Multidose Hyaluronidase Administration as an Optimal Procedure to Degrade Resilient Hyaluronic Acid Soft Tissue Fillers
Abstract
:1. Introduction
2. Results
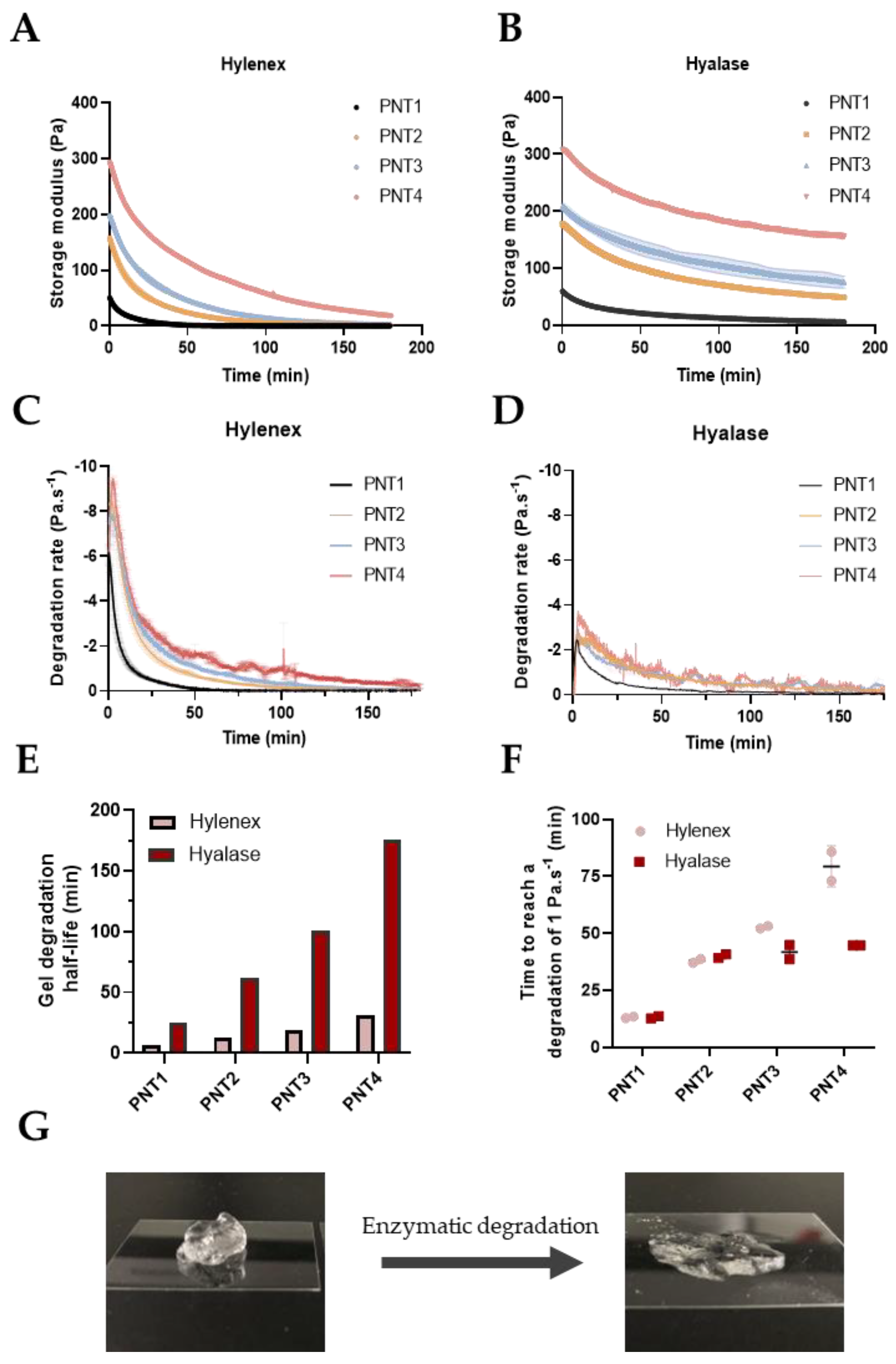
2.1. Development of the Degradation Protocol Assessed by Rheological Time Sweeps
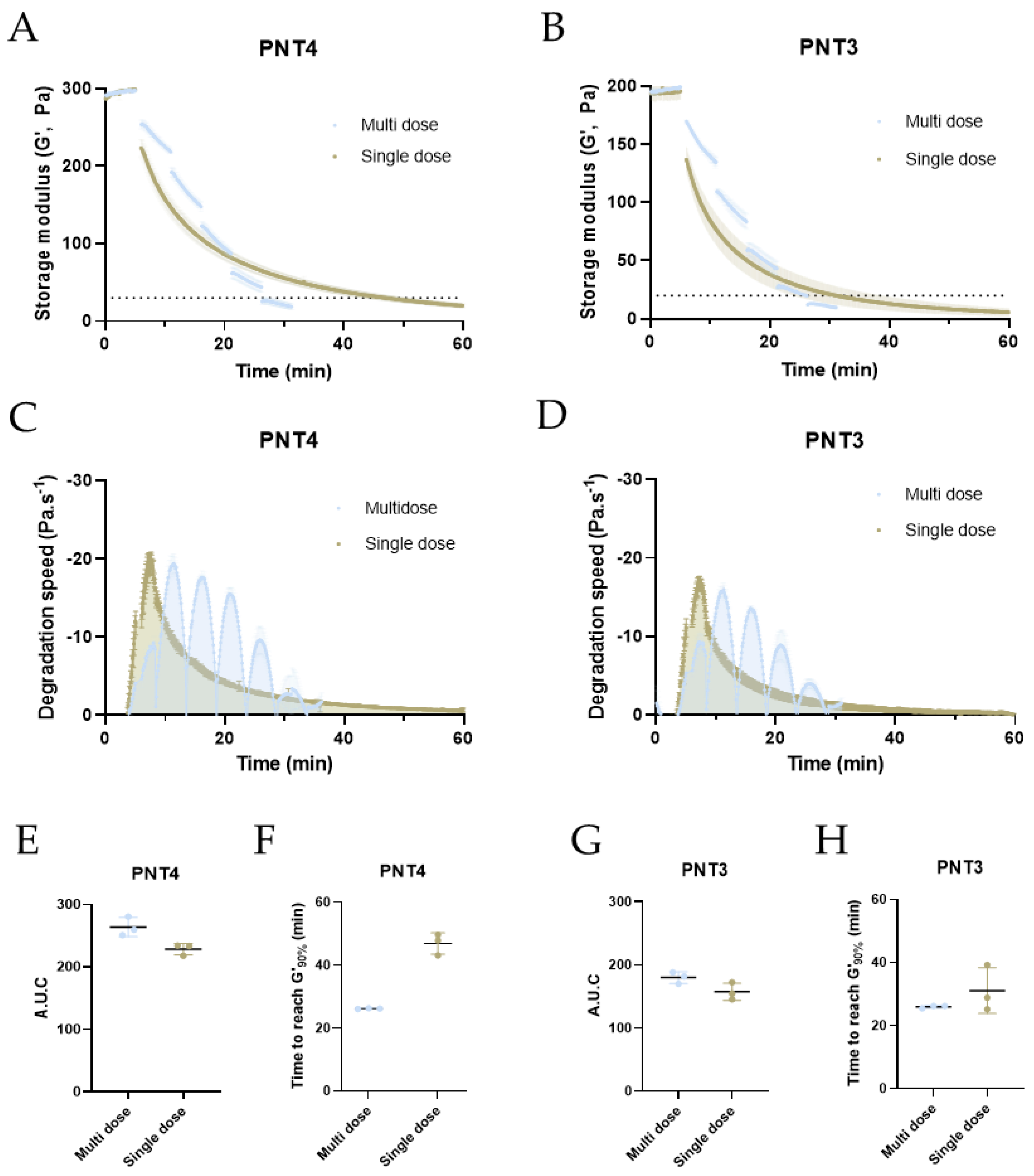
2.2. Degradation of PNT4 Gels with Single, Double, or Triple Doses of Hylenex and Hyalase Enzymes
2.3. Refining Multidose Parameters: Injection Spans and Enzymatic Units
2.4. Optimized Degradation of PNT Gels Using a Multidose Approach
3. Materials and Methods
3.1. Materials
3.2. Single Dose Degradation Kinetics of PNT Gels Measured by Rheology
3.3. Determination of Half-Lives and Gel Degradation G’090%
3.4. Single versus Multi-Dose Degradation Kinetics of PNT4 Gels Measured by Rheology
3.5. Refinment Study of Multi-Dose Degradation Kinetics of PNT4 Gels Measured by Rheology
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Beasley, K.L.; Weiss, M.A.; Weiss, R.A. Hyaluronic acid fillers: A comprehensive review. Facial Plast. Surg. 2009, 25, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Laurent, U.B.G.; Reed, R.K. Turnover of hyaluronan in the tissues. Adv. Drug Deliv. Rev. 1991, 7, 237–256. [Google Scholar] [CrossRef]
- Zhong, S.P.; Campoccia, D.; Doherty, P.J.; Williams, R.L.; Benedetti, L.; Williams, D.F. Biodegradation of hyaluronic acid derivatives by hyaluronidase. Biomaterials 1994, 15, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.H. International standard for hyaluronidase. Bull. World Health Organ. 1957, 16, 291–294. [Google Scholar] [PubMed]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Soltes, L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Sall, I.; Férard, G. Comparison of the sensitivity of 11 crosslinked hyaluronic acid gels to bovine testis hyaluronidase. Polym. Degrad. Stab. 2007, 92, 915–919. [Google Scholar] [CrossRef]
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking hyaluronic acid soft-tissue fillers: Current status and perspectives from an industrial point of view. Expert Rev. Med. Devices 2021, 18, 1175–1187. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [Green Version]
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced Concepts in Rheology for the Evaluation of Hyaluronic Acid-Based Soft Tissue Fillers. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2021, 47, e159–e167. [Google Scholar] [CrossRef]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, T.C.; Thompson, D.H.; Hyun, S.H. Molecular weight analyses and enzymatic degradation profiles of the soft-tissue fillers Belotero Balance, Restylane, and Juvéderm Ultra. Plast. Reconstr. Surg. 2013, 132, 22s–32s. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Tezel, A.; Borrell, M. In Vitro Resistance to Degradation of Hyaluronic Acid Dermal Fillers by Ovine Testicular Hyaluronidase. Dermatol. Surg. 2010, 36, 804–809. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Convery, C.; Davies, E. This month’s guideline: The Use of Hyaluronidase in Aesthetic Practice (v2.4). J. Clin. Aesthet. Dermatol. 2018, 11, E61–E68. [Google Scholar]
- Pierre, A.; Levy, P.M. Hyaluronidase offers an efficacious treatment for inaesthetic hyaluronic acid overcorrection. J. Cosmet. Dermatol. 2007, 6, 159–162. [Google Scholar] [CrossRef]
- Soares, D.J. Bridging a Century-Old Problem: The Pathophysiology and Molecular Mechanisms of HA Filler-Induced Vascular Occlusion (FIVO)—Implications for Therapeutic Interventions. Molecules 2022, 27, 5398. [Google Scholar]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.H.; Fagien, S.; Rohrich, R.J. Changing role of hyaluronidase in plastic surgery. Plast. Reconstr. Surg. 2014, 133, 127e–132e. [Google Scholar] [CrossRef]
- Rzany, B.; Becker-Wegerich, P.; Bachmann, F.; Erdmann, R.; Wollina, U. Hyaluronidase in the correction of hyaluronic acid-based fillers: A review and a recommendation for use. J. Cosmet. Dermatol. 2009, 8, 317–323. [Google Scholar] [CrossRef] [Green Version]
- DeLorenzi, C. New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events. Aesthet. Surg. J. 2017, 37, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.; Convery, C.; Walker, L.; Davies, E. Guideline for the Management of Hyaluronic Acid Filler-induced Vascular Occlusion. J. Clin. Aesthet. Dermatol. 2021, 14, E61–E69. [Google Scholar] [PubMed]
- Ryu, C.; Lu, J.E.; Zhang-Nunes, S. Response of twelve different hyaluronic acid gels to varying doses of recombinant human hyaluronidase. J. Plast. Reconstr. Aesthetic Surg. 2020, 8, P881–P889. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Papagni, M.; Trocchi, G. Sensitivity of hyaluronic acid fillers to hyaluronidase: An in vitro analysis. J. Clin. Exp. 2020, 11, 517. [Google Scholar]
- Rao, V.; Chi, S.; Woodward, J. Reversing facial fillers: Interactions between hyaluronidase and commercially available hyaluronic-acid based fillers. J. Drugs Dermatol. 2014, 13, 1053–1056. [Google Scholar]
- La Gatta, A.; Salzillo, R.; Catalano, C.; D’Agostino, A.; Pirozzi, A.V.A.; De Rosa, M.; Schiraldi, C. Hyaluronan-based hydrogels as dermal fillers: The biophysical properties that translate into a “volumetric” effect. PLoS ONE 2019, 14, e0218287. [Google Scholar] [CrossRef] [Green Version]
- Buhren, B.A.; Schrumpf, H.; Bolke, E.; Kammers, K.; Gerber, P.A. Standardized in vitro analysis of the degradability of hyaluronic acid fillers by hyaluronidase. Eur. J. Med. Res. 2018, 23, 37. [Google Scholar] [CrossRef]
- Juhász, M.L.W.; Levin, M.K.; Marmur, E.S. The Kinetics of Reversible Hyaluronic Acid Filler Injection Treated With Hyaluronidase. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2017, 43, 841–847. [Google Scholar] [CrossRef]
- Zhang-Nunes, S.; Ryu, C.; Cahill, K.; Straka, D.; Nabavi, C.; Czyz, C.; Foster, J. Prospective in vivo evaluation of three different hyaluronic acid gels to varying doses of hyaluronidase with long-term follow-up. J. Plast. Reconstr. Aesthetic Surg. 2020, 74, P874–P880. [Google Scholar] [CrossRef]
- Shumate, G.T.; Chopra, R.; Jones, D.; Messina, D.J.; Hee, C.K. In Vivo Degradation of Crosslinked Hyaluronic Acid Fillers by Exogenous Hyaluronidases. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2018, 44, 1075–1083. [Google Scholar] [CrossRef]
- Paap, M.K.; Silkiss, R.Z. The interaction between hyaluronidase and hyaluronic acid gel fillers—A review of the literature and comparative analysis. Plast. Aesthetic Res. 2020, 7, 36. [Google Scholar] [CrossRef]
- DeLorenzi, C. Commentary on: Efficacy of Percutaneous Intraarterial Facial/Supratrochlear Arterial Hyaluronidase Injection for Treatment of Vascular Embolism Resulting From Hyaluronic Acid Filler Cosmetic Injection. Aesthet. Surg. J. 2022, 42, 656–659. [Google Scholar] [CrossRef]
- Monheit, G.; Kaufman-Janette, J.; Joseph, J.H.; Shamban, A.; Dover, J.S.; Smith, S. Efficacy and Safety of Two Resilient Hyaluronic Acid Fillers in the Treatment of Moderate-to-Severe Nasolabial Folds: A 64-Week, Prospective, Multicenter, Controlled, Randomized, Double-Blinded, and Within-Subject Study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2020, 46, 1521–1529. [Google Scholar] [CrossRef]
- Kaufman-Janette, J.; Taylor, S.C.; Cox, S.E.; Weinkle, S.H.; Smith, S.; Kinney, B.M. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: A 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J. Cosmet. Dermatol. 2019, 18, 1244–1253. [Google Scholar] [CrossRef]
- Rzany, B.; Converset-Viethel, S.; Hartmann, M.; Larrouy, J.C.; Ribe, N.; Sito, G.; Noize-Pin, C. Efficacy and Safety of 3 New Resilient Hyaluronic Acid Fillers, Crosslinked With Decreased BDDE, for the Treatment of Dynamic Wrinkles: Results of an 18-Month, Randomized Controlled Trial Versus Already Available Comparators. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2019, 45, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, H.; Shamban, A.; Schlessinger, J.; Kaufman-Janette, J.; Joseph, J.H.; Lupin, M.; Draelos, Z.; Carey, W.; Smith, S.; Eaton, L. Efficacy and Safety of a New Resilient Hyaluronic Acid Filler in the Correction of Moderate-to-Severe Dynamic Perioral Rhytides: A 52-Week Prospective, Multicenter, Controlled, Randomized, Evaluator-Blinded Study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2021, 48, 87–93. [Google Scholar] [CrossRef]
- Ryu, H.-J.; Kwak, S.-S.; Rhee, C.-H.; Yang, G.-H.; Yun, H.-y.; Kang, W.-h. Model-Based Prediction to Evaluate Residence Time of Hyaluronic Acid Based Dermal Fillers. Pharmaceutics 2021, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Muckenschnabel, I.; Bernhardt, G.; Spruss, T.; Buschauer, A. Pharmacokinetics and tissue distribution of bovine testicular hyaluronidase and vinblastine in mice: An attempt to optimize the mode of adjuvant hyaluronidase administration in cancer chemotherapy. Cancer Lett. 1998, 131, 71–84. [Google Scholar] [CrossRef]
- Lee, W.; Oh, W.; Oh, S.M.; Yang, E.J. Comparative Effectiveness of Different Interventions of Perivascular Hyaluronidase. Plast. Reconstr. Surg. 2020, 145, 957–964. [Google Scholar] [CrossRef]
- DeLorenzi, C. Transarterial Degradation of Hyaluronic Acid Filler by Hyaluronidase. Dermatol. Surg. 2014, 40, 832–841. [Google Scholar] [CrossRef]
- Skippen, B.; Baldelli, I.; Hartstein, M.; Casabona, G.; Montes, J.R.; Bernardini, F. Rehabilitation of the Dysmorphic Lower Eyelid From Hyaluronic Acid Filler: What to Do After a Good Periocular Treatment Goes Bad. Aesthet. Surg. J. 2020, 40, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Gonzalez, M.; Davo-Cabrera, J.; Rausell-Fontestad, N.; Botella-Estrada, R.; Espana-Gregori, E.; Perez-Lopez, M. Does hyaluronidase injected in periocular area change skin ultrastructure?: Standardized in vitro analysis. J. Cosmet. Dermatol. 2022, 21, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Walker, L.; Convery, C.; Davies, E. Management of a Vascular Occlusion Associated with Cosmetic Injections. J. Clin. Aesthet. Dermatol. 2020, 13, E53–E58. [Google Scholar] [PubMed]


| Filler | Abbreviation | Storage Modulus (Pa) a | Delta (°) a | HA Concentration (mg/mL) | Degree of Modification (%) b | Batch References |
|---|---|---|---|---|---|---|
| TEOSYAL PNT1 | PNT1 | 58.6 | 19.5 | 15 | 1.9 | TPRL_22142AA |
| TEOSYAL PNT2 | PNT2 | 138.9 | 16.2 | 23 | 3.1 | TP30L_220916A TP30L_22142AA TP30L_22121DA |
| TEOSYAL PNT3 | PNT3 | 145.8 | 12.1 | 23 | 3.6 | TP27L_221117A TP27L2214IA |
| TEOSYAL PNT4 | PNT4 | 263.3 | 6.6 | 23 | 4.1 | TPUL_22142CA TPUL_22142BA TPUL_221029A TPUL_22232IA TPUL_222232H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flégeau, K.; Jing, J.; Brusini, R.; Gallet, M.; Moreno, C.; Walker, L.; Bourdon, F.; Faivre, J. Multidose Hyaluronidase Administration as an Optimal Procedure to Degrade Resilient Hyaluronic Acid Soft Tissue Fillers. Molecules 2023, 28, 1003. https://doi.org/10.3390/molecules28031003
Flégeau K, Jing J, Brusini R, Gallet M, Moreno C, Walker L, Bourdon F, Faivre J. Multidose Hyaluronidase Administration as an Optimal Procedure to Degrade Resilient Hyaluronic Acid Soft Tissue Fillers. Molecules. 2023; 28(3):1003. https://doi.org/10.3390/molecules28031003
Chicago/Turabian StyleFlégeau, Killian, Jing Jing, Romain Brusini, Mélanie Gallet, Capucine Moreno, Lee Walker, François Bourdon, and Jimmy Faivre. 2023. "Multidose Hyaluronidase Administration as an Optimal Procedure to Degrade Resilient Hyaluronic Acid Soft Tissue Fillers" Molecules 28, no. 3: 1003. https://doi.org/10.3390/molecules28031003
APA StyleFlégeau, K., Jing, J., Brusini, R., Gallet, M., Moreno, C., Walker, L., Bourdon, F., & Faivre, J. (2023). Multidose Hyaluronidase Administration as an Optimal Procedure to Degrade Resilient Hyaluronic Acid Soft Tissue Fillers. Molecules, 28(3), 1003. https://doi.org/10.3390/molecules28031003





