||||||||||
| 4-hydroxybenzaldehyde | + | + | ||||||||||||||||||||||
| 4-hydroxybenzoic acid 4-(6-O-sulfo)glucoside | + | |||||||||||||||||||||||
| Syringaldehyde | + | + | ||||||||||||||||||||||
| Vanillin | + | + | + | |||||||||||||||||||||
| Class: Other polyphenol. Sub Class: Hydroxycoumarins | ||||||||||||||||||||||||
| 4-methylumbelliferone | + | |||||||||||||||||||||||
| 6,7-Dihydroxycoumarin 3O-glucoside (Aesculin) | + | |||||||||||||||||||||||
| Coumarin | + | |||||||||||||||||||||||
| Class: Other polyphenol. Sub Class: Tyroslos | ||||||||||||||||||||||||
| Oleuropein | + | |||||||||||||||||||||||
| Class: Lignans. Sub Class: Lignans | ||||||||||||||||||||||||
| Carnosic acid | + | + | ||||||||||||||||||||||
| Carnosol | + | + | + | |||||||||||||||||||||
| Isolariciresinol 3-glucoside | + | |||||||||||||||||||||||
| Methyl carnosic acid | + | |||||||||||||||||||||||
| Pinoresinol | + | + | ||||||||||||||||||||||
| Rosmanol | + | |||||||||||||||||||||||
| Rosmanol derivate | + | |||||||||||||||||||||||
| Sagerinic acid | + | |||||||||||||||||||||||
| Thymol | + | |||||||||||||||||||||||
| Class: Other polyphenols. Sub Class: Other polyphenols | ||||||||||||||||||||||||
| 5-Nonadecylresorcinol | + | |||||||||||||||||||||||
| Arbutin | + | |||||||||||||||||||||||
| Catechol | + | + | + | + | ||||||||||||||||||||
| Coniferaldehyde | + | + | ||||||||||||||||||||||
| Hydroquinone derivative | + | |||||||||||||||||||||||
| Salvianolic acid | + | |||||||||||||||||||||||
| Salvianolic acid A | + | + | ||||||||||||||||||||||
| salvianolic acid B (lithospermic acid B) | + | + | + | |||||||||||||||||||||
| Salvianolic acid B/E/L | + | |||||||||||||||||||||||
| Salvianolic acid C | + | |||||||||||||||||||||||
| Salvianolic acid C isomer | + | |||||||||||||||||||||||
| Salvianolic acid F | + | |||||||||||||||||||||||
| Salvianolic acid K | + | |||||||||||||||||||||||
| Salvianolic acid I | + | |||||||||||||||||||||||
| Sculetin | + | |||||||||||||||||||||||
| Class: Stilbenes. Sub Class: Stilbenes | ||||||||||||||||||||||||
| Piceid | + | |||||||||||||||||||||||
| Resveratrol | + | + | + | |||||||||||||||||||||
| Stilbericoside | + | |||||||||||||||||||||||
| References | [75,78,79,80,81,82,83,84,85,86] | [81,87,88] | [81,83,88,89,90] | [81] | [81,88,91] | [81,82] | [92,93,94,95,96] | [97,98,99] | [100] | [100] | [101] | [102,103] | [101] | [83,104] | [105,106,107] | [108,109] | [110,111,112,113,114,115] | [116,117,118] | [117] | [119,120,121,122,123,124,125,126,127,128,129,130,131,132,133] | [134,135,136,137,138,139] | [140,141,142] | [143,144,145,146,147,148,149,150,151,152] | [92,153,154,155,156,157,158,159,160,161,162] |
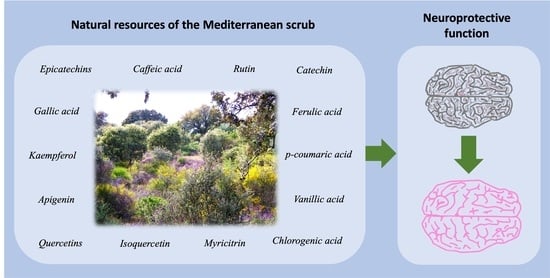
4. Neuroprotective Effect of the Most Represented Phenolic Compounds in the Selected Species
5. Main Species with Neuroprotective Activity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, P.; Oliverio, M.; Maiuolo, J.; Bonacci, S.; De Luca, G.; Masullo, M.; Arcone, R.; Procopio, A. Novel hydroxytyrosol-donepezil hybrids as potential antioxidant and neuroprotective agents. Front. Chem. 2021, 9, 741444. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Dilberger, B.; Weppler, S.; Eckert, G.P. Phenolic acid metabolites of polyphenols act as inductors for hormesis in C. elegans. Mech. Ageing Dev. 2021, 198, 111518. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Kanazawa, I.; Inaba, M.; Inoue, D.; Uenishi, K.; Saito, M.; Shiraki, M.; Suzuki, A.; Takeuchi, Y.; Hagino, H.; Fujiwara, S.; et al. Executive summary of clinical practice guide on fracture risk in lifestyle diseases. J. Bone Miner. Metab. 2020, 38, 746–758. [Google Scholar] [CrossRef]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Marzetti, E.; Csiszar, A.; Dutta, D.; Balagopal, G.; Calvani, R.; Leeuwenburgh, C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: From mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H459–H476. [Google Scholar] [CrossRef]
- Dilberger, B.; Baumanns, S.; Schmitt, F.; Schmiedl, T.; Hardt, M.; Wenzel, U.; Eckert, G.P. Mitochondrial oxidative stress impairs energy metabolism and reduces stress resistance and longevity of C. elegans. Oxid. Med. Cell. Longev. 2019, 2019, 6840540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mehta, V.; Raj, U.; Varadwaj, P.K.; Udayabanu, M.; Yennamalli, R.M.; Singh, T.R. Computational and in-vitro validation of natural molecules as potential acetylcholinesterase inhibitors and neuroprotective agents. Curr. Alzheimer Res. 2019, 16, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective potency of some spice herbs, a literature review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e62. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-C.; Providencia, R.; Sofat, R.; Pujades-Rodriguez, M.; Torralbo, A.; Fatemifar, G.; Fitzpatrick, N.K.; Taylor, J.; Li, K.; Dale, C.; et al. Incidence, morbidity, mortality and disparities in dementia: A population linked electronic health records study of 4.3 million individuals. Alzheimer’s Dement. 2023, 19, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Chen, Z.; Cai, P.; Wang, R.; Yang, Q.; Li, L.; Yang, H.; Zhu, R. Targeting microRNA-125b promotes neurite outgrowth but represses cell apoptosis and inflammation via blocking PTGS2 and CDK5 in a FOXQ1-dependent way in Alzheimer disease. Front. Cell. Neurosci. 2020, 14, 587747. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.R. Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol, F.S.; Ercetin, T.; Kahraman, A.; Celep, F.; Akaydin, G.; Sener, B.; Dogan, M. Assessment of anticholinesterase and antioxidant properties of selected sage (Salvia) species with their total phenol and flavonoid contents. Ind. Crops Prod. 2013, 41, 21–30. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Iqbal, K.; Alonso, A.d.C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.-X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1739, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M. β-Site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Jeandel, C.; Nicolas, M.B.; Dubois, F.; Nabet-Belleville, F.; Penin, F.; Cuny, G. Lipid peroxidation and free radical scavengers in Alzheimer’s disease. Gerontology 1989, 35, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gabbita, S.P.; Lovell, M.A.; Markesbery, W.R. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J. Neurochem. 1998, 71, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.; Snyder, P.; Giacobini, E.; Khachaturian, Z.; Cholinergic System Working Group, and for the Alzheimer Precision Medicine Initiative (APMI). Revisiting the cholinergic hypothesis in Alzheimer’s disease: Emerging evidence from translational and clinical research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar]
- Dou, K.-X.; Tan, M.-S.; Tan, C.-C.; Cao, X.-P.; Hou, X.-H.; Guo, Q.-H.; Tan, L.; Mok, V.; Yu, J.-T. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: A network meta-analysis of 41 randomized controlled trials. Alzheimer’s Res.Ther. 2018, 10, 126. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- González-Casacuberta, I.; Juárez-Flores, D.L.; Morén, C.; Garrabou, G. Bioenergetics and Autophagic Imbalance in Patients-Derived Cell Models of Parkinson Disease Supports Systemic Dysfunction in Neurodegeneration. Front. Neurosci. 2019, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Parkinson’s Disease: From pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef] [PubMed]
- Naveen, K.; Bhattacharjee, A. Medicinal herbs as neuroprotective agents. WJPPS 2021, 10, 675–689. [Google Scholar]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Graves, S.M.; Xie, Z.; Stout, K.A.; Zampese, E.; Burbulla, L.F.; Shih, J.C.; Kondapalli, J.; Patriarchi, T.; Tian, L.; Brichta, L.; et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat. Neurosci. 2020, 23, 15–20. [Google Scholar] [CrossRef]
- Junn, E.; Mouradian, M.M. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci. Lett. 2002, 320, 146–150. [Google Scholar] [CrossRef]
- . Perfeito, R.; Ribeiro, M.; Rego, A.C. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch. Toxicol. 2017, 91, 1245–1259. [Google Scholar] [CrossRef]
- Gómez, L.A.; Tovar, H.C.; Agudelo, C. Utilización de servicios de salud y perfiles epidemiológicos como parámetros de adecuación del Plan Obligatorio de Salud en Colombia. Rev. Salud Pública 2003, 5, 246–262. [Google Scholar] [CrossRef]
- López Locanto, Ó. Tratamiento farmacológico de la enfermedad de Alzheimer y otras demencias. Arch. Med. Interna 2015, 37, 61–67. [Google Scholar]
- Blesa, J.; Lanciego, J.; Obeso, J.A. Editorial: Parkinson’s disease: Cell vulnerability and disease progression. Front. Neuroanat. 2015, 9, 125. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-S.; Cho, J.-y.; Kim, D.-H.; Yan, J.-J.; Lee, H.-K.; Suh, H.-W.; Song, D.-K. Inhibitory Effects of Long-Term Administration of Ferulic Acid on Microglial Activation Induced by Intracerebroventricular Injection of β-Amyloid Peptide (1—42) in Mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Lee, S.; Hwang, E.-S.; Maeng, S.; Park, J.-H. p-Coumaric acid enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Biochem. Biophys. Res. Commun. 2017, 492, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.E.; Jung, K.; Kang, K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Buonfiglio, M.; Corona, G.; Chirafisi, J.; Vafeiadou, K.; Angeloni, C.; Hrelia, S.; Hrelia, P.; Spencer, J.P.E. Sulforaphane protects cortical neurons against 5-S-cysteinyl-dopamine-induced toxicity through the activation of ERK1/2, NrF-2 and the upregulation of detoxification enzymes. Mol. Nutr. Food Res. 2010, 54, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Russo, G.L. Neuroprotective role of natural polyphenols. Curr.Top. Med. Chem. 2016, 16, 1943–1950. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.; Costa, I.; Almeida, A.; Tavares, L.; Pais, T.; Pinto, P.; Ventura, M. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, M.A. Interaction of dietary antioxidants in vivo: How fruit and vegetables prevent disease? QJM 1999, 92, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Katan, M.B. Health effects and bioavailability of dietary flavonols. Free Radic. Res. 1999, 31, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.-S.; Lee, M.-K.; Park, Y.B.; Choi, M.-S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Eghorn, L.F.; Hoestgaard-Jensen, K.; Kongstad, K.T.; Bay, T.; Higgins, D.; Frølund, B.; Wellendorph, P. Positive allosteric modulation of the GHB high-affinity binding site by the GABAA receptor modulator monastrol and the flavonoid catechin. Eur. J. Pharmacol. 2014, 740, 570–577. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Bilan, A.R. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen. Res. 2018, 13, 955. [Google Scholar]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- Katalinić, M.; Rusak, G.; Barović, J.D.; Šinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Choi, J.H.; Yang, H.; Jeong, E.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Domanowska, A.; Wódkiewicz, E.; Malinowski, B. Neuroprotective Properties of Resveratrol and Its Derivatives—Influence on Potential Mechanisms Leading to the Development of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2749. [Google Scholar] [CrossRef]
- Pereira, L.R.; Fritzen, M.; Yunes, R.A.; Leal, P.C.; Creczynski-Pasa, T.B.; Pereira, A.F. Inhibitory effects of gallic acid ester derivatives on Saccharomyces cerevisiae multidrug resistance protein Pdr5p. FEMS Yeast Res. 2010, 10, 244–251. [Google Scholar] [CrossRef]
- Iwaki, A.; Ohnuki, S.; Suga, Y.; Izawa, S.; Ohya, Y. Vanillin inhibits translation and induces messenger ribonucleoprotein (mRNP) granule formation in Saccharomyces cerevisiae: Application and validation of high-content, image-based profiling. PLoS ONE 2013, 8, e61748. [Google Scholar] [CrossRef]
- Sunthonkun, P.; Palajai, R.; Somboon, P.; Suan, C.L.; Ungsurangsri, M.; Soontorngun, N. Life-span extension by pigmented rice bran in the model yeast Saccharomyces cerevisiae. Sci. Rep. 2019, 9, 18061. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Cui, X.; Lin, Q.; Liang, Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Front. aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, M.; Fiumano, V.; D’Abrosca, B.; Esposito, A.; Choi, Y.H.; Verpoorte, R.; Fiorentino, A. Chemical interactions between plants in Mediterranean vegetation: The influence of selected plant extracts on Aegilops geniculata metabolome. Phytochemistry 2014, 106, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, M.; Graziani, V.; Tsafantakis, N.; Esposito, A.; Fiorentino, A.; D’Abrosca, B. NMR-based metabolomics and bioassays to study phytotoxic extracts and putative phytotoxins from Mediterranean plant species. Phytochem. Anal. 2019, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Chaves, N.; Alías, J.; Sosa, T. Phytotoxicity of Cistus ladanifer L.: Role of allelopathy. Allelopath. J. 2016, 38, 113–131. [Google Scholar] [CrossRef]
- Rivas-Martínez, S. Memoria y Mapas de la Series de Vegetación de España.1:400.000; ICONA. Serie Técnica; MAPA: Madrid, Spain, 1987. [Google Scholar]
- Plan Forestal de Extremadura. Análisis y Estudio del Paisaje Vegetal y su Dinámica en la región de Extremadura. Available online: http://extremambiente.juntaex.es/index.php?option=com_content&view=article&id=3609&Itemid=307 (accessed on 8 November 2023).
- Chaves, N.; Ríos, J.J.; Gutierrez, C.; Escudero, J.C.; Olías, J.M. Analysis of secreted flavonoids of Cistus ladanifer L. by high-performance liquid chromatography–particle beam mass spectrometry. J. Chromatogr. A 1998, 799, 111–115. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Einhellig, F.A.; Galindo, J.C.G.; Molinillo, J.M.G.; Cutler, H.G. Mode of allelochemical action of phenolic compounds. In Allelopathy: Chemistry and Mode of Action of Allelochemicals; CRC Press: Boca Raton, FL, USA, 2004; pp. 217–238. [Google Scholar]
- Barrajõn-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sendra, E.; Alvarez, J.A.P.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of flavonoid content and chemical composition of the essential oils of moroccan herbs: Myrtle (Myrtus communis L.), rockrose (Cistus ladanifer L.) and montpellier cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Alves-Ferreira, J.; Miranda, I.; Duarte, L.C.; Roseiro, L.B.; Lourenço, A.; Quilhó, T.; Cardoso, S.; Fernandes, M.C.; Carvalheiro, F.; Pereira, H. Cistus ladanifer as a source of chemicals: Structural and chemical characterization. Biomass Convers. Biorefinery 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Carev, I.; Maravić, A.; Ilić, N.; Čulić, V.Č.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS phytochemical analysis of two Croatian Cistus species and their biological activity. Life 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef] [PubMed]
- Ben Jemia, M.; Kchouk, M.E.; Senatore, F.; Autore, G.; Marzocco, S.; De Feo, V.; Bruno, M. Antiproliferative activity of hexane extract from Tunisian Cistus libanotis, Cistus monspeliensis and Cistus villosus. Chem. Cent. J. 2013, 7, 47. [Google Scholar] [CrossRef]
- Salomé-Abarca, L.F.; Mandrone, M.; Sanna, C.; Poli, F.; van der Hondel, C.A.M.J.J.; Klinkhamer, P.G.L.; Choi, Y.H. Metabolic variation in Cistus monspeliensis L. ecotypes correlated to their plant-fungal interactions. Phytochemistry 2020, 176, 112402. [Google Scholar] [CrossRef]
- Tahiri, O.; Atmani-Kilani, D.; Sanchez-Fidalgo, S.; Aparicio-Soto, M.; Alarcón-de-la-Lastra, C.; Barrajón-Catalán, E.; Micol, V.; Atmani, D. The flavonol-enriched Cistus albidus chloroform extract possesses in vivo anti-inflammatory and anti-nociceptive activity. J. Ethnopharmacol. 2017, 209, 210–218. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- Pereira, O.R.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Identification of phenolic constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef]
- Pereira, O.R.; Macias, R.I.R.; Perez, M.J.; Marin, J.J.G.; Cardoso, S.M. Protective effects of phenolic constituents from Cytisus multiflorus, Lamium album L. and Thymus citriodorus on liver cells. J. Funct. Foods 2013, 5, 1170–1179. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Pereira, E.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokovic, M.; Simal-Gandara, J.; Prieto, M.A.; et al. From Tradition to Health: Chemical and Bioactive Characterization of Five Traditional Plants. Molecules 2022, 27, 6495. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Ribeiro, D.; Fernandes, E.; Nogueira, D.R.; Conde, E.; Moure, A.; Vinardell, M.P.; Mitjans, M.; Domínguez, H. Potential use of Cytisus scoparius extracts in topical applications for skin protection against oxidative damage. J. Photochem. Photobiol. B Biol. 2013, 125, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Otero, A.; Conde, E.; Falqué, E.; Moure, A.; Domínguez, H. Extraction of phenolics from broom branches using green technologies. J. Chem. Technol. Biotechnol. 2017, 92, 1345–1352. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; Van Wamel, W.J.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef]
- Bekkai, D.; El Majdoub, Y.O.; Bekkai, H.; Cacciola, F.; Miceli, N.; Taviano, M.F.; Cavò, E.; Errabii, T.; Vinci, R.L.; Mondello, L. Determination of the phenolic profile by liquid chromatography, evaluation of antioxidant activity and toxicity of moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules 2022, 27, 3979. [Google Scholar] [CrossRef]
- Márquez-García, B.; Fernández, M.A.; Córdoba, F. Phenolics composition in Erica sp. differentially exposed to metal pollution in the Iberian Southwestern Pyritic Belt. Bioresour. Technol. 2009, 100, 446–451. [Google Scholar] [CrossRef]
- Caleja, C.; Finimundy, T.C.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Ivanov, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951. [Google Scholar] [CrossRef]
- Yangui, I.; Younsi, F.; Ghali, W.; Boussaid, M.; Messaoud, C. Phytochemicals, antioxidant and anti-proliferative activities of Myrtus communis L. genotypes from Tunisia. S. Afr. J. Bot. 2021, 137, 35–45. [Google Scholar] [CrossRef]
- Amel, Z.; Nabila, B.B.; Nacéra, G.; Fethi, T.; Fawzia, A.B. Assessment of phytochemical composition and antioxidant properties of extracts from the leaf, stem, fruit and root of Pistacia lentiscus L. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 627–633. [Google Scholar]
- Yemmen, M.; Landolsi, A.; Ben Hamida, J.; Mégraud, F.; Trabelsi Ayadi, M. Antioxidant activities, anticancer activity and polyphenolics profile, of leaf, fruit and stem extracts of Pistacia lentiscus from Tunisia. Cell. Mol. Biol. 2017, 63, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sehaki, C.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. A Review of Pistacia lentiscus Polyphenols: Chemical Diversity and Pharmacological Activities. Plants 2023, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al Juhaimi, F.; Uslu, N.; Ahmed, I.A.M.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; Alqah, H.A.S.; Ghafoor, K. Effect of sonication process of terebinth (Pistacia terebinthus L.) fruits on antioxidant activity, phenolic compounds, fatty acids and tocopherol contents. J. Food Sci. Technol. 2020, 57, 2017–2025. [Google Scholar] [CrossRef]
- Uysal, S.; Sinan, K.I.; Jekő, J.; Cziáky, Z.; Zengin, G. Chemical characterization, comprehensive antioxidant capacity, and enzyme inhibitory potential of leaves from Pistacia terebinthus L. (Anacardiaceae). Food Biosci. 2022, 48, 101820. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Falade, A.O.; Omolaiye, G.I.; Adewole, K.E.; Agunloye, O.M.; Ishola, A.A.; Okaiyeto, K.; Oboh, G.; Oguntibeju, O.O. Aqueous Extracts of Bay Leaf (Laurus nobilis) and Rosemary (Rosmarinus officinalis) Inhibit Iron-Induced Lipid Peroxidation and Key-Enzymes Implicated in Alzheimer’s Disease in Rat Brain-in Vitro. Am. J. Biochem. Biotechnol. 2022, 18, 9–22. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Congiu, F.; Cencetti, G.; Raio, A.; Menicucci, F.; Mulinacci, N.; Michelozzi, M. Within-plant variation in Rosmarinus officinalis L. Terpenes and phenols and their antimicrobial activity against the rosemary phytopathogens Alternaria alternata and Pseudomonas viridiflava. Molecules 2021, 26, 3425. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. An overview of the chemistry and anticancer properties of rosemary extract and its diterpenes. J. HerbMed Pharmacol. 2022, 11, 10–19. [Google Scholar] [CrossRef]
- Shaymaa, M.H.; Adnan, M.M.; Muthanna, J.M. The Effect of Extracts and Phenolic Compounds Isolation from Rosmarinus officinalis Plant Leaves on Tribolium castaneum Mortality. Int. J. Drug Deliv. Technol. 2022, 12, 814–819. [Google Scholar] [CrossRef]
- Takayama, K.S.; Monteiro, M.C.; Saito, P.; Pinto, I.C.; Nakano, C.T.; Martinez, R.M.; Thomaz, D.V.; Verri, W.A.; Baracat, M.M.; Arakawa, N.S. Rosmarinus officinalis extract-loaded emulgel prevents UVB irradiation damage to the skin. Anais Acad. Brasil. Ciências 2022, 94, e20201058. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Sokovic, M.; Ciric, A.; Koukoulitsa, C.; Bilia, A.R.; Skaltsa, H. Antimicrobial properties of Quercus ilex L. proanthocyanidin dimers and simple phenolics: Evaluation of their synergistic activity with conventional antimicrobials and prediction of their pharmacokinetic profile. J. Agric. Food Chem. 2011, 59, 6412–6422. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind. Crops Prod. 2013, 45, 114–120. [Google Scholar] [CrossRef]
- Hadidi, L.; Babou, L.; Zaidi, F.; Valentão, P.; Andrade, P.B.; Grosso, C. Quercus ilex L.: How season, Plant Organ and Extraction Procedure Can Influence Chemistry and Bioactivities. Chem. Biodivers. 2017, 14, e1600187. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus unedo L.(Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Castaldi, S.; D’Abrosca, B.; Natale, A.; Carfora, A.; Messere, A.; Monaco, P. Polyphenols from the hydroalcoholic extract of Arbutus unedo living in a monospecific Mediterranean woodland. Biochem. Syst. Ecol. 2007, 35, 809. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; Del Castillo, M.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Maleš, Ž.; Šarić, D.; Bojić, M. Quantitative determination of flavonoids and chlorogenic acid in the leaves of Arbutus unedo L. using thin layer chromatography. J. Anal. Methods Chem. 2013, 2013, 385473. [Google Scholar] [CrossRef]
- Kachkoul, R.; Housseini, T.S.; Mohim, M.; El Habbani, R.; Miyah, Y.; Lahrichi, A. Chemical compounds as well as antioxidant and litholytic activities of Arbutus unedo L. leaves against calcium oxalate stones. J. Integr. Med. 2019, 17, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; D’Urso, G.; Pagliuca, G.; Petretto, G.L.; Foddai, M.; Gallo, F.R.; Multari, G.; Caruso, D.; Montoro, P.; Pintore, G. HPTLC-PCA complementary to HRMS-PCA in the case study of Arbutus unedo antioxidant phenolic profiling. Foods 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- El Cadi, H.; El Cadi, A.; Kounnoun, A.; El Majdoub, Y.O.; Lovillo, M.P.; Brigui, J.; Dugo, P.; Mondello, L.; Cacciola, F. Wild strawberry (Arbutus unedo): Phytochemical screening and antioxidant properties of fruits collected in northern Morocco. Arab. J. Chem. 2020, 13, 6299–6311. [Google Scholar] [CrossRef]
- Zitouni, H.; Hssaini, L.; Messaoudi, Z.; Ourradi, H.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Hanine, H. Phytochemical components and bioactivity assessment among twelve strawberry (Arbutus unedo L.) genotypes growing in Morocco using chemometrics. Foods 2020, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.T.; Luís, Â.F.; Batista, M.T.; Ferreira, S.M.; Duarte, A.P.C. Phytochemical characterization, bioactivities evaluation and synergistic effect of Arbutus unedo and Crataegus monogyna extracts with Amphotericin B. Curr. Microbiol. 2020, 77, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, V.; Santarelli, V.; Carbone, K. Phytochemical profile, antiradical capacity and α-glucosidase inhibitory potential of wild Arbutus unedo L. Fruits from central italy: A chemometric approach. Plants 2020, 9, 1785. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; Casado, N.; Sierra, I. Study of the Phenolic Compound Profile of Arbutus unedo L. Fruits at Different Ripening Stages by HPLC-TQ-MS/MS. Appl. Sci. 2021, 11, 11616. [Google Scholar] [CrossRef]
- Martins, J.; Batista, T.; Pinto, G.; Canhoto, J. Seasonal variation of phenolic compounds in Strawberry tree (Arbutus unedo L.) leaves and inhibitory potential on Phytophthora cinnamomi. Trees 2021, 35, 1571–1586. [Google Scholar] [CrossRef]
- Brčić Karačonji, I.; Jurica, K.; Gašić, U.; Dramićanin, A.; Tešić, Ž.; Milojković Opsenica, D. Comparative study on the phenolic fingerprint and antioxidant activity of strawberry tree (Arbutus unedo L.) leaves and fruits. Plants 2022, 11, 25. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Algieri, F.; Rodriguez-Nogales, A.; Gálvez, J.; Segura-Carretero, A. Phytochemical profiling of anti-inflammatory Lavandula extracts via RP–HPLC–DAD–QTOF–MS and –MS/MS: Assessment of their qualitative and quantitative differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Karan, T. Metabolic profile and biological activities of Lavandula stoechas L. Cell. Mol. Biol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef] [PubMed]
- Sriti, J.; Fares, N.; Msaada, K.; Zarroug, Y.; Boulares, M.; Djebbi, S.; Selmi, S.; Limam, F. Phenological stage effect on phenolic composition, antioxidant, and antibacterial activity of Lavandula stoechas extract. Riv. Ital. Sostanze Grasse 2022, 99, 225–234. [Google Scholar]
- Karabagias, I.K.; Karabagias, V.K.; Riganakos, K.A. Physico-Chemical Parameters, Phenolic Profile, In Vitro Antioxidant Activity and Volatile Compounds of Ladastacho (Lavandula stoechas) from the Region of Saidona. Antioxidants 2019, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; Mozos-Pascual, M.D.L.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crops Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Delgado, T.; Marinero, P.; Asensio-S.-Manzanera, M.C.; Asensio, C.; Herrero, B.; Pereira, J.A.; Ramalhosa, E. Antioxidant activity of twenty wild Spanish Thymus mastichina L. populations and its relation with their chemical composition. LWT Food Sci. Technol. 2014, 57, 412–418. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Sponza, S.; Asensio-S-Manzanera, M.C.; Novak, J. Contribution of the main polyphenols of Thymus mastichina subsp: Mastichina to its antioxidant properties. Ind. Crops Prod. 2015, 66, 291–298. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al-Raqmi, K.A.S.; Al-Mijizy, Z.H.; Weli, A.M.; Al-Riyami, Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed. 2013, 3, 705–710. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). J. Agric. Food Chem. 2012, 60, 10920–10929. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Kogiannou, D.A.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of Greek herbal infusions; Balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Antonio, A.L.; Cabo Verde, S.; Santos-Buelga, C.; Ferreira, I.C.F.R. Infusions from Thymus vulgaris L. treated at different gamma radiation doses: Effects on antioxidant activity and phenolic composition. LWT 2016, 74, 34–39. [Google Scholar] [CrossRef]
- Pereira, E.; Pimenta, A.I.; Barros, L.; Calhelha, R.C.; Antonio, A.L.; Cabo Verde, S.; Ferreira, I.C.F.R. Effects of gamma radiation on the bioactivity of medicinal and aromatic plants: Mentha × piperita L., Thymus vulgaris L. and Aloysia citrodora Paláu as case studies. Food Funct. 2018, 9, 5150–5161. [Google Scholar] [CrossRef] [PubMed]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of bioactive and volatile profiles of thyme (Thymus vulgaris L.) teas as affected by infusion times. J. Food Meas. Charact. 2018, 12, 2570–2580. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, C. Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind. Crops Prod. 2020, 151, 112448. [Google Scholar] [CrossRef]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef]
- Mokhtari, R.; Kazemi Fard, M.; Rezaei, M.; Moftakharzadeh, S.A.; Mohseni, A. Antioxidant, Antimicrobial Activities, and Characterization of Phenolic Compounds of Thyme (Thymus vulgarisL.), Sage (Salvia officinalis L.), and Thyme–Sage Mixture Extracts. J. Food Qual. 2023, 2602454. [Google Scholar] [CrossRef]
- Martini, S.; D’Addario, C.; Colacevich, A.; Focardi, S.; Borghini, F.; Santucci, A.; Figura, N.; Rossi, C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents 2009, 34, 50–59. [Google Scholar] [CrossRef]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, B.M.; Sánchez-Moreno, C.; Ancos, B.D.; Sánchez-Mata, M.D.; Fernández-Ruíz, V.; Cámara, M.; Tardío, J. Wild Arbutus unedo L. and Rubus ulmifolius Schott fruits are underutilized sources of valuable bioactive compounds with antioxidant capacity. Fruits 2014, 69, 435–448. [Google Scholar] [CrossRef]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, S.; Aouadhi, C.; Mechergui, K.; Hammi, K.M.; Ksouri, R.; Raies, A.; Toumi, L. Comparison of Phytochemical Composition and Biological Activities of Rubus ulmifolius Extracts Originating from Four Regions of Tunisia. Chem. Biodivers. 2017, 14, e1600168. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Pereira, E.; Pires, T.C.S.P.; Alves, M.J.; Pereira, O.R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott fruits: A detailed study of its nutritional, chemical and bioactive properties. Food Res. Int. 2019, 119, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Seraglio, S.K.T.; Della Betta, F.; Nehring, P.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int. 2019, 122, 627–634. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Nicácio, A.E.; Boeing, J.S.; Garcia, F.P.; Nakamura, C.V.; Visentainer, J.V.; Maldaner, L. Rapid extraction method followed by a d-SPE clean-up step for determination of phenolic composition and antioxidant and antiproliferative activities from berry fruits. Food Chem. 2020, 309, 125694. [Google Scholar] [CrossRef]
- Candela, R.G.; Lazzara, G.; Piacente, S.; Bruno, M.; Cavallaro, G.; Badalamenti, N. Conversion of organic dyes into pigments: Extraction of flavonoids from blackberries (Rubus ulmifolius) and stabilization. Molecules 2021, 26, 6278. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; F Nabavi, S.; S Talas, Z.; M Nabavi, S. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm.Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Transferrin-functionalized liposomes for the delivery of gallic acid: A therapeutic approach for Alzheimer’s disease. Pharmaceutics 2022, 14, 2163. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Mirshekar, M.A.; Sarkaki, A.; Farbood, Y.; Gharib Naseri, M.K.; Badavi, M.; Mansouri, M.T.; Haghparast, A. Neuroprotective effects of gallic acid in a rat model of traumatic brain injury: Behavioral, electrophysiological, and molecular studies. Iran. J. Basic Med. Sci. 2018, 21, 1056–1063. [Google Scholar] [PubMed]
- Maya, S.; Prakash, T.; Goli, D. Effect of wedelolactone and gallic acid on quinolinic acid-induced neurotoxicity and impaired motor function: Significance to sporadic amyotrophic lateral sclerosis. NeuroToxicology 2018, 68, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.-X.; Shan, J.-L.; Hu, W.-Q.; Zeng, J.-X.; Shu, J.-C. Gallic acid activates hippocampal BDNF-Akt-mTOR signaling in chronic mild stress. Metab. Brain Dis. 2019, 34, 93–101. [Google Scholar] [CrossRef]
- Diaz, A.; Muñoz-Arenas, G.; Caporal-Hernandez, K.; Vázquez-Roque, R.; Lopez-Lopez, G.; Kozina, A.; Espinosa, B.; Flores, G.; Treviño, S.; Guevara, J. Gallic acid improves recognition memory and decreases oxidative-inflammatory damage in the rat hippocampus with metabolic syndrome. Synapse 2021, 75, e22186. [Google Scholar] [CrossRef]
- Kim, M.-J.; Seong, A.-R.; Yoo, J.-Y.; Jin, C.-H.; Lee, Y.-H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.-G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808. [Google Scholar] [CrossRef]
- Maya, S.; Prakash, T.; Goli, D. Evaluation of neuroprotective effects of wedelolactone and gallic acid on aluminium-induced neurodegeneration: Relevance to sporadic amyotrophic lateral sclerosis. Eur. J. Pharmacol. 2018, 835, 41–51. [Google Scholar] [CrossRef]
- Ogunlade, B.; Adelakun, S.A.; Agie, J.A. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem. Toxicol. 2022, 45, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Jabeen, S.; Imran, I.; Zulfiqar, I.; Bilal, K. Protective effect of gallic acid against arsenic-induced anxiety−/depression-like behaviors and memory impairment in male rats. Metab. Brain Dis. 2019, 34, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic acid and mental diseases: From chemistry to medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Kaur, S.; Dhiman, M.; Mantha, A.K. Ferulic Acid: A Natural Antioxidant with Application towards Neuroprotection against Alzheimer’s Disease. In Functional Food and Human Health; Rani, V., Yadav, U.C.S., Eds.; Springer: Singapore, 2018; pp. 575–586. [Google Scholar]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. Water-soluble ferulic acid derivatives improve amyloid-β-induced neuronal cell death and dysmnesia through inhibition of amyloid-β aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553. [Google Scholar] [CrossRef]
- Wang, E.-J.; Wu, M.-Y.; Lu, J.-H. Ferulic Acid in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Cells 2021, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-J.; Cho, J.-Y.; Kim, H.-S.; Kim, K.-L.; Jung, J.-S.; Huh, S.-O.; Suh, H.-W.; Kim, Y.-H.; Song, D.-K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Kim, H.-S.; Kim, D.-H.; Yan, J.-J.; Suh, H.-W.; Song, D.-K. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-Y.; Li, J.-N.; Liu, W.-L.; Huang, Q.; Li, W.-X.; Tan, Y.-H.; Liu, F.; Song, Z.-H.; Wang, M.-Y.; Xie, N.; et al. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef] [PubMed]
- AAlikhani, M.; Khalili, M.; Jahanshahi, M. The natural iron chelators’ ferulic acid and caffeic acid rescue mice’s brains from side effects of iron overload. Front. Neurol. 2022, 13, 951725. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 2016, 131, 142–154. [Google Scholar] [CrossRef]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-Derived Neurotropic Factor in Neurodegenerative Disorders. Biomedicines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective effect of caffeic acid against Alzheimer’s disease pathogenesis via modulating cerebral insulin signaling, β-amyloid accumulation, and synaptic plasticity in hyperinsulinemic rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K.S.; Saify, Z.S. Gallic and vanillic acid suppress inflammation and promote myelination in an in vitro mouse model of neurodegeneration. Mol. Biol. Rep. 2019, 46, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. BioMed Res. Int. 2015, 814068. [Google Scholar] [CrossRef] [PubMed]
- Sakamula, R.; Thong-asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Youn, K.; Ho, C.-T.; Karwe, M.V.; Jeong, W.-S.; Jun, M. p-Coumaric Acid and Ursolic Acid from Corni fructus Attenuated β-Amyloid25–35-Induced Toxicity through Regulation of the NF-κB Signaling Pathway in PC12 Cells. J. Agric. Food Chem. 2014, 62, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-R.; Kim, M.-J.; Choi, E.-J.; Kim, Y.; Lee, H.-S.; Bae, D.; Choi, C. Protective Effects of p-Coumaric Acid Isolated from Vaccinium bracteatum Thunb. Leaf Extract on Corticosterone-Induced Neurotoxicity in SH-SY5Y Cells and Primary Rat Cortical Neurons. Processes 2021, 9, 869. [Google Scholar] [CrossRef]
- Daroi, P.A.; Dhage, S.N.; Juvekar, A.R. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. J. Pharm. Pharmacol. 2021, 74, 556–564. [Google Scholar] [CrossRef]
- Rashno, M.; Gholipour, P.; Salehi, I.; Komaki, A.; Rashidi, K.; Esmaeil Khoshnam, S.; Ghaderi, S. p-Coumaric acid mitigates passive avoidance memory and hippocampal synaptic plasticity impairments in aluminum chloride-induced Alzheimer’s disease rat model. J. Funct. Foods 2022, 94, 105117. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Tsoi, B.; Qi, S.; Gu, B.; Wang, Z.; Peng, C.; Shen, J. Alpinia oxyphylla Miq. and Its Active Compound P-Coumaric Acid Promote Brain-Derived Neurotrophic Factor Signaling for Inducing Hippocampal Neurogenesis and Improving Post-cerebral Ischemic Spatial Cognitive Functions. Front. Cell Develop. Biol. 2021, 8, 577790. [Google Scholar] [CrossRef]
- Mughal, E.U.; Sadiq, A.; Ashraf, J.; Zafar, M.N.; Sumrra, S.H.; Tariq, R.; Javed, C.O. Flavonols and 4-thioflavonols as potential acetylcholinesterase and butyrylcholinesterase inhibitors: Synthesis, structure-activity relationship and molecular docking studies. Bioorg. Chem. 2019, 91, 103124. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; dos Santos, C.N. Polyphenols beyond barriers: A glimpse into the brain. Curr. Neuropharmacol.. 2017, 15, 562–594. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Martinoli, M.-G. Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Ishisaka, A.; Ichikawa, S.; Sakakibara, H.; Piskula, M.K.; Nakamura, T.; Kato, Y.; Ito, M.; Miyamoto, K.-I.; Tsuji, A.; Kawai, Y. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Rad. Biol. Med. 2011, 51, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Abin-Carriquiry, J.A.; Arredondo, F.; Blasina, F.; Echeverry, C.; Martínez, M.; Rivera, F.; Vaamonde, L. Quercetin in brain diseases: Potential and limits. Neurochem. Int. 2015, 89, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhuang, J.; Li, Y.; Shen, Y.; Zheng, X. Myricitrin protects against peroxynitrite-mediated DNA damage and cytotoxicity in astrocytes. Food Chem. 2013, 141, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; DOnofrio, G.; Fazel Nabavi, S.; Daglia, M.; Rastrelli, L.; Mohammad Nabavi, S. Neuroprotective effects of quercetin: From chemistry to medicine. CNS Neurol. Disord. Drug Targets 2016, 15, 964–975. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Azib, L.; Debbache-Benaida, N.; Costa, G.D.; Atmani-Kilani, D.; Saidene, N.; Ayouni, K.; Richard, T.; Atmani, D. Pistacia lentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind. Crops Prod. 2019, 137, 576–584. [Google Scholar] [CrossRef]
- Moneim, A.E.A. Antioxidant activities of Punica granatum (pomegranate) peel extract on brain of rats. J. Med. Plants Res. 2012, 6, 195–199. [Google Scholar]
- Martín, S.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem. 2011, 128, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Munoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacol. 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Nunes, M.A.G.; Rubin, M.A.; da Cruz, I.B. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+, K+-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H.; Orhan, I.; Şenol, F.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Biol. Interact. 2009, 181, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Cuong, T.D.; Lee, J.-S.; Shin, B.-S.; Woo, M.H.; Hung, T.M. Cholinesterase inhibitors from Cleistocalyx operculatus buds. Arch. Pharmacal Res. 2010, 33, 1665–1670. [Google Scholar] [CrossRef]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorji, N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med. 2019, 85, 1326–1350. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial effects of green tea catechins on neurodegenerative diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Ribeiro, G.A.C.; da Rocha, C.Q.; Veloso, W.B.; Fernandes, R.N.; da Silva, I.S.; Tanaka, A.A. Determination of the catechin contents of bioactive plant extracts using disposable screen-printed carbon electrodes in a batch injection analysis (BIA) system. Microchem. J. 2019, 146, 1249–1254. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abbasabadi, Z.; Braidy, N.; Nabavi, S.M. Role of green tea catechins in prevention of age-related cognitive decline: Pharmacological targets and clinical perspective. J. Cell. Physiol. 2019, 234, 2447–2459. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Yazdi, H.S.; Samarghandian, S. The protective effects of green tea catechins in the management of neurodegenerative diseases: A review. Curr. Drug Discov. Technol. 2019, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, S.P.; Ramalingayya, G.V.; Chamallamudi, M.R.; Biswas, S.; Nandakumar, K.; Nampoothiri, M.; Gourishetti, K.; Kumar, N. Catechin ameliorates doxorubicin-induced neuronal cytotoxicity in in vitro and episodic memory deficit in in vivo in Wistar rats. Cytotechnology 2018, 70, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of tea catechins on Alzheimer’s disease: Recent updates and perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.; Mandel, S. Green tea polyphenol (–)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Limón, D.I.; Pérez-Severiano, F.; Díaz, A.; Ortega, L.; Zenteno, E.; Guevara, J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur. J. Pharmacol. 2009, 616, 122–127. [Google Scholar] [CrossRef]
- Nan, S.; Wang, P.; Zhang, Y.; Fan, J. Epigallocatechin-3-Gallate Provides Protection Against Alzheimer’s Disease-Induced Learning and Memory Impairments in Rats. Drug Des. Devel. Ther. 2021, 13, 2013–2024. [Google Scholar] [CrossRef]
- Ali, A.A.; Abd El-Fattah, A.I.; Abu-Elfotuh, K.; Elariny, H.A. Natural antioxidants enhance the power of physical and mental activities versus risk factors inducing progression of Alzheimer’s disease in rats. Int. Immunopharmacol. 2021, 96, 107729. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Farah, A.; Zaroual, H.; El Ouali Lalami, A. Antimicrobial activity of Lavandula stoechas phenolic extracts against pathogenic bacteria isolated from a hospital in Morocco. Vegetos 2020, 33, 703–711. [Google Scholar] [CrossRef]
- Sharma, D.R.; Wani, W.Y.; Sunkaria, A.; Kandimalla, R.J.; Verma, D.; Cameotra, S.S.; Gill, K.D. Quercetin protects against chronic aluminum-induced oxidative stress and ensuing biochemical, cholinergic, and neurobehavioral impairments in rats. Neurotox. Res. 2013, 23, 336–357. [Google Scholar] [CrossRef]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Assemian, I.C.C.; Abrini, J.; Dakka, N. In vitro antiproliferative activity of selected medicinal plants from the North-West of Morocco on several cancer cell lines. Eur. J. Integr. Med. 2018, 18, 23–29. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Moussaoui, N.E.; Abrini, J.; Bakri, Y. Phenolic contents and antiradical capacity of vegetable oil from Pistacia lentiscus (L). J. Mater. Environ. Sci. 2018, 9, 1518–1524. [Google Scholar]
- Ghasemi Pirbalouti, A.; Emami Bistghani, Z.; Malekpoor, F. An overview on genus Thymus. J. Med. Herb 2015, 6, 93–100. [Google Scholar]



| N° Compound | Percentage (%) | |
|---|---|---|
| Class: Phenolic acid | 144 | 35.72% |
| Sub Class: Hydroxybenzoic acids | 82 | 20.34% |
| Sub Class: Hydroxycinnamic acids | 59 | 14.64% |
| Sub Class: Hydroxyphenylacetic acids | 3 | 0.74% |
| Class: Flavonoid | 224 | 55.56% |
| Sub Class: Flavanones | 13 | 3.22% |
| Sub Class: Flavones | 59 | 14.64% |
| Sub Class: Flavonols | 103 | 25.55% |
| Sub Class: Flavanols | 28 | 6.94% |
| Sub Class: Anthocyanins | 16 | 3.97% |
| Sub Class: Isoflavonoids | 5 | 1.24% |
| Class: Other polyphenols | 23 | 5.69% |
| Sub Class: Hydroxybenzaldehydes | 4 | 0.99% |
| Sub Class: Hydroxycoumarins | 3 | 0.74% |
| Sub Class: Tyrosols | 1 | 0.24% |
| Sub Class: Other polyphenols | 15 | 3.72% |
| Class: Lignans. Sub Class: Lignans | 9 | 2.23% |
| Class: Stilbenes. Sub Class: Stilbenes | 3 | 0.74% |
| Phenolic Compound | Species in Which It Appears | Percentage (%) |
|---|---|---|
| Class: Phenolic acid. Sub Class: Hydroxybenzoic acids | ||
| 4-hydroxybenzoic acid | 7 | 29.16% |
| Ellagic acid | 8 | 33.33% |
| Gallic acid (3,4,5-trihydroxybenzoic acid) | 17 | 70.83% |
| Hexahydroxydiphenoyl-glucose | 6 | 25.00% |
| Protocatechuic acid (3,4-dihydroxy-benzoic acid) | 9 | 37.50% |
| Syringic acid | 8 | 33.33% |
| Vanillic acid | 10 | 41.66% |
| Class: Phenolic acid. Sub Class: Hydroxycinnamic acids | ||
| Caffeic acid (3,4-dihydroxycinnamic acid) | 12 | 50.00% |
| Chlorogenic acid (3-O-caffeoylquinic acid) | 13 | 54.16% |
| Cinnamic acid | 7 | 29.16% |
| p-Coumaric acid | 10 | 41.66% |
| Coumaroyl quinic acid | 5 | 20.83% |
| Ferulic Acid | 12 | 50.00% |
| Rosmarinic acid | 5 | 20.83% |
| Methylrosmaric acid | 6 | 25.00% |
| Class: Flavonoid. Sub Class: Flavanones | ||
| Hesperetin 7-O-rutinoside (Hesperidin) | 5 | 20.83% |
| Naringenin | 8 | 33.33% |
| Naringenin 7-O-glucoside (Naringin-Prunina) | 9 | 37.50% |
| Class: Flavonoid. Sub Class: Flavones | ||
| Apigenin | 11 | 45.83% |
| Apigenin 7-O-glucoside | 6 | 25.00% |
| Luteolin (3′.4′.5.7-Tetrahydroxyflavone) | 9 | 37.50% |
| Luteolin 7-O-glucoside | 5 | 20.83% |
| Luteolin-7-O-rutinoside | 6 | 25.00% |
| Class: Flavonoid. Sub Class: Flavonols | ||
| Kaempferol | 13 | 54.16% |
| Kaempferol O-hexoside | 5 | 20.83% |
| Myricetin (3.3′.4′.5.5′.7-Hexahydroxyflavone) | 9 | 37.50% |
| Myricetin 3-O-hexoside | 6 | 25.00% |
| Myricetin-O-rhamnoside (Myricitrin) | 12 | 50.00% |
| Myricetin-O-rutinoside | 5 | 20.83% |
| Quercetin (3.3′.4′.5.7-Pentahydroxyflavone) | 15 | 62.50% |
| Quercetin 3-O-glucoside (Isoquercetin) | 16 | 66.66% |
| Quercetin-O-hexoside | 8 | 33.33% |
| Quercetin-O-rhamnoside (Quercetrin) | 11 | 45.83% |
| Quercetin 3-O-rutinoside (Rutin) | 16 | 66.66% |
| Class: Flavonoid. Sub Class: Flavanols | ||
| Catechin | 11 | 45.83% |
| Epicatechin | 10 | 41.66% |
| Epicatechin gallate | 5 | 20.83% |
| Class: Flavonoid. Sub Class: Anthocyanins | ||
| Cyanidin 3-O-glucoside | 6 | 25.00% |
| Phenolic Compounds | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | VA | CA | CHA | p-CA | FA | Ap | K | MOR | Q | QOG | QOR | QORU | Ca | Epi | |
| C. ladanifer | + | + | + | + | + | + | + | + | + | + | |||||
| C. salviifolius | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| C. monspeliensis | + | + | + | + | + | + | + | ||||||||
| C. crispus | + | + | + | ||||||||||||
| C. albidus | + | + | + | + | + | + | + | + | |||||||
| C. populifolius | + | + | |||||||||||||
| C. multiflorus | + | + | + | + | + | + | + | + | + | + | |||||
| C. scoparius | + | + | + | + | + | + | + | + | |||||||
| C. striatus | + | ||||||||||||||
| E. multiflora | + | + | + | + | |||||||||||
| E. scoparia | + | + | |||||||||||||
| E. australis | + | + | + | + | + | + | + | + | + | ||||||
| C. vulgaris | + | + | + | ||||||||||||
| M. communis | + | + | + | ||||||||||||
| P. lentiscus | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| P. terebinthus | + | + | + | + | + | + | + | + | + | + | |||||
| R.officinalis | + | + | + | + | + | + | + | + | + | ||||||
| Q. ilex | + | + | + | + | + | + | + | + | + | + | |||||
| Q. suber | + | + | + | + | |||||||||||
| A. unedo | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| L. stoechas | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| T. mastichina | + | + | + | + | + | + | |||||||||
| T. vulgaris | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| R. ulmifolius | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, N.; Nogales, L.; Montero-Fernández, I.; Blanco-Salas, J.; Alías, J.C. Mediterranean Shrub Species as a Source of Biomolecules against Neurodegenerative Diseases. Molecules 2023, 28, 8133. https://doi.org/10.3390/molecules28248133
Chaves N, Nogales L, Montero-Fernández I, Blanco-Salas J, Alías JC. Mediterranean Shrub Species as a Source of Biomolecules against Neurodegenerative Diseases. Molecules. 2023; 28(24):8133. https://doi.org/10.3390/molecules28248133
Chicago/Turabian StyleChaves, Natividad, Laura Nogales, Ismael Montero-Fernández, José Blanco-Salas, and Juan Carlos Alías. 2023. "Mediterranean Shrub Species as a Source of Biomolecules against Neurodegenerative Diseases" Molecules 28, no. 24: 8133. https://doi.org/10.3390/molecules28248133
APA StyleChaves, N., Nogales, L., Montero-Fernández, I., Blanco-Salas, J., & Alías, J. C. (2023). Mediterranean Shrub Species as a Source of Biomolecules against Neurodegenerative Diseases. Molecules, 28(24), 8133. https://doi.org/10.3390/molecules28248133