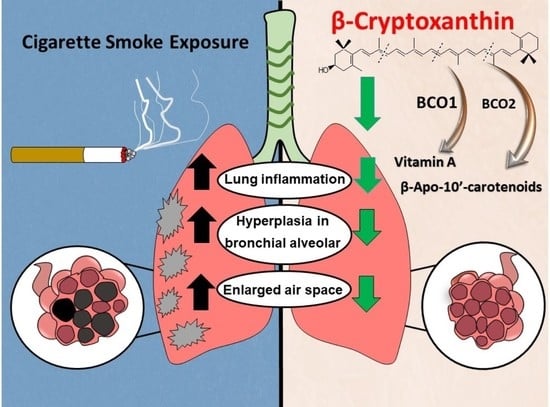
β-Cryptoxanthin Attenuates Cigarette-Smoke-Induced Lung Lesions in the Absence of Carotenoid Cleavage Enzymes (BCO1/BCO2) in Mice
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Urinary Cotinine Levels
2.2. Hepatic Vitamin A and BCX Levels in WT and DKO Mice fed Control and BCX Diets with or without CS Exposure
2.3. Effects of BCX Supplementation on CS-Induced Inflammatory Cell Infiltration in WT and DKO Mice
2.4. Effects of BCX Supplementation on CS-Induced Enlargement of Alveolar Airspace and Bronchiolar Epithelium Hyperplasia in WT and DKO Mice
2.5. Effects of BCX and BCX Metabolite, 3OH-BA10C, on Inflammatory Responses in Human Bronchial Epithelial Cells Stimulated with Lipopolysaccharide (LPS)
3. Discussion
4. Materials and Methods
4.1. Animals, Diets, and CS-Exposure
4.2. Histological Analysis
4.3. High-Performance Liquid Chromatography (HPLC)
4.4. Cell Culture and Reagents
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Casetta, B.; Videla, A.J.; Bardach, A.; Morello, P.; Soto, N.; Lee, K.; Camacho, P.A.; Hermoza Moquillaza, R.V.; Ciapponi, A. Association between cigarette smoking prevalence and income level: A systematic review and meta-analysis. Nicotine Tob Res. 2017, 19, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Erdman, J.W. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef] [PubMed]
- Mustra Rakic, J.; Wang, X.D. Role of lycopene in smoke-promoted chronic obstructive pulmonary disease and lung carcinogenesis. Arch. Biochem. Biophys. 2020, 689, 108439. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits-where are we now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef]
- Lim, J.Y.; Wang, X.D. Mechanistic understanding of beta-cryptoxanthin and lycopene in cancer prevention in animal models. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158652. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. 2014, 105, 736–743. [Google Scholar] [CrossRef]
- Yuan, J.M.; Ross, R.K.; Chu, X.D.; Gao, Y.T.; Yu, M.C. Prediagnostic levels of serum beta-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2001, 10, 767–773. [Google Scholar]
- Yuan, J.M.; Stram, D.O.; Arakawa, K.; Lee, H.P.; Yu, M.C. Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 890–898. [Google Scholar]
- Mannisto, S.; Smith-Warner, S.A.; Spiegelman, D.; Albanes, D.; Anderson, K.; van den Brandt, P.A.; Cerhan, J.R.; Colditz, G.; Feskanich, D.; Freudenheim, J.L.; et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Bronson, R.T.; Russell, R.M.; Wang, X.D. beta-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev. Res. 2011, 4, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.R.; Miao, B.; Li, X.; Hu, K.Q.; Liu, C.; Wang, X.D. beta-Cryptoxanthin Reduced Lung Tumor Multiplicity and Inhibited Lung Cancer Cell Motility by Downregulating Nicotinic Acetylcholine Receptor alpha7 Signaling. Cancer Prev. Res. 2016, 9, 875–886. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Vogt, K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 2000, 275, 11915–11920. [Google Scholar] [CrossRef]
- von Lintig, J. Colors with functions: Elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 2010, 30, 35–56. [Google Scholar] [CrossRef]
- Harrison, E.H.; Kopec, R.E. Enzymology of vertebrate carotenoid oxygenases. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158653. [Google Scholar] [CrossRef]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; von Lintig, J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef]
- Hu, K.Q.; Liu, C.; Ernst, H.; Krinsky, N.I.; Russell, R.M.; Wang, X.D. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 2006, 281, 19327–19338. [Google Scholar] [CrossRef]
- Mein, J.R.; Dolnikowski, G.G.; Ernst, H.; Russell, R.M.; Wang, X.D. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and β-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch. Biochem. Biophys. 2011, 506, 109–121. [Google Scholar] [CrossRef]
- Amengual, J.; Widjaja-Adhi, M.A.K.; Rodriguez-Santiago, S.; Hessel, S.; Golczak, M.; Palczewski, K.; von Lintig, J. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 2013, 288, 34081–34096. [Google Scholar] [CrossRef]
- Harrison, E.H.; Quadro, L. Apocarotenoids: Emerging Roles in Mammals. Annu. Rev. Nutr. 2018, 38, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Lietz, G.; Oxley, A.; Leung, W.; Hesketh, J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J. Nutr. 2012, 142, 161S–165S. [Google Scholar] [CrossRef]
- Meyers, K.J.; Mares, J.A.; Igo, R.P., Jr.; Truitt, B.; Liu, Z.; Millen, A.E.; Klein, M.; Johnson, E.J.; Engelman, C.D.; Karki, C.K.; et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Investig. Ophthalmol. Vis. Sci. 2014, 55, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2012, 56, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.A.; Clinton, S.K.; von Lintig, J.; Wyss, A.; Erdman, J.W. Loss of Carotene-9’, 10’-Monooxygenase Expression Increases Serum and Tissue Lycopene Concentrations in Lycopene-Fed Mice. J. Nutr. 2010, 140, 2134–2138. [Google Scholar] [CrossRef]
- Tian, R.; Pitchford, W.S.; Morris, C.A.; Cullen, N.G.; Bottema, C.D.K. Genetic variation in the beta, beta-carotene-9’, 10’-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim. Genet. 2010, 41, 253–259. [Google Scholar] [CrossRef]
- Praud, C.; Al Ahmadieh, S.; Voldoire, E.; Le Vern, Y.; Godet, E.; Courousse, N.; Graulet, B.; Duval, E.L.; Berri, C.; Duclos, M.J. Beta-carotene preferentially regulates chicken myoblast proliferation withdrawal and differentiation commitment via BCO1 activity and retinoic acid production. Exp. Cell Res. 2017, 358, 140–146. [Google Scholar] [CrossRef]
- Amengual, J.; Gouranton, E.; van Helden, Y.G.J.; Hessel, S.; Ribot, J.; Kramer, E.; Kiec-Wilk, B.; Razny, U.; Lietz, G.; Wyss, A.; et al. Beta-Carotene reduces body adiposity of mice via BCMO1. PLoS ONE 2011, 6, e20644. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Lyu, Y.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; Hildebrand, D.; Wang, W.; Medeiros, D.M.; Shen, X.; et al. Targeted Metabolomics Reveals Abnormal Hepatic Energy Metabolism by Depletion of beta-Carotene Oxygenase 2 in Mice. Sci. Rep. 2017, 7, 14624. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Liu, C.; Hu, K.Q.; Smith, D.E.; Wang, X.D. Ablation of carotenoid cleavage enzymes (BCO1 and BCO2) induced hepatic steatosis by altering the farnesoid X receptor/miR-34a/sirtuin 1 pathway. Arch. Biochem. Biophys. 2018, 654, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Liu, C.; Hu, K.Q.; Smith, D.E.; Wu, D.; Lamon-Fava, S.; Ausman, L.M.; Wang, X.D. Xanthophyll beta-Cryptoxanthin Inhibits Highly Refined Carbohydrate Diet-Promoted Hepatocellular Carcinoma Progression in Mice. Mol. Nutr. Food Res. 2020, 64, e1900949. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.J.; Fidler, J.; Mindell, J.; Feyerabend, C.; West, R. Assessing smoking status in children, adolescents and adults: Cotinine cut-points revisited. Addiction 2008, 103, 1553–1561. [Google Scholar] [CrossRef]
- Hendrix, A.Y.; Kheradmand, F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog. Mol. Biol. Transl. Sci. 2017, 148, 1–29. [Google Scholar]
- Martini, D.; Negrini, L.; Marino, M.; Riso, P.; Del Bo, C.; Porrini, M. What Is the Current Direction of the Research on Carotenoids and Human Health? An Overview of Registered Clinical Trials. Nutrients 2022, 14, 1191. [Google Scholar] [CrossRef]
- Chen, D.; Steele, A.D.; Lindquist, S.; Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 2005, 310, 1641. [Google Scholar] [CrossRef]
- Couillard, C.; Lemieux, S.; Vohl, M.C.; Couture, P.; Lamarche, B. Carotenoids as biomarkers of fruit and vegetable intake in men and women. Br. J. Nutr. 2016, 116, 1206–1215. [Google Scholar] [CrossRef]
- Billatos, E.; Faiz, A.; Gesthalter, Y.; LeClerc, A.; Alekseyev, Y.O.; Xiao, X.; Liu, G.; ten Hacken, N.H.T.; Heijink, I.H.; Timens, W.; et al. Impact of acute exposure to cigarette smoke on airway gene expression. Physiol. Genom. 2018, 50, 705–713. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.Q.; Choi, S.W.; Ausman, L.M.; Wang, X.D. beta-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev. Res. 2013, 6, 309–320. [Google Scholar] [CrossRef]
- Herfs, M.; Hubert, P.; Poirrier, A.L.; Vandevenne, P.; Renoux, V.; Habraken, Y.; Cataldo, D.; Boniver, J.; Delvenne, P. Proinflammatory Cytokines Induce Bronchial Hyperplasia and Squamous Metaplasia in Smokers Implications for Chronic Obstructive Pulmonary Disease Therapy. Am. J. Resp. Cell Mol. 2012, 47, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; Manicone, A.M.; Parks, W.C. Matrix metalloproteinases in emphysema. Matrix Biol. 2018, 73, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Meshi, B.; Vitalis, T.Z.; Ionescu, D.; Elliott, W.M.; Liu, C.; Wang, X.D.; Hayashi, S.; Hogg, J.C. Emphysematous lung destruction by cigarette smoke. The effects of latent adenoviral infection on the lung inflammatory response. Am. J. Respir. Cell Mol. Biol. 2002, 26, 52–57. [Google Scholar] [CrossRef]
- Schmitz, H.H.; Poor, C.L.; Wellman, R.B.; Erdman, J.W., Jr. Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J. Nutr. 1991, 121, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D. Biological Activities of Carotenoid Metabolites. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhauser: Basel, Belgium, 2009; Volume 5, pp. 383–408. [Google Scholar]
- Kelly, M.E.; Ramkumar, S.; Sun, W.; Colon Ortiz, C.; Kiser, P.D.; Golczak, M.; von Lintig, J. The biochemical basis of vitamin A production from the asymmetric carotenoid beta-cryptoxanthin. ACS Chem. Biol. 2018, 13, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kinnula, V.L.; Gorbunova, V.; Yao, H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev. Med. 2012, 54, S20–S28. [Google Scholar] [CrossRef]
- Balansky, R.; Ganchev, G.; Iltcheva, M.; Dimitrova, E.; Micale, R.T.; La Maestra, S.; De Flora, S. Carcinogenic response and other histopathological alterations in mice exposed to cigarette smoke for varying time periods after birth. Carcinogenesis 2018, 39, 580–587. [Google Scholar] [CrossRef]
- Mustra Rakic, J.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C.O.; Ausman, L.M.; Wang, X.D. Lycopene Inhibits Smoke-Induced Chronic Obstructive Pulmonary Disease and Lung Carcinogenesis by Modulating Reverse Cholesterol Transport in Ferrets. Cancer Prev. Res. 2019, 12, 421–432. [Google Scholar] [CrossRef]
- Troiano, C.; Jaleel, Z.; Spiegel, J.H. Association of Electronic Cigarette Vaping and Cigarette Smoking with Decreased Random Flap Viability in Rats. JAMA Facial Plast. Surg. 2019, 21, 5–10. [Google Scholar] [CrossRef] [Green Version]






| Wild Type | BCO1/BCO2 DKO | |||||||
|---|---|---|---|---|---|---|---|---|
| Experimental Group | Control | BCX | CS | CS+BCX | Control | BCX | CS | CS + BCX |
| Animal (n) | 6 | 6 | 6 | 6 | 8 | 7 | 9 | 8 |
| Male | 3 | 3 | 3 | 3 | 5 | 4 | 5 | 4 |
| Female | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 |
| Age (day) | 206 | 206 | 206 | 206 | 200 | 201 | 205 | 210 |
| Final BW (g) | 36 ± 9 | 37 ± 7 | 38 ± 8 | 35 ± 7 | 33 ± 9 | 34 ± 6 | 31 ± 6 | 32 ± 8 |
| UC (μg/mL) | 0.5 ± 0.2 a | 0.4 ± 0.2 a | 3.9 ± 1.3 b | 4.0 ± 3.1 b | 0.4 ± 0.4 a | 0.2 ± 0.2 a | 4.1 ± 2.3 b | 4.0 ± 3.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaverelli, R.A.; Hu, K.-Q.; Liu, C.; Lim, J.Y.; Daniels, M.S.; Xia, H.; Mein, J.; von Lintig, J.; Wang, X.-D. β-Cryptoxanthin Attenuates Cigarette-Smoke-Induced Lung Lesions in the Absence of Carotenoid Cleavage Enzymes (BCO1/BCO2) in Mice. Molecules 2023, 28, 1383. https://doi.org/10.3390/molecules28031383
Chiaverelli RA, Hu K-Q, Liu C, Lim JY, Daniels MS, Xia H, Mein J, von Lintig J, Wang X-D. β-Cryptoxanthin Attenuates Cigarette-Smoke-Induced Lung Lesions in the Absence of Carotenoid Cleavage Enzymes (BCO1/BCO2) in Mice. Molecules. 2023; 28(3):1383. https://doi.org/10.3390/molecules28031383
Chicago/Turabian StyleChiaverelli, Rachel A., Kang-Quan Hu, Chun Liu, Ji Ye Lim, Michael S. Daniels, Hui Xia, Jonathan Mein, Johannes von Lintig, and Xiang-Dong Wang. 2023. "β-Cryptoxanthin Attenuates Cigarette-Smoke-Induced Lung Lesions in the Absence of Carotenoid Cleavage Enzymes (BCO1/BCO2) in Mice" Molecules 28, no. 3: 1383. https://doi.org/10.3390/molecules28031383
APA StyleChiaverelli, R. A., Hu, K.-Q., Liu, C., Lim, J. Y., Daniels, M. S., Xia, H., Mein, J., von Lintig, J., & Wang, X.-D. (2023). β-Cryptoxanthin Attenuates Cigarette-Smoke-Induced Lung Lesions in the Absence of Carotenoid Cleavage Enzymes (BCO1/BCO2) in Mice. Molecules, 28(3), 1383. https://doi.org/10.3390/molecules28031383