Synthesis, Characterization, and Antiproliferative Properties of New Bio-Inspired Xanthylium Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
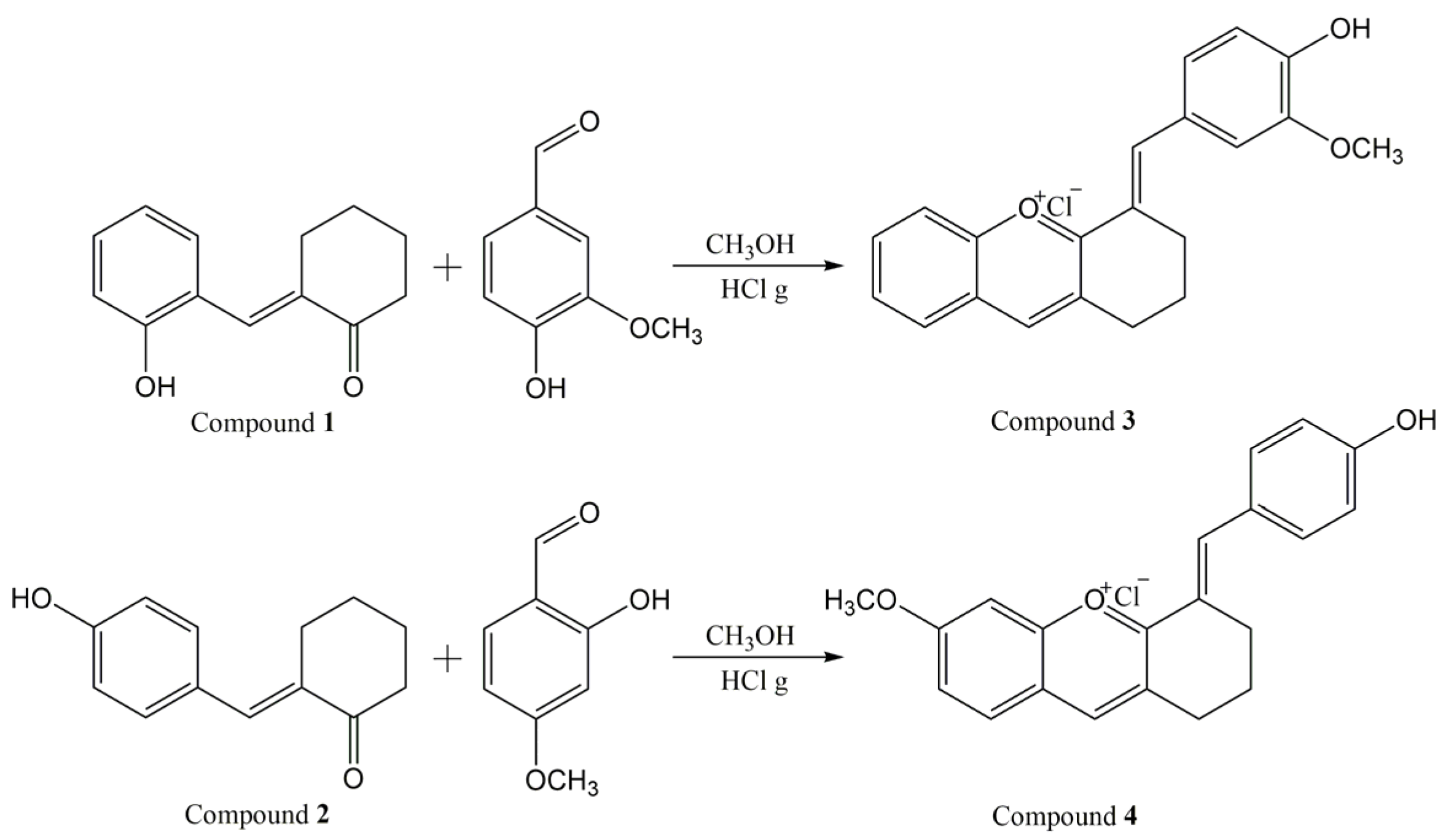
2.2.1. Synthesis and Characterization of the New Xanthylium Derivatives
2.2.2. UV-Vis and FT-IR Spectroscopy
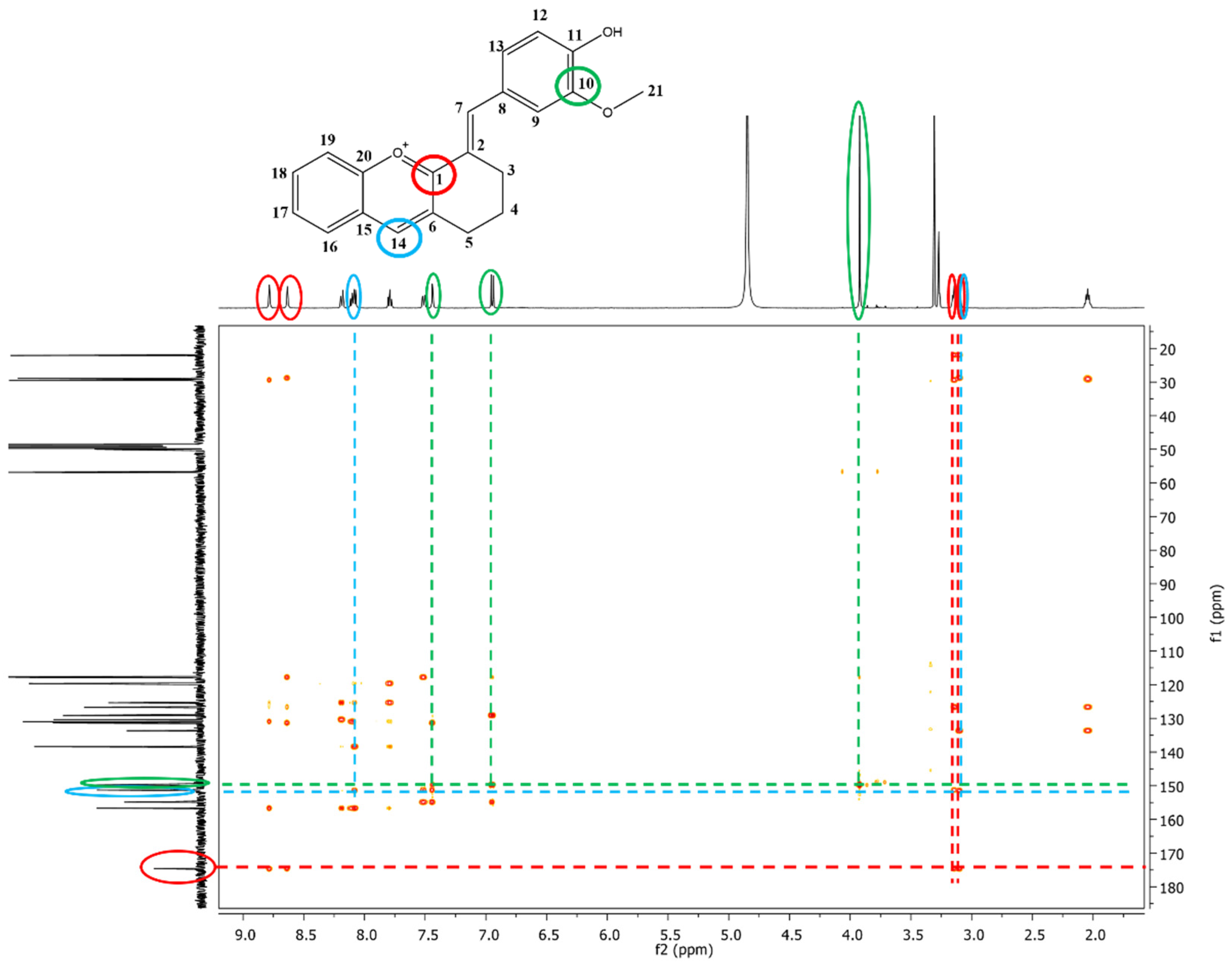
2.2.3. NMR Analysis
2.2.4. Study of pH Dependent Photochromic Properties
2.2.5. Biological Activity Study
3. Results and Discussions
3.1. Synthesis and Characterization of the Xanthylium Dyes
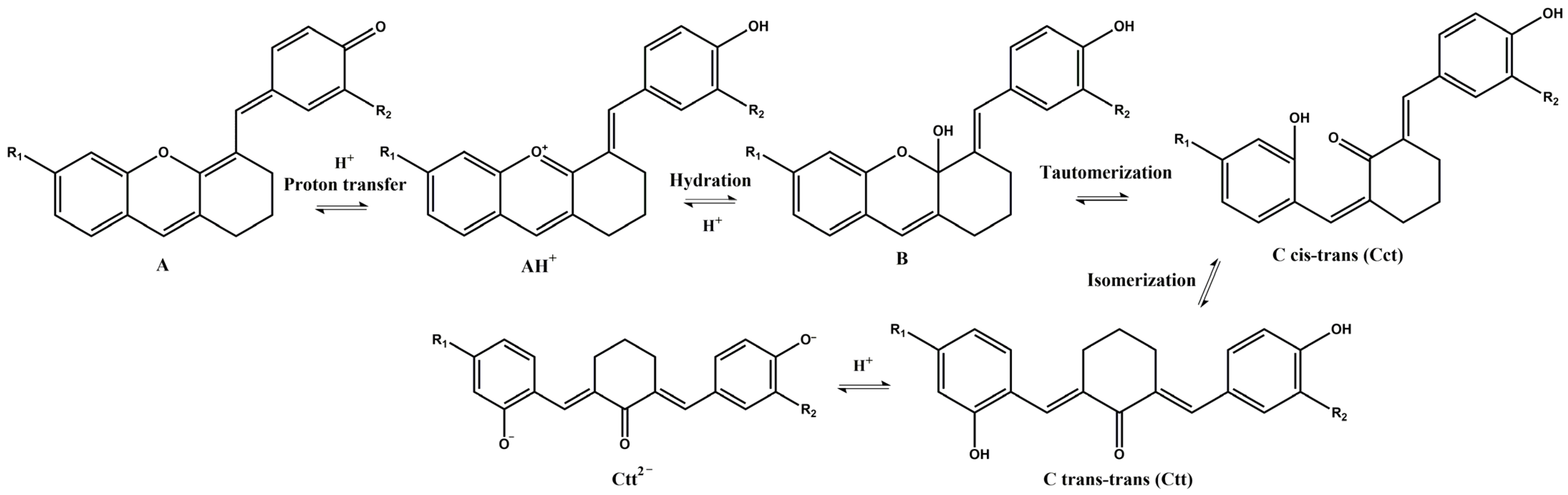
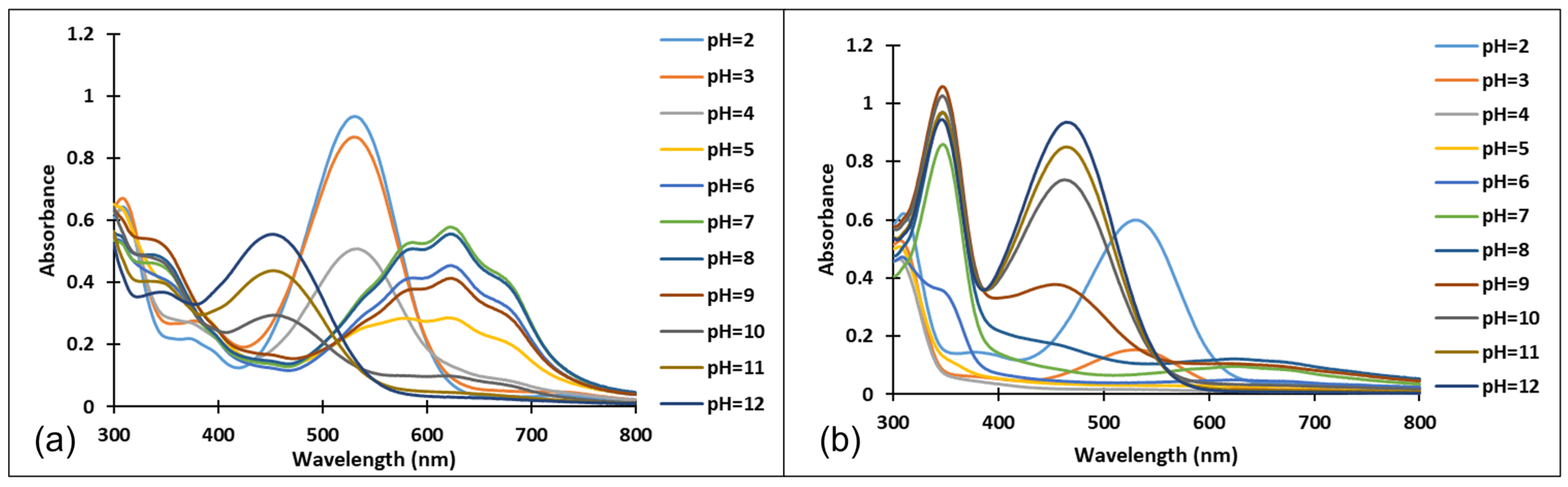
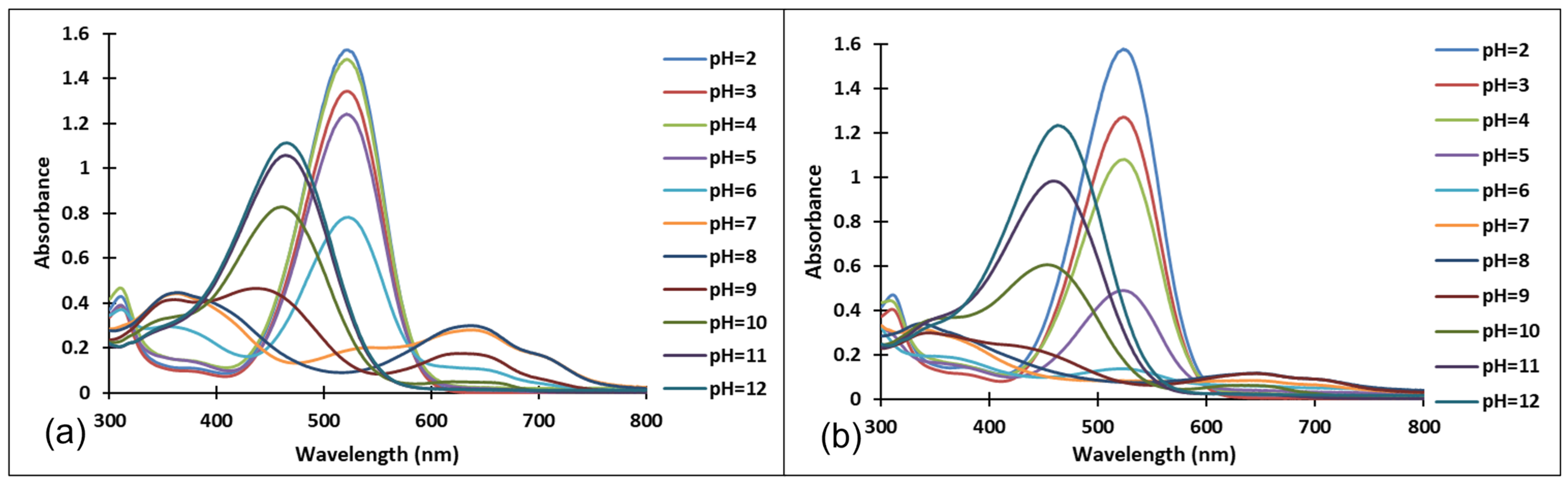
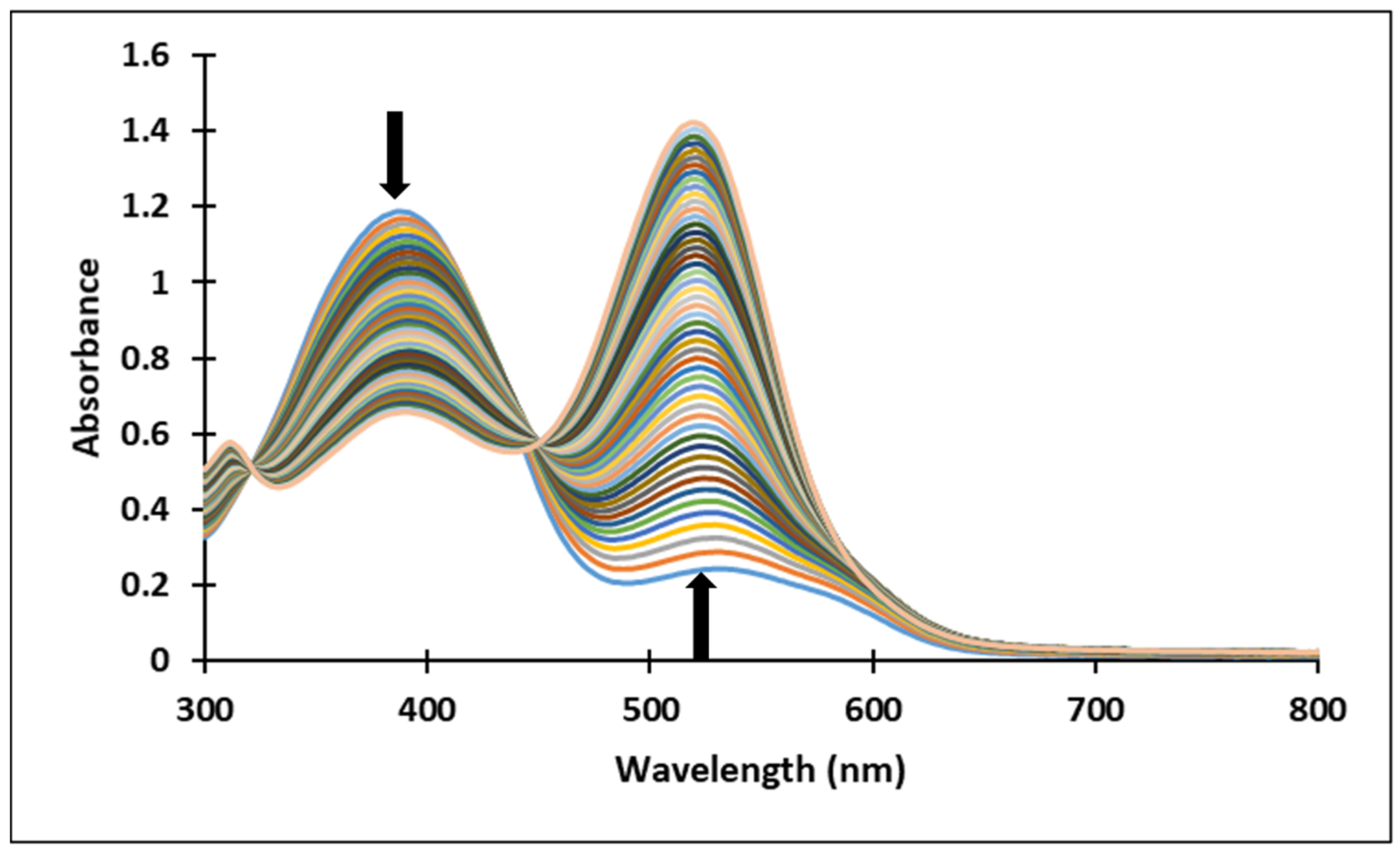
3.2. pH-Dependent Photochromic Properties
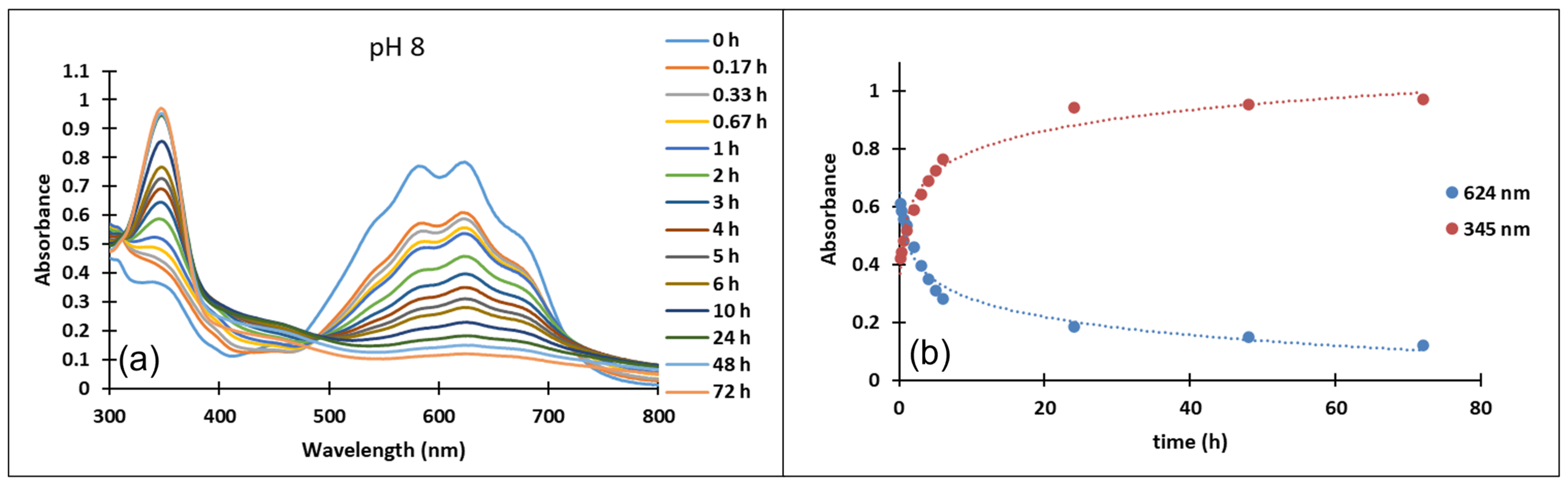
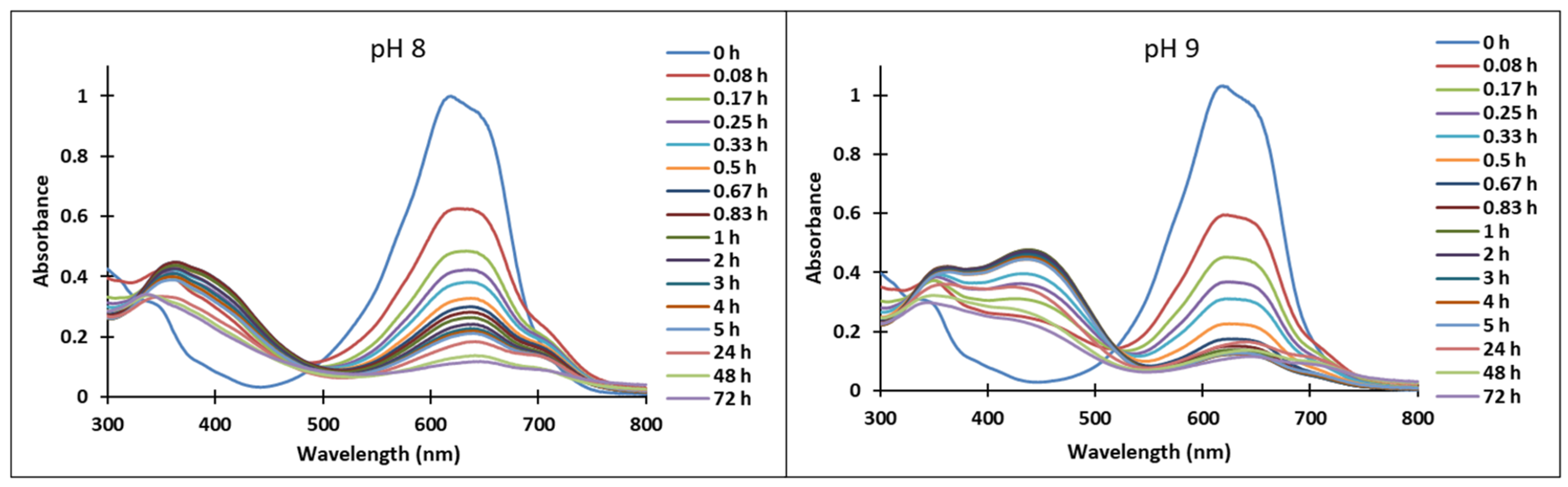
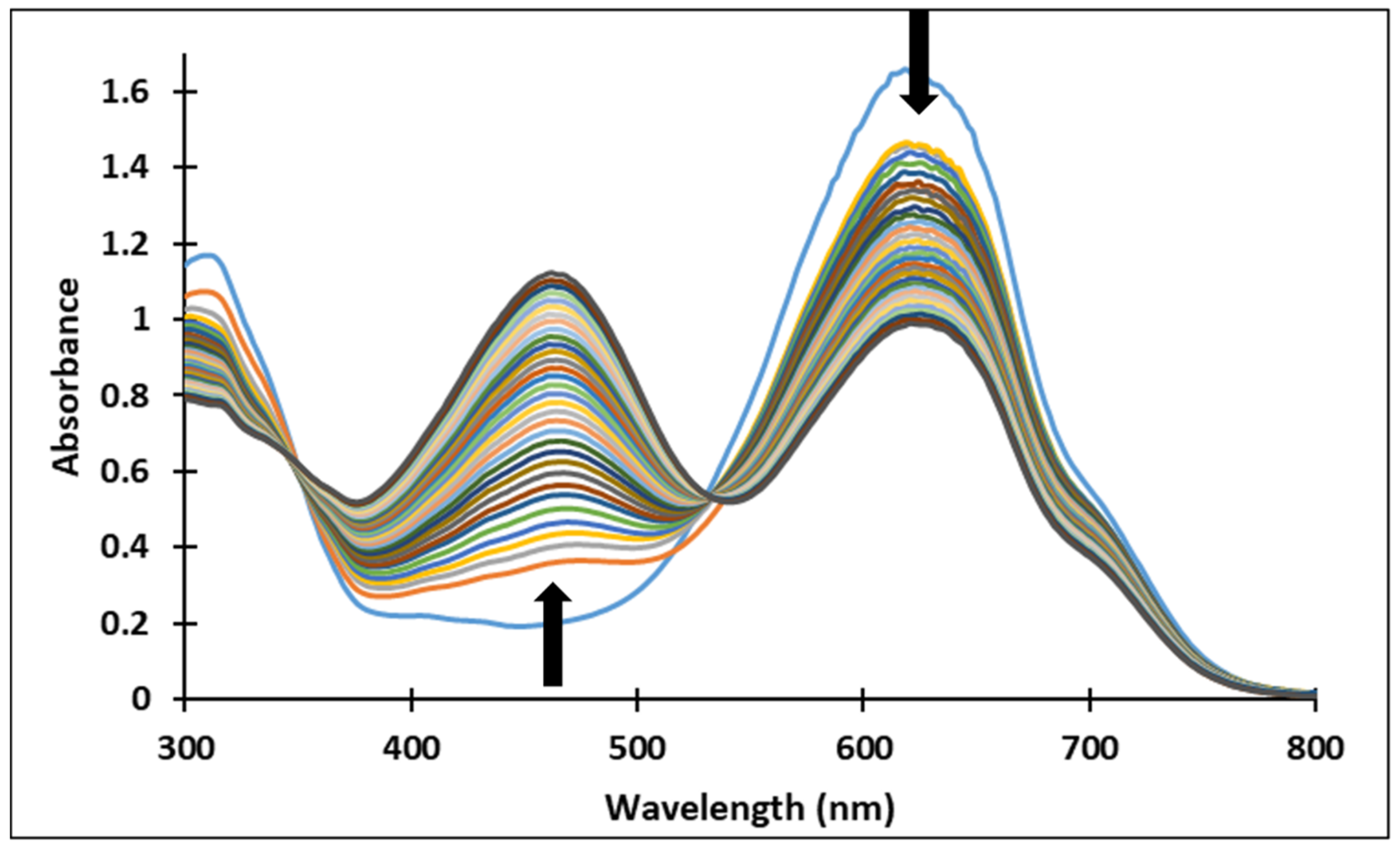
3.3. pH-Jumps Study
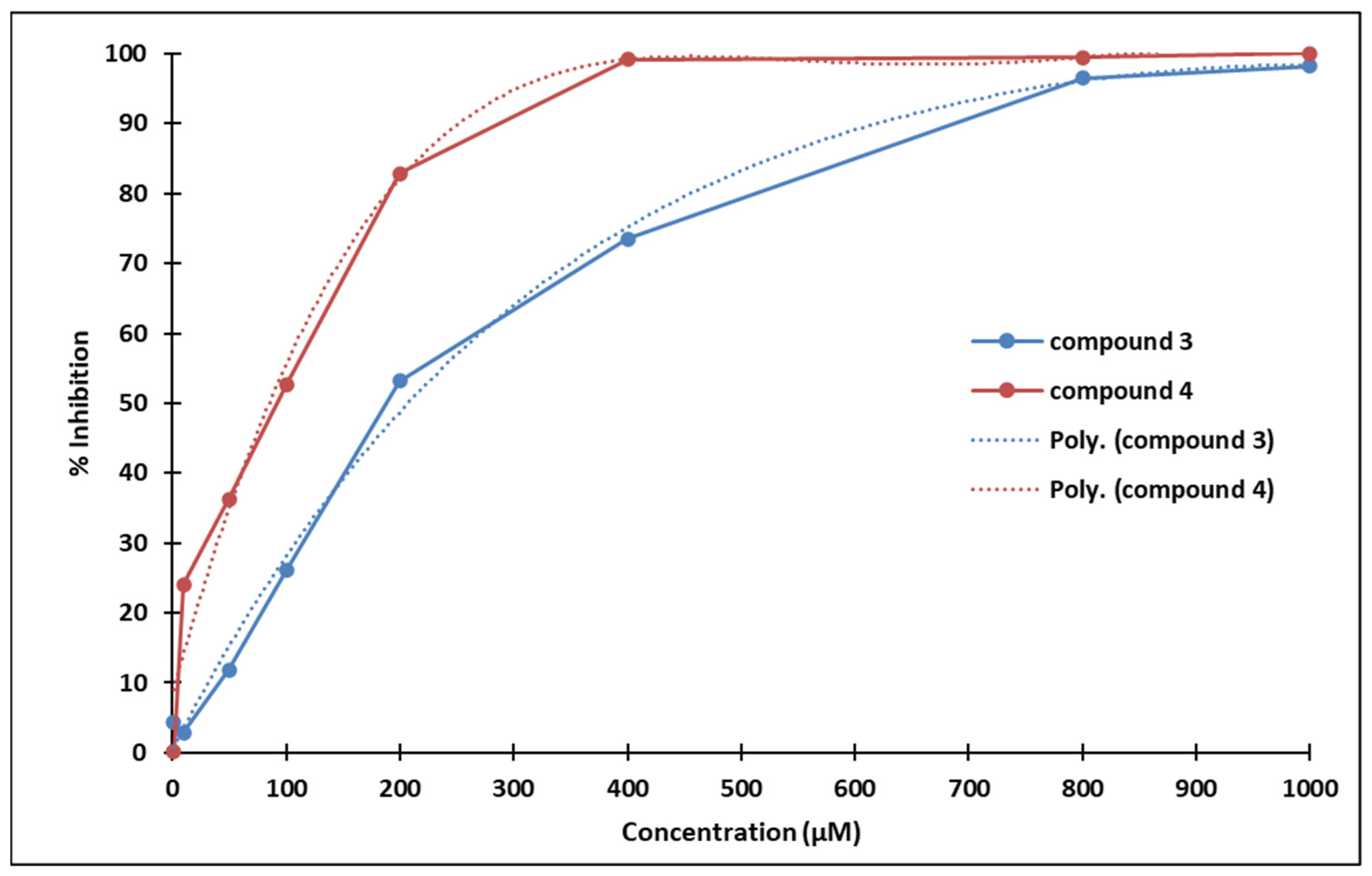
3.4. Inhibition of Cell Proliferation
3.5. The Effect on Cell Cycle Progression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, M.S.; Swamy, U.R.; Sinniah, U.R. (Eds.) Natural Bioactive Compound, Vol. 1: Production and Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akthar, M.S. (Eds.) Natural Bioactive Compounds, Vol. 2: Chemistry, Pharmacology and Health Care Practices; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M. the International Natural Products Sciences Taskforce, Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Preedy, V.; Zibadi, S. (Eds.) Polyphenols in Prevention and Treatment of Human Disease, 2nd ed.; Academic Press: New York, NY, USA, 2018. [Google Scholar]
- Colomer, R.; Sarrats, A.; Lupu, R.; Puig, T. Natural polyphenols and their synthetic analogs as emerging anticancer agents. Curr. Drug Targ. 2017, 18, 147–159. [Google Scholar] [CrossRef]
- Crous-Masó, J.; Palomeras, S.; Relat, J.; Camó, C.; Martínez-Garza, Ú.; Planas, M.; Feliu, L.; Puig, T. (−)-Epigallocatechin 3-gallate synthetic analogues inhibit fatty acid synthase and show anticancer activity in triple negative breast cancer. Molecules 2018, 23, 1160. [Google Scholar] [CrossRef] [Green Version]
- Păușescu, I.; Kántor, I.; Babos, G.; May, Z.; Fodor-Kardos, A.; Miskolczy, Z.; Biczók, L.; Peter, F.; Medeleanu, M.; Feczkó, T. Halochromic behavior and anticancer effect of new synthetic anthocyanidins complexed with β-cyclodextrin derivatives. Int. J. Mol. Sci. 2022, 23, 8103. [Google Scholar] [CrossRef]
- Feng, L.-S.; Cheng, J.-B.; Su, W.-Q.; Li, H.-Z.; Xiao, T.; Chen, D.-A.; Zhang, Z.-L. Cinnamic acid hybrids as anticancer agents: A mini-review. Arch. Pharm. 2022, 355, 2200052. [Google Scholar] [CrossRef]
- Spatafora, C.; Tringali, C. Natural-derived polyphenols as potential anticancer agents. Anti-Cancer Agents Med. Chem. 2012, 12, 902–918. [Google Scholar] [CrossRef] [PubMed]
- Fardad, F.; Ghasemi, K.; Ansarinejad, N.; Khodakarim, N.; Nasiripour, S.; Farasatinasab, M.A. Comparative study to assess the effectiveness of curcumin, mucosamin, and chlorhexidine in chemotherapy-induced oral mucositis. Explore 2022, 19, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.; Fadhlizil, M.; Mohd, F.; Rullah, K.; Wai, K. Insights on the synthesis of asymmetric curcumin derivatives and their biological activities. Eur. J. Med. Chem. 2019, 183, 111704. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, Y.; Wu, K.; Kang, K.; Wang, N.; Ma, Y.; Zhu, H.; Zhang, Y.; Zhou, Y.; Chen, C.; et al. Synthesis and biological evaluation of allylated mono-carbonyl analogues of curcumin (MACs) as anti-cancer agents for cholangiocarcinoma. Bioorganic Med. Chem. Lett. 2016, 26, 5971–5976. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, J.; Zheng, S.; Feng, Q.; Xiao, X. Curcumin, a polyphenolic curcuminoid with its protective effects and molecular mechanisms in diabetes and diabetic cardiomyopathy. Front. Pharmacol. 2018, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Choudhary, S.; Sharma, D.; Sainaga, V.G.S.; Baldi, A.; Kumar, N.; Jitender, M. Armamentarium of anticancer analogues of curcumin: Portray of structural insight, bioavailability, drug-target interaction and therapeutic efficacy. J. Mol. Struct. 2021, 1231, 129691. [Google Scholar] [CrossRef]
- Moreira, J.; Saraiva, L.; Pinto, M.M.; Cidade, H. Diarylpentanoids with antitumor activity: A critical review of structure-activity relationship studies. Eur. J. Med. Chem. 2020, 192, 112177. [Google Scholar] [CrossRef] [PubMed]
- Nasery, M.M.; Varzandeh, M.; Pahlavanneshan, S.; Mohamadi, N.; Sarhadi, S.; Fekri, H.S.; Mohammadinejad, R.; Ahn, K.S. Curcumin: A potential therapeutic natural product for adenocarcinomas. Phytochem. Lett. 2022, 49, 45–55. [Google Scholar] [CrossRef]
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C. Curcumin selectively induces colon cancer cell apoptosis and S cell cycle arrest by regulates Rb/E2F/p53 pathway. J. Mol. Struct. 2022, 1263, 133180. [Google Scholar] [CrossRef]
- Revalde, J.L.; Li, Y.; Hawkins, B.C.; Rosengren, R.J.; Paxton, J.W. Heterocyclic cyclohexanone monocarbonyl analogs of curcumin can inhibit the activity of ATP-binding cassette transporters in cancer multidrug resistance. Biochem. Pharmacol. 2015, 93, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Păușescu, I.; Todea, A.; Badea, V.; Peter, F.; Medeleanu, M.; Ledeți, I. Optical and thermal properties of intelligent pH indicator films based on chitosan / PVA and a new xanthylium dye. J. Therm. Anal. Calorim. 2020, 141, 999–1008. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Moro, A.J.; Parola, A.J.; Lima, J.C.; Pina, F.; Corici, L.; Shova, S.; Cseh, L. Generalization of the anthocyanins kinetics and thermodynamics multistate to 2,6-bis(2-hydroxybenzylidene)cyclohexanones. Dyes. Pigm. 2019, 163, 573–588. [Google Scholar] [CrossRef]
- Moro, A.J.; Parola, A.J.; Pina, F.; Pana, A.M.; Badea, V.; Pausescu, I.; Shova, S.; Cseh, L. 2,2′-Spirobis[chromene] derivatives chemistry and their relation with the multistate system of antocyanins. J. Org. Chem. 2017, 82, 5301–5309. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Ning, H.; Ge, Y.; Yin, Z.; Chen, L.; Hu, D.; Shen, S.; Wang, X.; Wang, S.; Li, R.; et al. Bisphenol A induces apoptosis in response to DNA damage through c-Abl / YAP Y357 / p73 pathway in P19 embryonal carcinoma stem cells. Toxicology 2022, 70, 153138. [Google Scholar] [CrossRef]
- Monzo, H.J.; Park, T.I.H.; Montgomery, J.M.; Faull, R.L.M.; Dragunow, M.; Curtis, M.A. A method for generating high-yield enriched neuronal cultures from P19 embryonal carcinoma cells. J. Neurosci. Methods 2012, 204, 87–103. [Google Scholar] [CrossRef]
- Jasmin; Spray, D.C.; Campos de Carvalho, A.C.; Mendez-Otero, R. Chemical Induction of Cardiac Differentiation in P19 Embryonal Carcinoma Stem Cells. Stem. Cells Dev. 2009, 19, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Pana, A.M.; Pausescu, I.; Shova, S.; Badea, V.; Tudose, R.; Silion, M.; Costisor, O.; Cseh, L. pH dependent structural interconversion of 2-(2-hydroxy-benzylidene)-cyclohexan-1-one: Crystal structures and spectroscopic investigation. J. Mol. Struct. 2017, 1137, 9–16. [Google Scholar] [CrossRef]
- Pana, A.M.; Badea, V.; Banica, R.; Bora, A.; Dudas, Z.; Cseh, L.; Costisor, O. Network reaction of 2,6-bis(2-hydroxybenzilidene)cyclohexanone by external stimuli. J. Photochem. Photobiol. 2014, 283, 22–28. [Google Scholar] [CrossRef]
- Carmody, W.R. An Easily Prepared Wide Range Buffer Series. J. Chem. Educ. 1961, 38, 559. [Google Scholar] [CrossRef]
- Seeley, M.R.; Faustman, E.M. Evaluation of P19 cells for studying mechanisms of developmental toxicity: Application to four direct-acting alkylating agents. Toxicology 1998, 127, 49–58. [Google Scholar] [CrossRef]
- Soltanian, S.; Sheikhbahaei, M.; Mohamadi, N. Cytotoxicity evaluation of methanol extracts of some medicinal plants on P19 embryonal carcinoma cells. J. Appl. Pharm. Sci. 2017, 7, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 15 February 2022).
- Vero, A.; Lines, M.C.F.C.; Prasetyaningrum, P.W.; Bahtiar, A. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci. Pharm. 2018, 86, 25. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Li, W.; Zhao, C. Da0324, an inhibitor of nuclear factor- κ B activation, demonstrates selective antitumor activity on human gastric cancer cells. Drug Des. Devel. Ther. 2016, 10, 979–995. [Google Scholar] [CrossRef]



| Compound 3 | Compound 4 | |||
|---|---|---|---|---|
| Concentration (µM) | % Inhibition | t-Test | % Inhibition | t-Test |
| 1000 | 98.19 | 6.49 × 10−2 | 100 | 8.97 × 10−3 |
| 800 | 96.51 | 1.72 × 10−4 | 99.45 | 1.82 × 10−2 |
| 400 | 73.51 | 2.38 × 10−2 | 99.22 | 7.68 × 10−2 |
| 200 | 53.14 | 3.23 × 10−2 | 82.85 | 1.83 × 10−3 |
| 100 | 26.03 | 4.25 × 10−1 | 52.59 | 3.48 × 10−2 |
| 50 | 11.84 | 6.53 × 10−1 | 36.33 | 9.40 × 10−5 |
| 10 | 2.86 | 8.47 × 10−1 | 24.11 | 1.08 × 10−1 |
| 1 | 4.29 | 8.29 × 10−1 | 0.16 | 9.92 × 10−1 |
| Control | Compound 3 | Compound 4 | 3 vs. 4 | |||
|---|---|---|---|---|---|---|
| % | t-Test | % | t-Test | t-Test | ||
| G0/G1 | 48.38 | 40.73 | 6.45 × 10−3 | 35.70 | 7.87 × 10−4 | 8.94 × 10−4 |
| S | 19.12 | 17.72 | 7.83 × 10−2 | 22.55 | 1.55 × 10−2 | 1.74 × 10−4 |
| G2/M | 32.42 | 41.43 | 1.98 × 10−5 | 41.63 | 2.28 × 10−5 | 5.91 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, C.; Dreavă, D.-M.; Todea, A.; Péter, F.; Medeleanu, M.; Păușescu, I.; Samoilă, C.; Sîrbu, I.O. Synthesis, Characterization, and Antiproliferative Properties of New Bio-Inspired Xanthylium Derivatives. Molecules 2023, 28, 1102. https://doi.org/10.3390/molecules28031102
Koch C, Dreavă D-M, Todea A, Péter F, Medeleanu M, Păușescu I, Samoilă C, Sîrbu IO. Synthesis, Characterization, and Antiproliferative Properties of New Bio-Inspired Xanthylium Derivatives. Molecules. 2023; 28(3):1102. https://doi.org/10.3390/molecules28031102
Chicago/Turabian StyleKoch, Claudia, Diana-Maria Dreavă, Anamaria Todea, Francisc Péter, Mihai Medeleanu, Iulia Păușescu, Corina Samoilă, and Ioan Ovidiu Sîrbu. 2023. "Synthesis, Characterization, and Antiproliferative Properties of New Bio-Inspired Xanthylium Derivatives" Molecules 28, no. 3: 1102. https://doi.org/10.3390/molecules28031102






