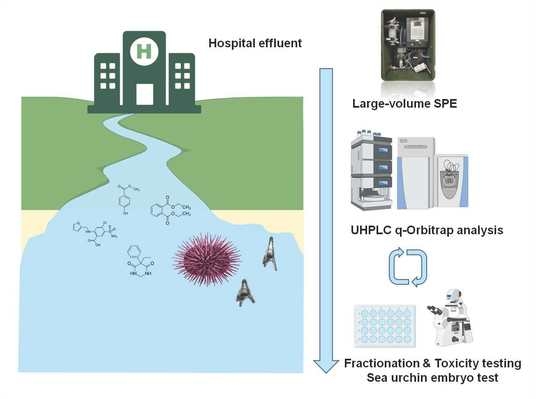
Suspect Screening of Chemicals in Hospital Wastewaters Using Effect-Directed Analysis Approach as Prioritization Strategy
Abstract
1. Introduction
2. Results and Discussion
2.1. Quality Assurance and Quality Control
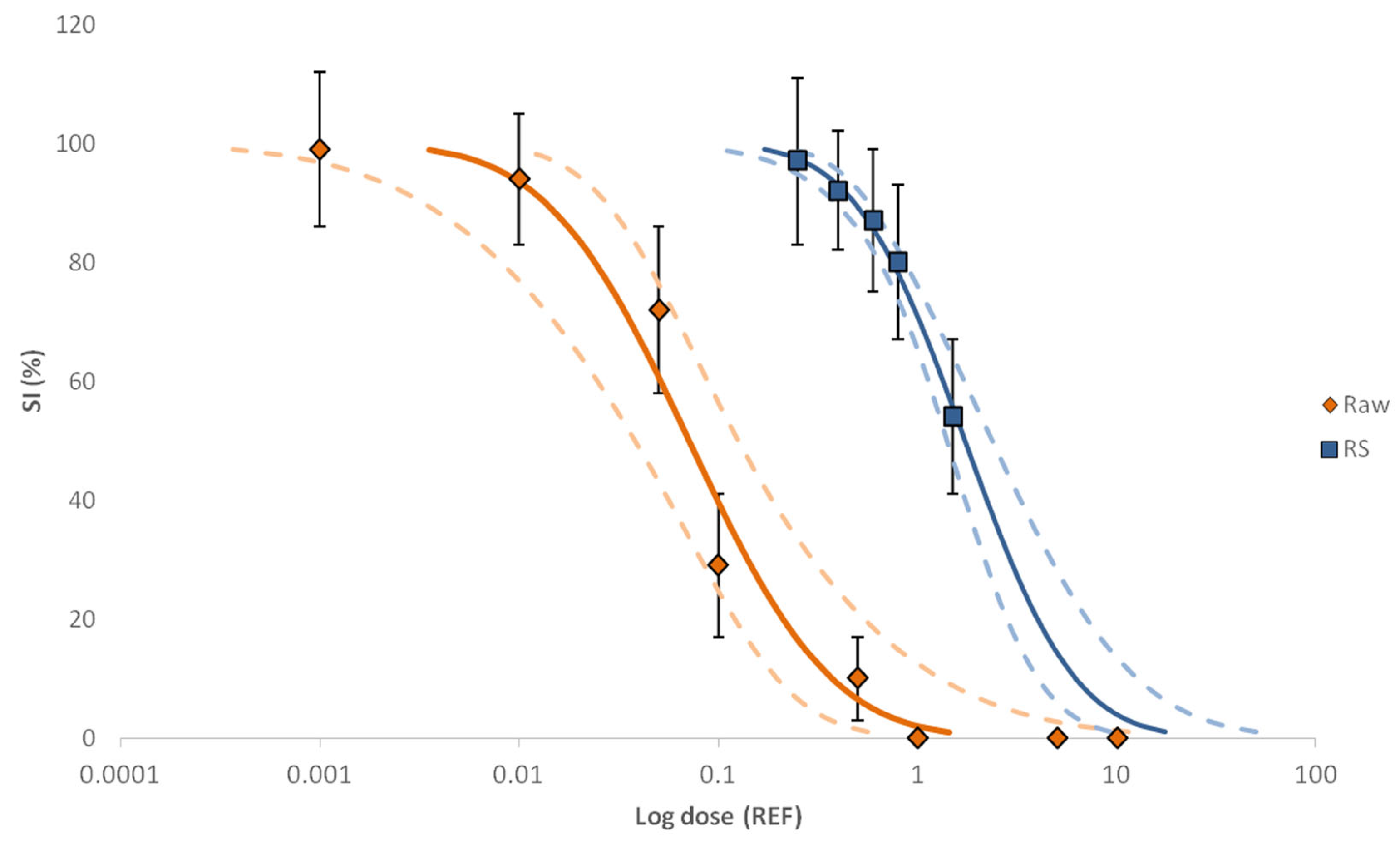
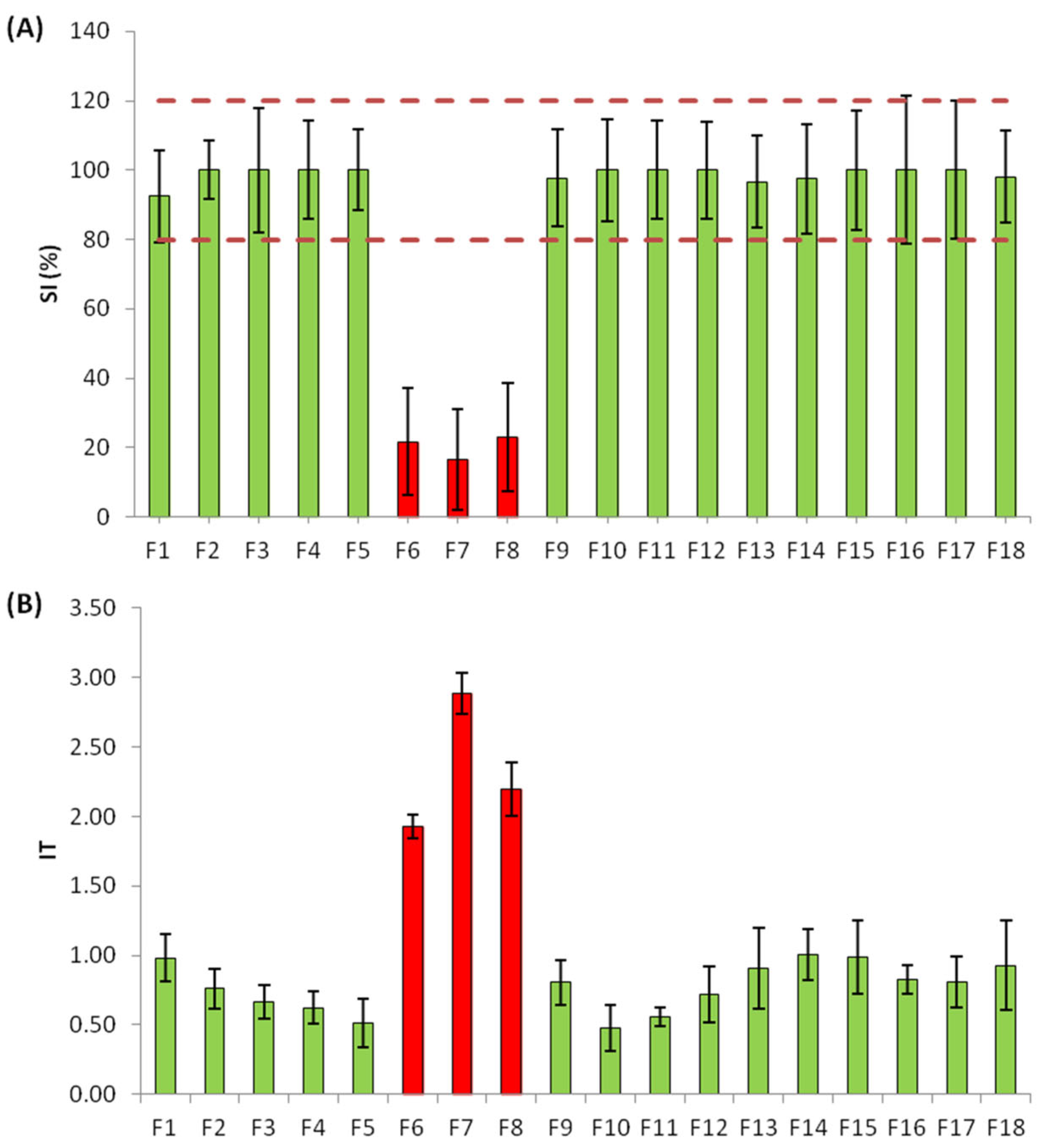
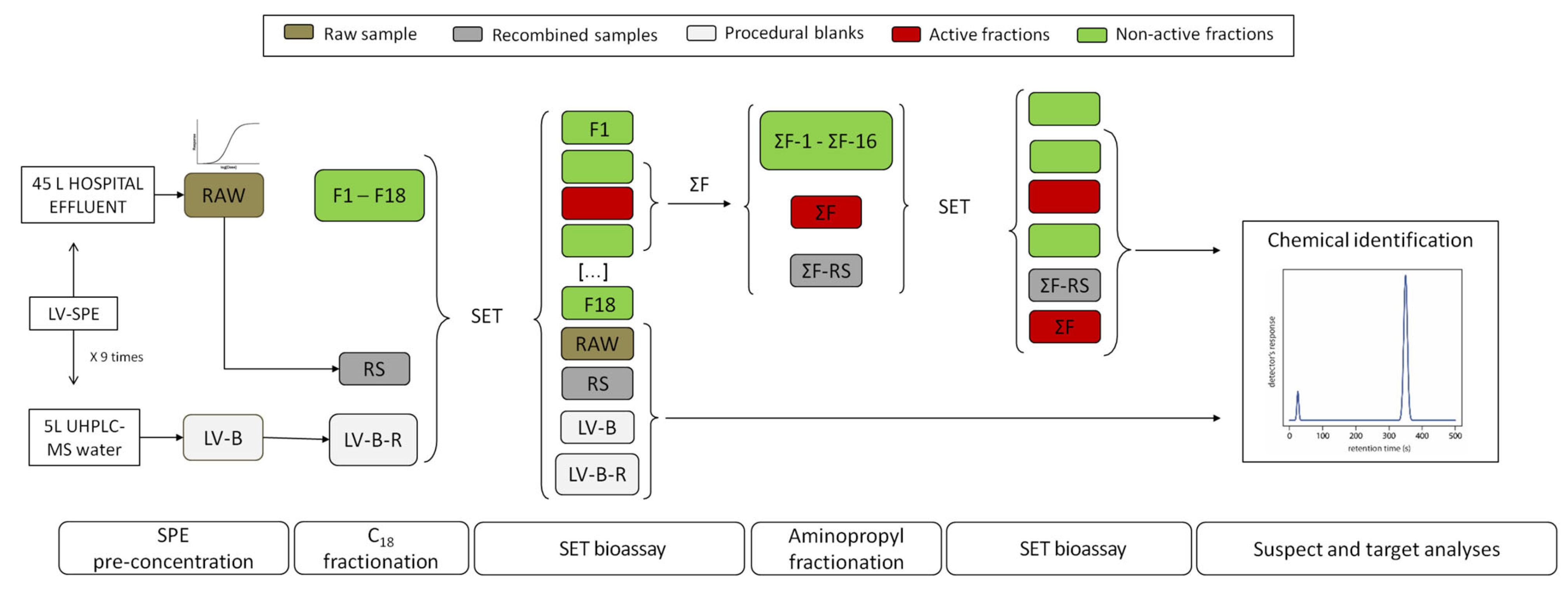
2.2. Hospital Effluent Toxicity Evaluation and Identification of Toxic Fractions through EDA
2.3. Identification of Unknown Compounds
Assessment of Potential Toxicity of Identified Compounds
3. Experimental Section
3.1. Reagents and Materials
3.2. Sampling
3.3. Effect-Directed Analysis (EDA)
3.3.1. Sea Urchin Embryo Test (SET)
3.3.2. Chemical Analysis
3.3.3. Potential Toxicity Assessment of Detected Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, J.; Wu, L.; Chang, A.C. Degradation and Adsorption of Selected Pharmaceuticals and Personal Care Products (PPCPs) in Agricultural Soils. Chemosphere 2009, 77, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.R.; Reemtsma, H.; Grimm, D.A.; Mitra, S. Pharmaceuticals and Personal Care Products (PPCPs) in Surface and Treated Waters of Louisiana, USA and Ontario, Canada. Sci. Total Environ. 2003, 311, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Lopez-Herguedas, N.; González-Gaya, B.; Castelblanco-Boyacá, N.; Rico, A.; Etxebarria, N.; Olivares, M.; Prieto, A.; Zuloaga, O. Characterization of the Contamination Fingerprint of Wastewater Treatment Plant Effluents in the Henares River Basin (Central Spain) Based on Target and Suspect Screening Analysis. Sci. Total Environ. 2021, 806, 151262. [Google Scholar] [CrossRef] [PubMed]
- Hug, C.; Ulrich, N.; Schulze, T.; Brack, W.; Krauss, M. Identification of Novel Micropollutants in Wastewater by a Combination of Suspect and Nontarget Screening. Environ. Pollut. 2014, 184, 25–32. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of Pharmaceuticals and Personal Care Products in Hospital effluent and Waste Water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518–519, 459–478. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-mozaz, S.; Delerue-matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of Hospital effluents to the Load of Pharmaceuticals in Urban Wastewaters: Identification of Ecologically Relevant Pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef]
- Fonseca, E.; Hernández, F.; Ibáñez, M.; Rico, A.; Pitarch, E.; Bijlsma, L. Occurrence and Ecological Risks of Pharmaceuticals in a Mediterranean River in Eastern Spain. Environ. Int. 2020, 144, 106004. [Google Scholar] [CrossRef]
- González Canal, I.; Muga Relloso, I.; Rodríguez Medina, J.; Blanco Miguel, M. Contaminantes Emergentes En Aguas Residuales Urbanas y Efluentes Hospitalarios; TECNOAQUA: Biscay, Spain, 2018. [Google Scholar]
- Mijangos, L.; Ziarrusta, H.; Ros, O.; Kortazar, L.; Fernández, L.A.; Olivares, M.; Zuloaga, O.; Prieto, A.; Etxebarria, N. Occurrence of Emerging Pollutants in Estuaries of the Basque Country: Analysis of Sources and Distribution, and Assessment of the Environmental Risk. Water Res. 2018, 147, 152–163. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Olivares, M.; Zuloaga, O.; Möder, M.; Etxebarria, N.; Prieto, A. Simultaneous Determination of 41 Multiclass Organic Pollutants in Environmental Waters by Means of Polyethersulfone Microextraction Followed by Liquid Chromatography–Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2018, 410, 615–632. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Decision (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified Under Document ((2015) 1756) Text with EEA Relevance); European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Bletsou, A.A.; Jeon, J.; Hollender, J.; Archontaki, E.; Thomaidis, N.S. Targeted and Non-Targeted Liquid Chromatography-Mass Spectrometric Workflows for Identification of Transformation Products of Emerging Pollutants in the Aquatic Environment. Trends Anal. Chem. 2015, 66, 32–44. [Google Scholar] [CrossRef]
- EUR 30297 EN; Selection of Substances for the 3rd Watch List under the Water Framework Directive. Publications Office of the European Union: Luxembourg, 2020.
- Weiss, J.M.; Simon, E.; Stroomberg, G.J.; De Boer, R.; De Boer, J.; Van Der Linden, S.C.; Leonards, P.E.G.; Lamoree, M.H. Identification Strategy for Unknown Pollutants Using High-Resolution Mass Spectrometry : Androgen-Disrupting Compounds Identified through Effect-Directed Analysis. Anal. Bioanal. Chem. 2011, 400, 3141–3149. [Google Scholar] [CrossRef]
- Brack, W.; Bandow, N.; Schwab, K.; Schulze, T.; Streck, G. Bioavailability in Effect-Directed Analysis of Organic Toxicants in Sediments. Trends Anall Chem. 2009, 28, 543–549. [Google Scholar] [CrossRef]
- Brack, W.; Ait-Aissa, S.; Burgess, R.M.; Busch, W.; Creusot, N.; Di Paolo, C.; Escher, B.I.; Mark Hewitt, L.; Hilscherova, K.; Hollender, J.; et al. Effect-Directed Analysis Supporting Monitoring of Aquatic Environments — An in-Depth Overview. Sci. Total Environ. 2016, 544, 1073–1118. [Google Scholar] [CrossRef]
- González-Gaya, B.; Lopez-Herguedas, N.; Bilbao, D.; Mijangos, L.; M. Iker, A.; Etxebarria, N.; Irazola, M.; Prieto, A.; Olivares, M.; Zuloaga, O. Suspect and Non-Target Screening: The Last Frontier in Environmental Analysis. Anal. Methods 2021, 13, 1876–1904. [Google Scholar] [CrossRef]
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking Complex Mixtures of Chemicals in Our Changing Environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Gennaro, M.C.; Marengo, E. Contaminants in Water: Non-Target UHPLC/MS Analysis. Environ. Chem. Lett. 2016, 14, 51–65. [Google Scholar] [CrossRef]
- Hollender, J.; Schymanski, E.L.; Singer, H.P.; Ferguson, P.L. Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ. Sci. Technol. 2017, 51, 11505–11512. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Bletsou, A.A.; Damalas, D.E.; Aalizadeh, R.; Alygizakis, N.A.; Singer, H.P.; Hollender, J.; Thomaidis, N.S. Wide-Scope Target Screening of >2000 Emerging Contaminants in Wastewater Samples with UPLC-Q-ToF-HRMS/MS and Smart Evaluation of Its Performance through the Validation of 195 Selected Representative Analytes. J. Hazard. Mater. 2020, 387, 121712. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Schymanski, E.L.; Bletsou, A.A.; Aalizadeh, R.; Hollender, J.; Thomaidis, N.S. Extended Suspect and Non-Target Strategies to Characterize Emerging Polar Organic Contaminants in Raw Wastewater with LC-HRMS/MS. Environ. Sci. Technol. 2015, 49, 12333–12341. [Google Scholar] [CrossRef] [PubMed]
- Brack, W. Effect-Directed Analysis: A Promising Tool for the Identification of Organic Toxicants in Complex Mixtures? Anal. Bioanal. Chem. 2003, 377, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.A.K.; Escher, B.I.; Krauss, M.; Teodorovic, I.; Brack, W. Effect-Directed Analysis (EDA) of Danube River Water Sample Receiving Untreated Municipal Wastewater from Novi Sad, Serbia. Sci. Total Environ. 2018, 624, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Zhao, J.-L.; Liu, Y.-S.; Hu, L.-X.; Liu, S.-S.; Ying, G.-G. Evaluation of Estrogenic Activity in the Pearl River by Using Effect-Directed Analysis. Environ. Sci. Pollut. Res. 2016, 23, 21692–21702. [Google Scholar] [CrossRef]
- Zwart, N.; Nio, S.L.; Houtman, C.J.; de Boer, J.; Kool, J.; Hamers, T.; Lamoree, M.H. High-Throughput Effect-Directed Analysis Using Downscaled in Vitro Reporter Gene Assays To Identify Endocrine Disruptors in Surface Water. Environ. Sci. Technol. 2018, 52, 4367–4377. [Google Scholar] [CrossRef]
- Di Paolo, C.; Seiler, T.; Keiter, S.; Hu, M.; Muz, M.; Brack, W. The Value of Zebrafish as an Integrative Model in Effect-Directed Analysis - a Review. Environ. Sci. Eur. 2015, 27, 8. [Google Scholar] [CrossRef]
- Mijangos, L.; Krauss, M.; de Miguel, L.; Ziarrusta, H.; Olivares, M.; Zuloaga, O.; Izagirre, U.; Schulze, T.; Brack, W.; Prieto, A.; et al. Application of the Sea Urchin Embryo Test in Toxicity Evaluation and Effect-Directed Analysis of Wastewater Treatment Plant Effluents. Environ. Sci. Technol. 2020, 54, 8890–8899. [Google Scholar] [CrossRef]
- Schmitt, C.; Streck, G.; Lamoree, M.; Leonards, P.; Brack, W.; de Deckere, E. Effect Directed Analysis of Riverine Sediments—The Usefulness of Potamopyrgus Antipodarum for in Vivo Effect Confirmation of Endocrine Disruption. Aquat. Toxicol. 2011, 101, 237–243. [Google Scholar] [CrossRef]
- Cunha, S.C.; Pena, A.; Fernandes, J.O. Mussels as Bioindicators of Diclofenac Contamination in Coastal Environments. Environ. Pollut. 2017, 225, 354–360. [Google Scholar] [CrossRef]
- Agnello, M. Introductory Chapter: Sea Urchin-Knowledge and Perspectives. In Sea Urchin-From Environment to Aquaculture and Biomedicine; IntechOpen: London, UK, 2017. [Google Scholar]
- ASTM. Standard Guide for Conducting Static Acute Toxicity Tests with Echinoid Embryos: Designation E1563-95; ASTM: West Conshohocken, PA, USA, 1995. [Google Scholar]
- Canada, E. Biological Test Method: Fertilization Assay Using Echinoids (Sea Urchins and Sand Dollars); Environment Canada: Gatineau, QC, Canada, 1992. [Google Scholar]
- USEPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms., 3rd ed.; EPA-821-R-02-014; Environmental Monitoring Systems Laboratory, U.S. Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Gambardella, C.; Ferrando, S.; Gatti, A.M.; Cataldi, E.; Ramoino, P.; Aluigi, M.G.; Faimali, M.; Diaspro, A.; Falugi, C. Review: Morphofunctional and Biochemical Markers of Stress in Sea Urchin Life Stages Exposed to Engineered Nanoparticles: Markers of Stress in Sea Urchin Exposed to Nanoparticles. Environ. Toxicol. 2016, 31, 1552–1562. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Hamers, T.; Martínez-Gómez, C.; Kamstra, J.H.; de Weert, J.; Leonards, P.E.G.; Smedes, F. Toxicity Profiling of Marine Surface Sediments: A Case Study Using Rapid Screening Bioassays of Exhaustive Total Extracts, Elutriates and Passive Sampler Extracts. Mar. Environ. Res. 2017, 124, 81–91. [Google Scholar] [CrossRef]
- Garmendia, J.M.; Menchaca, I.; Belzunce, M.J.; Franco, J.; Revilla, M. Seasonal Variability in Gonad Development in the Sea Urchin (Paracentrotus Lividus) on the Basque Coast (Southeastern Bay of Biscay). Mar. Pollut. Bull. 2010, 61, 259–266. [Google Scholar] [CrossRef]
- Alvarez-Mora, I.; Mijangos, L.; Lopez-Herguedas, N.; Amigo, J.M.; Eguiraun, H.; Salvoch, M.; Monperrus, M.; Etxebarria, N. SETApp: A Machine Learning and Image Analysis Based Application to Automate the Sea Urchin Embryo Test. Ecotoxicol. Environ. Saf. 2022, 241, 113728. [Google Scholar] [CrossRef]
- Lopez-Herguedas, N.; González-Gaya, B.; Cano, A.; Alvarez-Mora, I.; Mijangos, L.; Etxebarria, N.; Zuloaga, O.; Olivares, M.; Prieto, A. Effect-Directed Analysis of a Hospital Effluent Sample Using A-YES for the Identification of Endocrine Disrupting Compounds. Sci. Total Environ. 2022, 850, 157985. [Google Scholar] [CrossRef]
- Escher, B.; Neale, P.; Leusch, F. Bioanalytical Tools in Water Quality Assessment; IWA Publishing: London, UK, 2021. [Google Scholar]
- Brack, W.; Schirmer, K.; Erdinger, L.; Hollert, H. Effect-Directed Analysis of Mutagens and Ethoxyresorufin-O-Deethylase Inducers in Aquatic Sediments. Environ. Toxicol. Chem. 2005, 24, 2445–2458. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Singer, H.P.; Slobodnik, J.; Ipolyi, I.M.; Oswald, P.; Krauss, M.; Schulze, T.; Haglund, P.; Letzel, T.; Grosse, S.; et al. Non-Target Screening with High-Resolution Mass Spectrometry: Critical Review Using a Collaborative Trial on Water Analysis. Anal. Bioanal. Chem. 2015, 407, 6237–6255. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ru, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Sörengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass Loads, Source Apportionment, and Risk Estimation of Organic Micropollutants from Hospital and Municipal Wastewater in Recipient Catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, P.; Lu, S.; Liu, X.; Zhai, J.; Xu, J.; Wang, Y.; Zhang, G.; Liu, X.; Wan, Z. Occurrence and Risk Evaluation of Organophosphorus Pesticides in Typical Water Bodies of Beijing, China. Environ. Sci. Pollut. Res. 2021, 28, 1454–1463. [Google Scholar] [CrossRef]
- Maceira, A.; Marcé, R.M.; Borrull, F. Occurrence of Benzothiazole, Benzotriazole and Benzenesulfonamide Derivates in Outdoor Air Particulate Matter Samples and Human Exposure Assessment. Chemosphere 2018, 193, 557–566. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Wang, L.; Thomaidis, N.S.; Kannan, K. Benzotriazoles and Benzothiazoles in Human Urine from Several Countries: A Perspective on Occurrence, Biotransformation, and Human Exposure. Environ. Int. 2013, 59, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging Pollutants in Sewage, Surface and Drinking Water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Cousins, I.T.; Mackay, D.; Yamada, K. Observed Concentrations in the Environment. In Series Anthropogenic Compounds: Phtalate Esters; Staples, C.A., Ed.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2003; pp. 125–177. [Google Scholar] [CrossRef]
- Meng, D.; Fan, D.; Gu, W.; Wang, Z.; Chen, Y.; Bu, H.; Liu, J. Development of an Integral Strategy for Non-Target and Target Analysis of Site-Specific Potential Contaminants in Surface Water: A Case Study of Dianshan Lake, China. Chemosphere 2020, 243, 125367. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K. Hazard Assessment of Commonly Used Agricultural Antibiotics on Aquatic Ecosystems. Ecotoxicology 2008, 17, 526–538. [Google Scholar] [CrossRef]
- Godoy, A.A.; Domingues, I.; de Carvalho, L.B.; Oliveira, Á.C.; de Jesus Azevedo, C.C.; Taparo, J.M.; Assano, P.K.; Mori, V.; de Almeida Vergara Hidalgo, V.; Nogueira, A.J.A.; et al. Assessment of the Ecotoxicity of the Pharmaceuticals Bisoprolol, Sotalol, and Ranitidine Using Standard and Behavioral Endpoints. Environ. Sci. Pollut. Res. 2020, 27, 5469–5481. [Google Scholar] [CrossRef]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Zebrafish (Danio Rerio) Embryos as a Model for Testing Proteratogens. Toxicology 2011, 281, 25–36. [Google Scholar] [CrossRef]
- Grung, M.; Källqvist, T.; Sakshaug, S.; Skurtveit, S.; Thomas, K.V. Environmental Assessment of Norwegian Priority Pharmaceuticals Based on the EMEA Guideline. Ecotoxicol. Environ. Saf. 2008, 71, 328–340. [Google Scholar] [CrossRef]
- Goolsby, E.W.; Mason, C.M.; Wojcik, J.T.; Jordan, A.M.; Black, M.C. Acute and Chronic Effects of Diphenhydramine and Sertraline Mixtures in Ceriodaphnia Dubia. Environ. Toxicol. Chem. 2013, 32, 2866–2869. [Google Scholar] [CrossRef]
- Selderslaghs, Ingrid, W. T.; Blust, Ronny; Witters, Hilda, E. Feasibility Study of the Zebrafish Assay as an Alternative Method to Screen for Developmental Toxicity and Embryotoxicity Using a Training Set of 27 Compounds. Reprod. Toxicol. 2012, 33, 142–154. [Google Scholar] [CrossRef]
- Kim, J.W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute Toxicity of Pharmaceutical and Personal Care Products on Freshwater Crustacean (Thamnocephalus Platyurus) and Fish (Oryzias Latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef]
- Isidori, M.; Nardelli, A.; Parrella, A.; Pascarella, L.; Previtera, L. A Multispecies Study to Assess the Toxic and Genotoxic Effect of Pharmaceuticals: Furosemide and Its Photoproduct. Chemosphere 2006, 63, 785–793. [Google Scholar] [CrossRef]
- de Carvalho Penha, L.C.; Coimbra Rola, R.; da Silva Junior, F.M.; de Martinez Gaspar Martins, C. Toxicity and Sublethal Effects of Methylparaben on Zebrafish (Danio Rerio) Larvae and Adults. Environ. Sci. Pollut. Res. 2021, 28, 45534–45544. [Google Scholar] [CrossRef]
- Bazin, I.; Gadal, A.; Touraud, E.; Roig, B. Hydroxy Benzoate Preservatives (Parabens) in the Environment: Data for Environmental Toxicity Assessment. In Xenobiotics in the Urban Water Cycle: Mass Flows, Environmental Processes, Mitigation and Treatment Strategies; Fatta-Kassinos, D., Bester, K., Kümmerer, K., Eds.; Environmental Pollution; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 245–257. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and Genotoxic Evaluation of Six Antibiotics on Non-Target Organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef]
- Du, Z.; Wang, G.; Gao, S.; Wang, Z. Aryl Organophosphate Flame Retardants Induced Cardiotoxicity during Zebrafish Embryogenesis: By Disturbing Expression of the Transcriptional Regulators. Aquat. Toxicol. 2015, 161, 25–32. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, P.; Randak, T. Ecotoxocological Effects of Short-Term Exposure to a Human Pharmaceutical Verapamil in Juvenile Rainbow Trout (Oncorhynchus Mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 385–391. [Google Scholar] [CrossRef]
- Villegas-Navarro, A.; Rosas-L., E.; Reyes, J.L. The Heart of Daphnia Magna: Effects of Four Cardioactive Drugs. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 127–134. [Google Scholar] [CrossRef]
- Adams, W.J.; Biddinger, G.R.; Robillard, K.A.; Gorsuch, J.W. A Summary of the Acute Toxicity of 14 Phthalate Esters to Representative Aquatic Organisms. Environ. Toxicol. Chem. 1995, 14, 1569–1574. [Google Scholar] [CrossRef]
- Pauli, W.; Berger, S.; Schmitz, S.; Jaskulka, L.; Stadtlander, K. Validierung Toxikologischer Pruefparameter an Tetrahymena. Membranfunktionen, Chemotaxis, Rotation Im Elektrischen Drehfeld (Validation of Toxicological Endpoints with Tetrahymena. Membrane Functions, Chemotaxis, Cell Rotation in Electric Fields); Freie Universität Berlin: Berlin, Germany, 1993. [Google Scholar]
- Prieto-Amador, M. Ecotoxicological Assessment of Phthalates in the Freshwater Gastropod Physella Acuta and the Sea Urchin Paracentrotus Lividus. PhD. Thesis, Universidad Nacional de Educación a Distancia (UNED), Madrid, Spain, 2022. [Google Scholar]
- Ribeiro, S.; Torres, T.; Martins, R.; Santos, M.M. Toxicity Screening of Diclofenac, Propranolol, Sertraline and Simvastatin Using Danio Rerio and Paracentrotus Lividus Embryo Bioassays. Ecotoxicol. Environ. Saf. 2015, 114, 67–74. [Google Scholar] [CrossRef]
- Neale, P.A.; Ait-Aissa, S.; Brack, W.; Creusot, N.; Denison, M.S.; Deutschmann, B.; Hilscherová, K.; Hollert, H.; Krauss, M.; Novák, J.; et al. Linking in Vitro Effects and Detected Organic Micropollutants in Surface Water Using Mixture-Toxicity Modeling. Environ. Sci. Technol. 2015, 49, 14614–14624. [Google Scholar] [CrossRef]
- König, M.; Escher, B.I.; Neale, P.A.; Krauss, M.; Hilscherová, K.; Novák, J.; Teodorović, I.; Schulze, T.; Seidensticker, S.; Hashmi, M.A.K.; et al. Impact of Untreated Wastewater on a Major European River Evaluated with a Combination of in Vitro Bioassays and Chemical Analysis. Environ. Pollut. 2017, 220, 1220–1230. [Google Scholar] [CrossRef]
- Neale, P.A.; Munz, N.A.; Aїt-Aїssa, S.; Altenburger, R.; Brion, F.; Busch, W.; Escher, B.I.; Hilscherová, K.; Kienle, C.; Novák, J.; et al. Integrating Chemical Analysis and Bioanalysis to Evaluate the Contribution of Wastewater Effluent on the Micropollutant Burden in Small Streams. Sci. Total Environ. 2017, 576, 785–795. [Google Scholar] [CrossRef]
- Kienle, C.; Vermeirssen, E.L.M.; Schifferli, A.; Singer, H.; Stamm, C.; Werner, I. Effects of Treated Wastewater on the Ecotoxicity of Small Streams – Unravelling the Contribution of Chemicals Causing Effects. PLoS ONE 2019, 14, e0226278. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; van Daele, C.; Dutt, M.; Tang, J.Y.M.; Altenburger, R. Most Oxidative Stress Response In Water Samples Comes From Unknown Chemicals: The Need For Effect-Based Water Quality Trigger Values. Environ. Sci. Technol. 2013, 47, 7002–7011. [Google Scholar] [CrossRef]
- Välitalo, P.; Massei, R.; Heiskanen, I.; Behnisch, P.; Brack, W.; Tindall, A.J.; Du Pasquier, D.; Küster, E.; Mikola, A.; Schulze, T.; et al. Effect-Based Assessment of Toxicity Removal during Wastewater Treatment. Water Res. 2017, 126, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Saco-Álvarez, Liliana; Durán, Iria; Lorenzo, J. Ignacio; Beiras, Ricardo. Methodological Basis for the Optimization of a Marine Sea-Urchin Embryo Test (SET) for the Ecological Assessment of Coastal Water Quality. Ecotoxicol. Environ. Saf. 2010, 73, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, C.; Ramos-Gómez, J.; Martín-Díaz, L.; DelValls, T.A. Identification of Specific Malformations of Sea Urchin Larvae for Toxicity Assessment: Application to Marine Pisciculture Effluents. Mar. Environ. Res. 2012, 77, 12–22. [Google Scholar] [CrossRef]
- Prieto, A.; Zuloaga, O.; Usobiaga, A.; Etxebarria, N.; Fernández, L.A. Development of a Stir Bar Sorptive Extraction and Thermal Desorption–Gas Chromatography–Mass Spectrometry Method for the Simultaneous Determination of Several Persistent Organic Pollutants in Water Samples. J. Chromatogr. A 2007, 1174, 40–49. [Google Scholar] [CrossRef]
- Fernández, N.; Beiras, R. Combined Toxicity of Dissolved Mercury With Copper, Lead and Cadmium on Embryogenesis and Early Larval Growth of the Paracentrotus Lividus Sea-Urchin. Ecotoxicology 2001, 10, 263–271. [Google Scholar] [CrossRef]
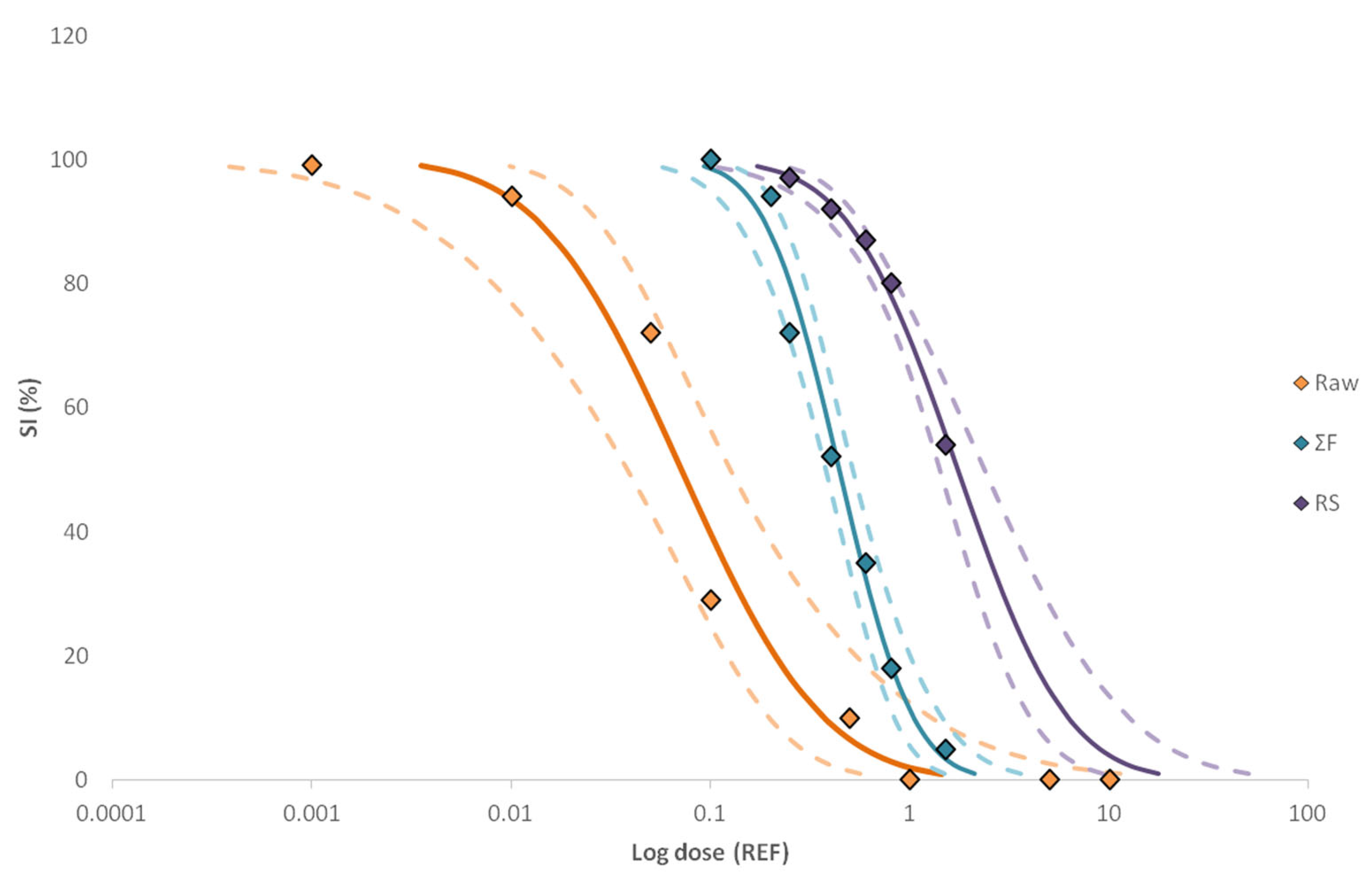
| Sample | Size Increase (SI) | |
|---|---|---|
| EC50 | TUbio | |
| Raw | 0.071 | 14.1 |
| (0.038–0.125) | (8.0–26.3) | |
| RS | 1.733 | 0.6 |
| (1.410–2.371) | (0.4–0.7) | |
| ∑F | 0.442 | 2.3 |
| (0.380–0.517) | (1.9–2.6) | |
| Compound | Raw (ng/L) | Application | MoA |
|---|---|---|---|
| 2,4-Di-tert-butylphenol * | 36,245 | Industrial product/Plasticizer | Unknown |
| 2-Hydroxybenzothiazole | 914 | Industrial product/Chemical manufacturing | Unknown |
| Acetamiprid | 24 | Pesticide/Insecticide | Nicotinic acetylcholine receptor (nAChR) |
| Bisoprolol | 1051 | Pharmaceutical/Antihypertensive | β-blocker |
| Clozapine | 8 | Pharmaceutical/Antipsychotic | Neurotransmitter receptor blocker |
| Cyclophosphamide | 138 | Pharmaceutical/Antineoplastic | DNA replication and protein synthesis inhibitor |
| Diethyl phthalate * | 63,097 | Industrial product/Plasticizer | Endocrine disruptor |
| Diphenhydramine | 3 | Pharmaceutical/Antihistamine | Histamine H1 receptor |
| Ediphenfos | 8 | Pesticide/Fungicide | Acetylcholinesterase and phospholipid biosynthesis inhibitor |
| Eprosartan | 602 | Pharmaceutical/Antihypertensive | Angiotensin receptor or enzyme |
| Fenpropidin | 20 | Pesticide/Fungicide | Sterol biosynthesis inhibition |
| Fluconazole | 4138 | Pharmaceutical/Antifungal | Sterol biosynthesis inhibition |
| Furosemide | 37,232 | Pharmaceutical/Diuretic | Ion channel modulation |
| Imazalil | 13 | Pesticide/Fungicide | Sterol biosynthesis inhibition |
| Isoproturon | 7 | Pesticide/Herbicide | Photosynthesis inhibition |
| Methylparaben | 15,614 | Personal care product/Preservative | DNA, RNA and enzymes synthesis inhibitor |
| Ofloxacin | 6154 | Pharmaceutical/Antibiotic | Nucleic acid biosynthesis |
| Omeprazole | 923 | Pharmaceutical/Gastric disorders | Proton pump inhibition |
| Pentoxifylline | 98 | Pharmaceutical/Anticoagulant | Signal transduction/Erythrocyte phosphodiesterase |
| Primidone | 6818 | Pharmaceutical/Antiepileptic | Neuroactive/GABA receptor |
| Propranolol | 3 | Pharmaceutical/Antihypertensive | β-blocker |
| Spiroxamine | 211 | Pesticide/Fungicide | Sterol biosynthesis inhibition |
| Sulfamethoxazole | 2133 | Pharmaceutical/Antibiotic | Dihydropteroate synthesis |
| Triethylphosphate | 15 | Industrial product/Flame retardant | Enzyme inhibition |
| Verapamil | 10 | Pharmaceutical/Antihypertensive | Calcium ion channel modulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Herguedas, N.; Mijangos, L.; Alvarez-Mora, I.; González-Gaya, B.; Uribe-Echeverria, T.; Etxebarria, N.; Zuloaga, O.; Olivares, M.; Prieto, A. Suspect Screening of Chemicals in Hospital Wastewaters Using Effect-Directed Analysis Approach as Prioritization Strategy. Molecules 2023, 28, 1212. https://doi.org/10.3390/molecules28031212
Lopez-Herguedas N, Mijangos L, Alvarez-Mora I, González-Gaya B, Uribe-Echeverria T, Etxebarria N, Zuloaga O, Olivares M, Prieto A. Suspect Screening of Chemicals in Hospital Wastewaters Using Effect-Directed Analysis Approach as Prioritization Strategy. Molecules. 2023; 28(3):1212. https://doi.org/10.3390/molecules28031212
Chicago/Turabian StyleLopez-Herguedas, Naroa, Leire Mijangos, Iker Alvarez-Mora, Belén González-Gaya, Teresa Uribe-Echeverria, Nestor Etxebarria, Olatz Zuloaga, Maitane Olivares, and Ailette Prieto. 2023. "Suspect Screening of Chemicals in Hospital Wastewaters Using Effect-Directed Analysis Approach as Prioritization Strategy" Molecules 28, no. 3: 1212. https://doi.org/10.3390/molecules28031212
APA StyleLopez-Herguedas, N., Mijangos, L., Alvarez-Mora, I., González-Gaya, B., Uribe-Echeverria, T., Etxebarria, N., Zuloaga, O., Olivares, M., & Prieto, A. (2023). Suspect Screening of Chemicals in Hospital Wastewaters Using Effect-Directed Analysis Approach as Prioritization Strategy. Molecules, 28(3), 1212. https://doi.org/10.3390/molecules28031212