Rapid Detection of Estrogens in Cosmetics by Chemical Derivatization and Paper-Spray Ionization Mass-Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
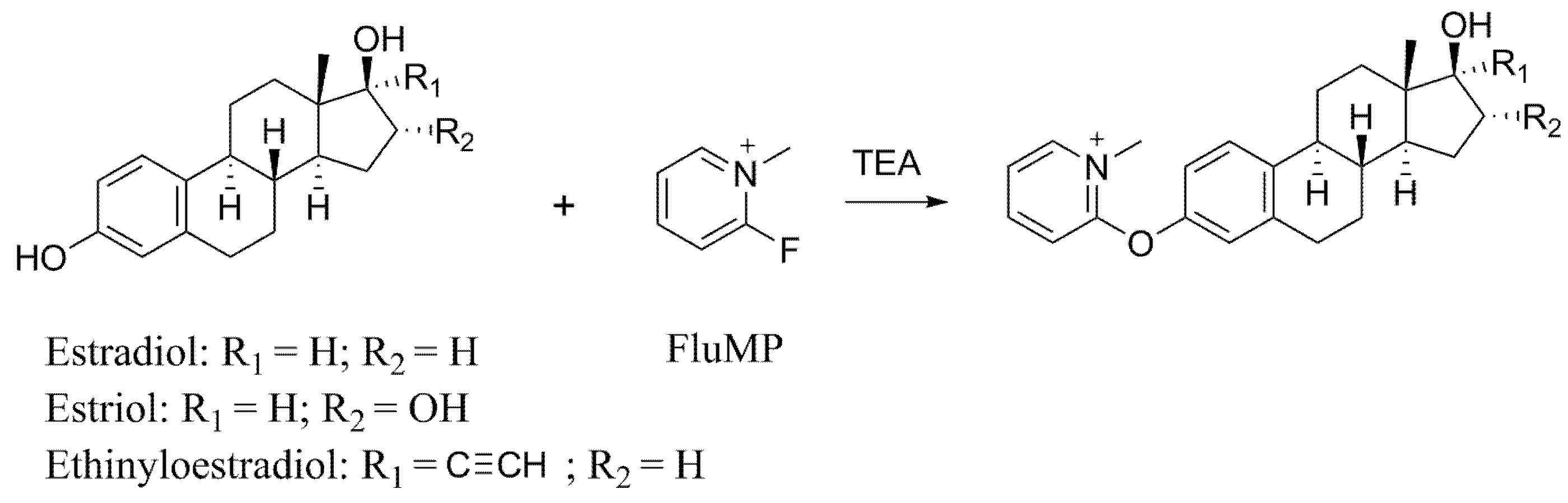
2.1. The Choose of Derivatization Reagents
2.2. The Optimization of Reaction Conditions
2.3. Optimization of Paper-Spray Mass Spectrometry Parameters
2.4. Other Important Parameters
2.5. Linearity, Lower Limits of Detection
2.6. Recovery
2.7. Detection Sample with Complex Matrix
2.8. Estimation of Measurement Uncertainty (MU)
2.9. Estimation of Greenness Character
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrument
3.3. Sample Preparation
3.4. Derivative Reaction
3.5. Mass Spectrometry Parameters
3.6. PSI Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. A role for estrogen in skin ageing and dermal biomechanics. Mech. Ageing Dev. 2021, 197, 111513. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.G.; Maibach, H.I. Estrogen and skin: An overview. Am. J. Clin. Dermatol. 2001, 2, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. The role of estrogen in cutaneous ageing and repair. Maturitas 2017, 103, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.-Z.; Wu, H.-L.; Li, Y.-N.; Zhang, J.; Li, Y.; Nie, C.-C.; Zhang, X.-H.; Yu, R.-Q. Measuring estriol and estrone simultaneously in liquid cosmetic samples using second-order calibration coupled with excitation–emission matrix fluorescence based on region selection. Anal. Methods 2012, 4, 222–229. [Google Scholar] [CrossRef]
- Komori, S.; Ito, Y.; Nakamura, Y.; Aoki, M.; Takashi, T.; Kinuta, T.; Tanaka, H.; Koyama, K. A long-term user of cosmetic cream containing estrogen developed breast cancer and endometrial hyperplasia. Menopause 2008, 15, 1191–1192. [Google Scholar] [CrossRef]
- Technical Specifications for Cosmetic Safety; China Standard Publishing House: Beijing, China, 2016.
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; The European Parliament and of the Council: Strasbourg, France, 2009.
- Hubinger, J.C. Determination of estriol, estradiol, estrone, and progesterone in cosmetic products. J. Cosmet. Sci. 2015, 66, 113–128. [Google Scholar]
- Wen, Y.; Wang, Y.; Zhou, B.; Xu, Y.; Feng, Y. Determination of Sexual Hormones in Liquid Cosmetics by Polymer Monolith Microextraction Coupled with High Performance Liquid Chromatography. Chin. J. Anal. Chem. 2007, 35, 681–684. [Google Scholar]
- Yilmaz, B. Simultaneous Determination of Estradiol Valerate and Medroxyprogesterone Acetate in a Tablet Formulation by Gas Chromatography-Mass Spectrometry. Anal. Sci. 2010, 26, 391–393. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Ye, L.; Han, J.; Feng, L.; Tan, Y.; Wang, X. Simultaneous determination of 25 sex hormones in cosmeceutical by liquid chromatography tandem mass spectrometry with isotope dilution. Chin. J. Health Lab. Technol. 2011, 21, 1048–1053. [Google Scholar]
- De Orsi, D.; Pellegrini, M.; Pichini, S.; Mattioli, D.; Marchei, E.; Gagliardi, L. High-performance liquid chromatography–diode array and electrospray-mass spectrometry analysis of non-allowed substances in cosmetic products for preventing hair loss and other hormone-dependent skin diseases. J. Pharm. Biomed. Anal. 2008, 48, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gu, J.H.; Soldin, O.P.; Singh, R.J.; Soldin, S.J. Rapid measurement of estrogens and their metabolites in human serum by liquid chromatography-tandem mass spectrometry without derivatization. Clin. Biochem. 2008, 41, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Denver, N.; Khan, S.; Homer, N.Z.; MacLean, M.R.; Andrew, R. Current strategies for quantification of estrogens in clinical research. J. Steroid Biochem. Mol. Biol. 2019, 192, 105373. [Google Scholar] [CrossRef]
- Bantscheff, M.; Schirle, M.; Sweetman, G.; Rick, J.; Kuster, B. Quantitative mass spectrometry in proteomics: A critical review. Anal. Bioanal. Chem. 2007, 389, 1017–1031. [Google Scholar] [CrossRef]
- Hoffmann, W.D.; Jackson, G.P. Forensic Mass Spectrometry. Annu. Rev. Anal. Chem. 2015, 8, 419–440. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Setou, M.; Niwa, T. Biomedical mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 1273–1274. [Google Scholar] [CrossRef] [Green Version]
- Takats, Z.; Wiseman, J.; Gologan, B.; Cooks, R. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, J.; Cooks, R.; Ouyang, Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew. Chem. 2010, 122, 889–892. [Google Scholar] [CrossRef]
- Keating, J.E.; Minges, J.T.; Randell, S.H.; Glish, G.L. Paper spray mass spectrometry for high-throughput quantification of nicotine and cotinine. Anal. Methods 2018, 10, 46–50. [Google Scholar] [CrossRef]
- Maciel, L.I.L.; Ramalho, R.R.F.; Ribeiro, R.I.; Pinto, M.C.X.; Pereira, I.; Vaz, B.G. Combining the Katritzky Reaction and Paper Spray Ionization Mass Spectrometry for Enhanced Detection of Amino Acid Neurotransmitters in Mouse Brain Sections. J. Am. Soc. Mass Spectrom. 2021, 32, 2513–2518. [Google Scholar] [CrossRef]
- Chamberlain, C.A.; Hatch, M.; Garrett, T. Extracellular Vesicle Analysis by Paper Spray Ionization Mass Spectrometry. Metabolites 2021, 11, 308. [Google Scholar] [CrossRef]
- Gonsalves, M.D.; Yevdokimov, A.; Brown-Nash, A.; Smith, J.L.; Oxley, J.C. Paper spray ionization–high-resolution mass spectrometry (PSI-HRMS) of peroxide explosives in biological matrices. Anal. Bioanal. Chem. 2021, 413, 3069–3079. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ifa, D.; Manicke, N.; Cooks, R. Rapid, Direct analysis of cholesterol by charge labeling in reactive de-sorption electrospray ionization. Anal. Chem. 2009, 81, 7618–7624. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.; Nakano, Y.; Mochizuki, K.; Nomoto, M.; Takahashi, Y.; Ito, R.; Saito, K.; Nakazawa, H. A new strategy for ionization enhancement by derivatization for mass spectrometry. J. Chromatogr. B 2011, 879, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zou, L.; Liu, Y.; Zhang, Z.; Ong, C.N. Enhancement of the capabilities of liquid chromatography–mass spectrometry with derivatization: General principles and applications. Mass Spectrom. Rev. 2011, 30, 1143–1172. [Google Scholar] [CrossRef]
- Quirke, J.; Hsu, Y.; Berkel, G. Selective detection of derivatized alcohols and phenols in essential oils by electrospray-tandem mass spectrometry. Essent. Oil Res. 2001, 13, 324–331. [Google Scholar] [CrossRef]
- Quirke, J.M.E.; Van Berkel, G.J. Electrospray tandem mass spectrometric study of alkyl 1-methylpyridinium ether derivatives of alcohols. J. Mass Spectrom. 2001, 36, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Beasley, E.; Francese, S.; Bassindale, T. Detection and Mapping of Cannabinoids in Single Hair Samples through Rapid Derivatization and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 10328–10334. [Google Scholar] [CrossRef] [Green Version]
- Thieme, D.; Sachs, H.; Thevis, M. Formation of the N-methylpyridinium derivative to improve the detection of buprenorphine by liquid chromatography-mass spectrometry. J. Mass Spectrom. 2008, 43, 974–979. [Google Scholar] [CrossRef] [PubMed]
- EURACHEM/CITAC. Quantifying Uncertainty in Analytical Measurement. EURACHEM/Co-Operation on International Traceability in Analytical Chemistry. UK. 2012. Available online: https://www.eurachem.org/images/stories/Guides/pdf/QUAM2012_P1.pdf (accessed on 14 January 2023).
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef]




| Estradiol | Estriol | Ethinyloestradiol | ||||||
|---|---|---|---|---|---|---|---|---|
| Added (μg/g, N = 3) | Found (μg/g, N = 3) | RSD% (N = 3) | Added (μg/g, N = 3) | Found (μg/g, N = 3) | RSD% (N = 3) | Added (μg/g, N = 3) | Found (μg/g, N = 3) | RSD% (N = 3) |
| 10 | 10.42 | 8.28 | 10 | 11.33 | 3.30 | 10 | 11.29 | 5.67 |
| 100 | 91.54 | 5.75 | 100 | 87.20 | 3.17 | 100 | 101.73 | 2.73 |
| 300 | 312.64 | 5.15 | 300 | 307.30 | 5.19 | 300 | 315.36 | 9.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Yuan, S.; Zhang, C.; Luan, L.; Liu, Y.; Zhang, Q. Rapid Detection of Estrogens in Cosmetics by Chemical Derivatization and Paper-Spray Ionization Mass-Spectrometry. Molecules 2023, 28, 1130. https://doi.org/10.3390/molecules28031130
Song D, Yuan S, Zhang C, Luan L, Liu Y, Zhang Q. Rapid Detection of Estrogens in Cosmetics by Chemical Derivatization and Paper-Spray Ionization Mass-Spectrometry. Molecules. 2023; 28(3):1130. https://doi.org/10.3390/molecules28031130
Chicago/Turabian StyleSong, Dongning, Song Yuan, Caiyu Zhang, Lin Luan, Yang Liu, and Qingsheng Zhang. 2023. "Rapid Detection of Estrogens in Cosmetics by Chemical Derivatization and Paper-Spray Ionization Mass-Spectrometry" Molecules 28, no. 3: 1130. https://doi.org/10.3390/molecules28031130






