Do Fungicides Affect Alkaloid Production in Catharanthus roseus (L.) G. Don. Seedlings?
Abstract
1. Introduction
2. Results
2.1. Optimization of Various Fungicides’ Concentrations on Chaetomium sp. Growth
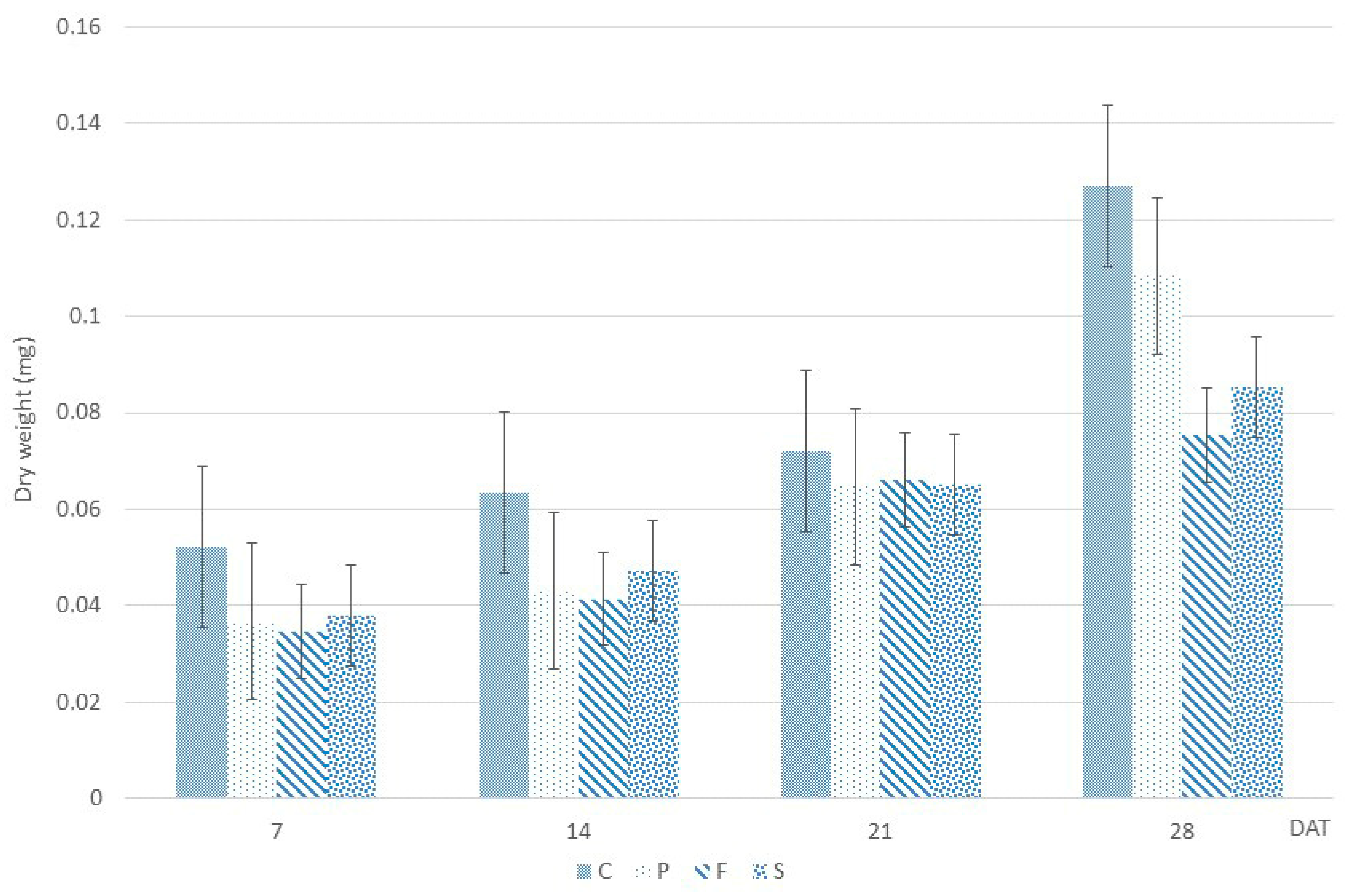
2.2. Dry Weight
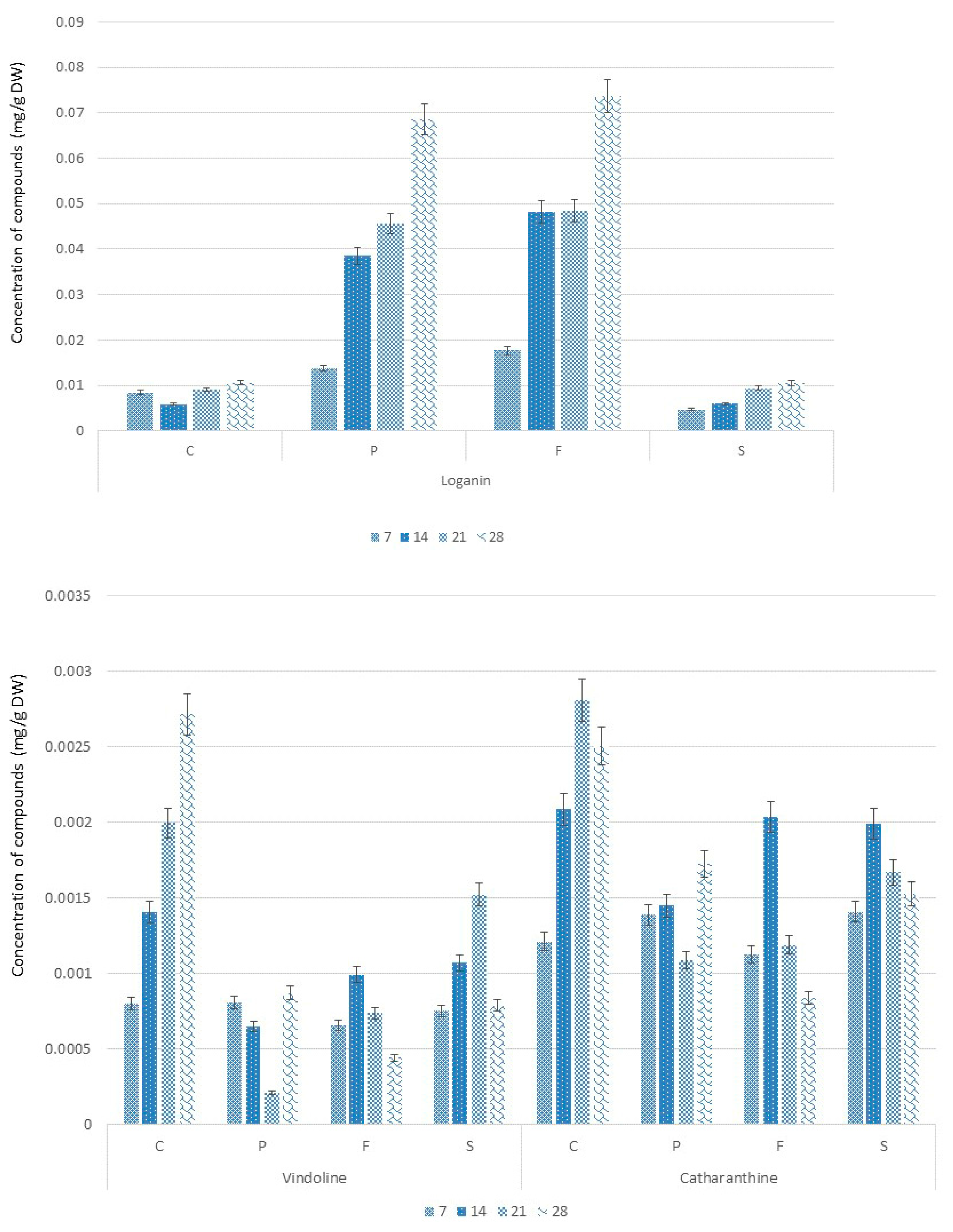
2.3. Alkaloids Accumulation
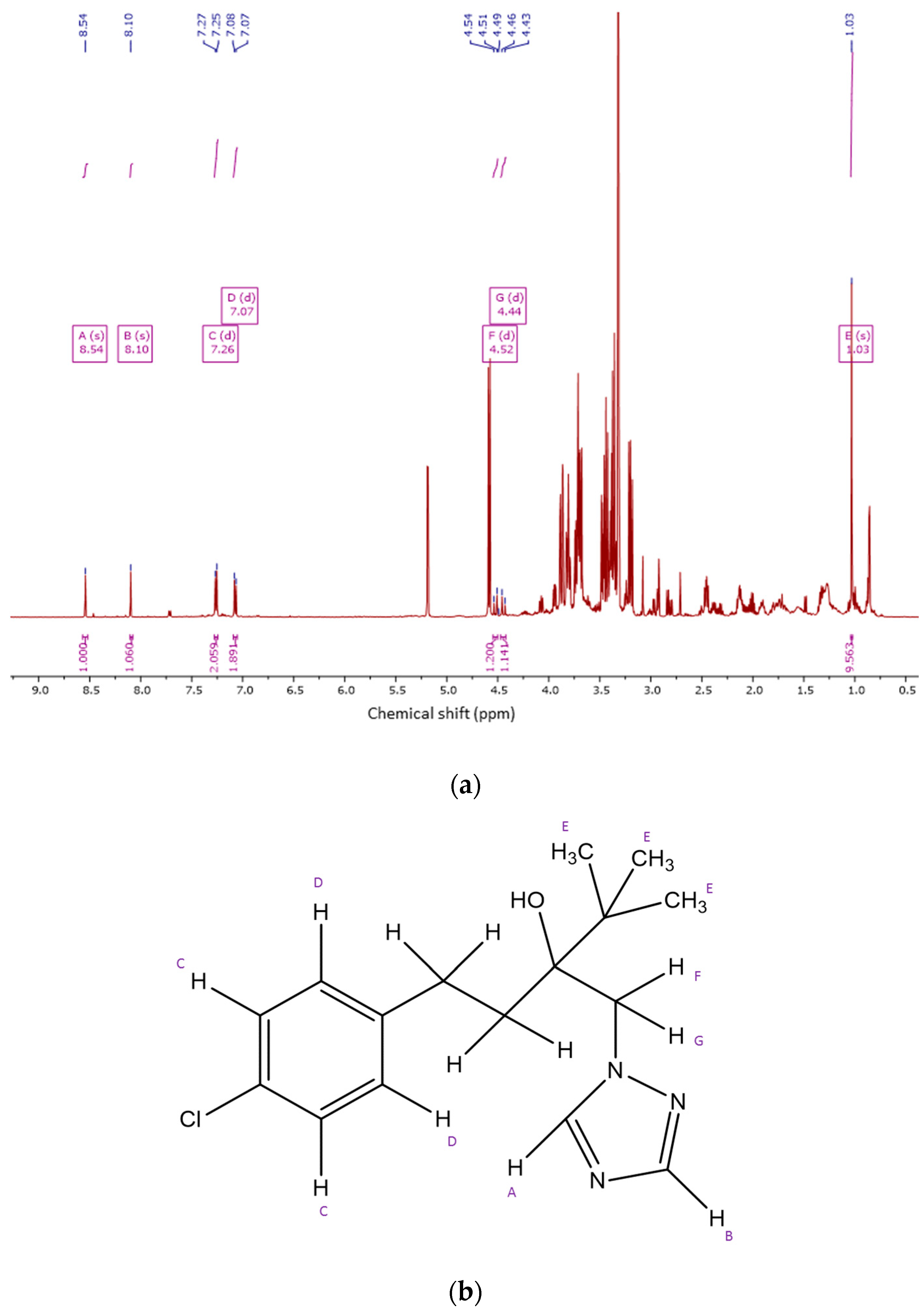
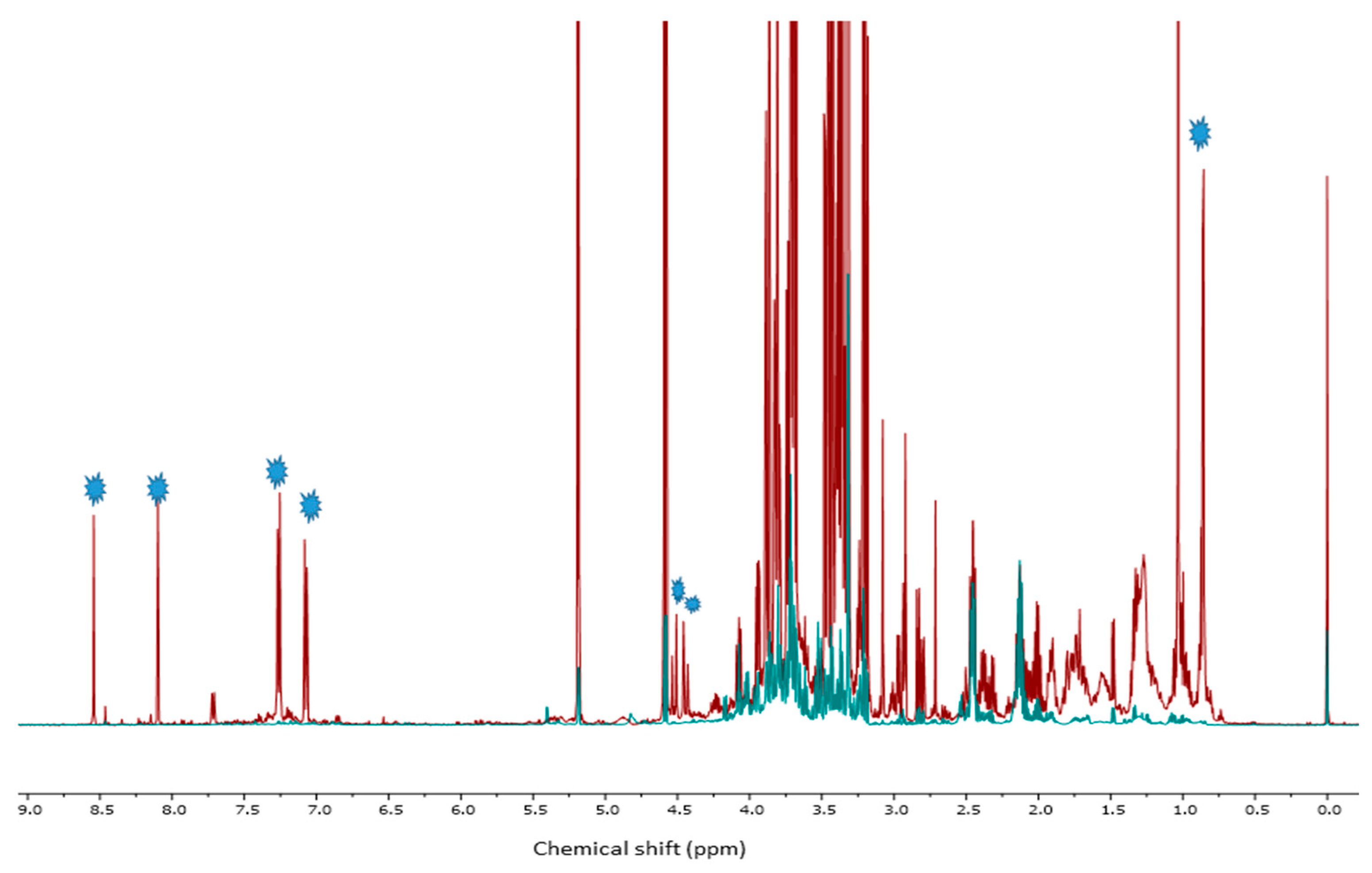
2.4. 1HNMR Measurements
2.4.1. Metabolite Identification
2.4.2. Multivariate Data Analysis
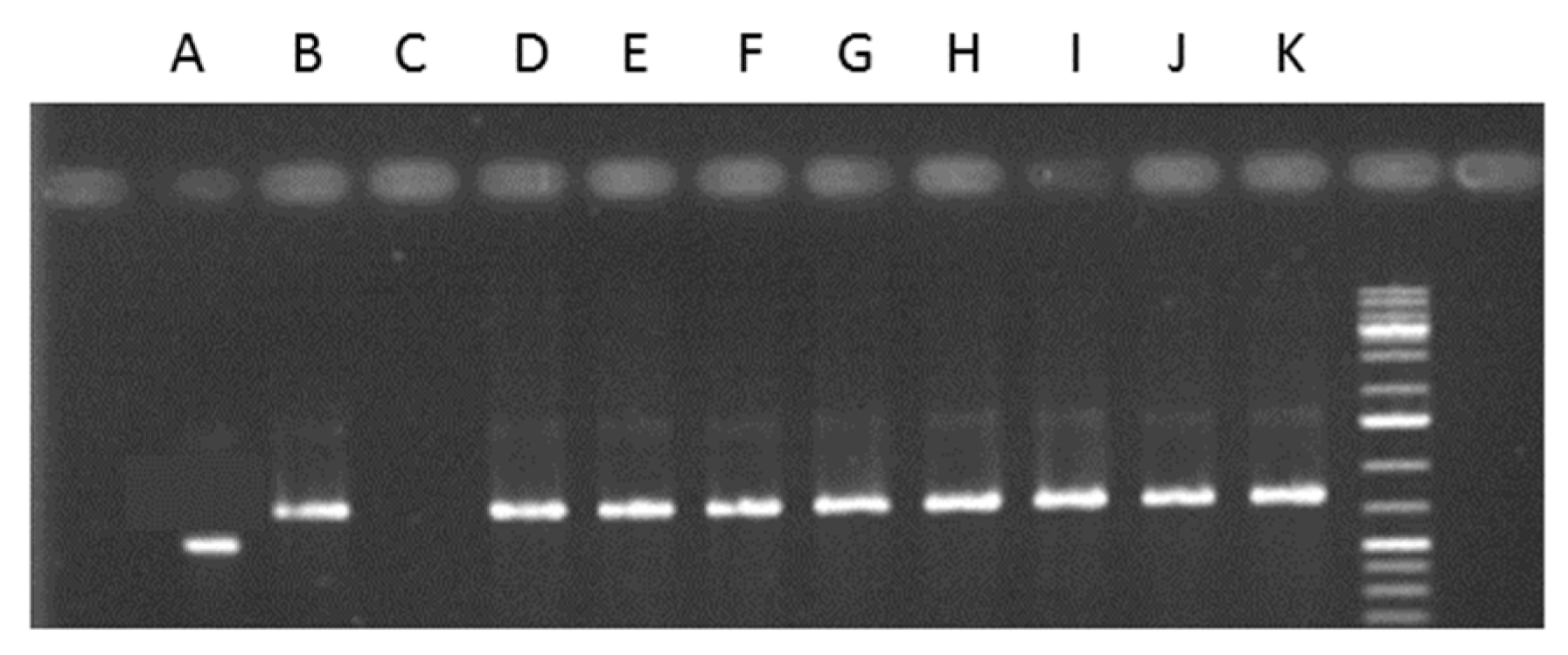
2.5. Endophytes Detection
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Effect of Fungicides on the Fungus Growth
4.3. Effect of Fungicides on C. roseus Seedlings
4.4. Seedling Germination
4.5. Standard Solution
4.6. Alkaloid Extraction
4.7. HPLC Analysis
4.8. Extraction and 1HNMR Measurement
4.9. Endophytes Detection
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Genet. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Verma, V.C.; Strobel, G.; Ezra, D. The endophytic fungal complex of Catharanthus roseus (L.) G. Don. Curr. Sci. 2008, 95, 228–232. [Google Scholar]
- Petrini, O.; Sieber, T.N.; Toti, L.; Viret, O. Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat. Toxins 1993, 1, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Ramani, S.; Jayabaskaran, C. Enhanced catharanthine and vindoline production in suspension cultures of C. roseus by ultraviolet-B light. J. Mol. Signal. 2008, 3, 9. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive Microbial Metabolites, a personal view. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Natural products from plant associated microorganism: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Muller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 7, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Daley, S.-K.; Cordell, G.A. Biologically Significant and Recently Isolated Alkaloids from Endophytic Fungi. J. Nat. Prod. 2021, 84, 871–897. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D.; Grothaus, P.; Bignami, G. The search for a taxol-producing microorganism among endophytic fungi of the Pacific yew, Taxus brevifolia. J. Nat. Prod. 1995, 58, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Kim, H.J.; Hur, B.K. Taxol promising fungal endophyt, Pestalotiopsis species isolated from Taxus cuspidata. J. Biosci. Bioeng. 2010, 110, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, R.S.; Muthumary, J.; Hur, B.-K. Taxol from Phyllosticta citricarpa, a leaf spot fungus of the angiosperm Citrus medica. J. Biosci. Bioeng. 2008, 106, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R.; Choi, Y.H. Effect of fungicide in galanthamine and metabolite profiles in Narcissus bulbs. Plant Physiol. Biochem. 2012, 58, 116–123. [Google Scholar] [CrossRef]
- Soliman, S.; Greenwood, J.S.; Bombarely, A.; Mueller, L.A.; Tsao, R.; Mosser, D.D.; Raizada, M.N. An Endophyte Constructs Fungicide-Containing Extracellular Barriers for Its Host Plant. Curr. Biol. 2015, 25, 2570–2576. [Google Scholar] [CrossRef]
- Kucht, S.; Hussein, Y.; Grothe, T.; Keller, U.; Basar, S.; Steiner, U.; Leistner, E.; Gross, J.; König, W.A. Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 2004, 219, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Markert, A.; Steffan, N.; PLoSs, K.; Hellwig, S.; Steiner, U.; Drewke, C.; Li, S.-M.; Boland, W.; Leistner, E. Biosynthesis and Accumulation of Ergoline Alkaloids in a Mutualistic Association between Ipomoea asarifolia (Convolvulaceae) and a Clavicipitalean Fungus. Plant Physiol. 2008, 147, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Steiner, U.; Hellwig, S.; Leistner, E. Specificity in the interaction between an epibiotic clavicipitalean fungus and its convolvulaceous host in a fungus/plant symbiotum. Plant Signal. Behav. 2008, 3, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Lester, D.G.; Luckenbill, B.K. Control of Clavicipitaceous Anamorphic Endophytes with Fungicides, Aerated Steam and Supercritical Fluid CO2-Seed Extraction. Plant Pathol. J. 2008, 7, 65–74. [Google Scholar] [CrossRef]
- Robert, T.R.; Hutson, D.H.; Lee, P.W.; Nicholls, P.H. Metabolic Pathway of Agrochemicals, Part 2: Insecticides and Fungicides; Royal Society of Chemistry: London, UK, 1999; pp. 1295–1296. [Google Scholar]
- Nuringtyas, T.R. Chapter 2, Role of Fungal Endophytes on Pyrrolizidine Alkaloids Metabolism in Jacobaea Plants. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2013; pp. 27–47. [Google Scholar]
- Rakotoniriana, E.F.; Chataigné, G.; Raoelison, G.; Rabemanantsoa, C.; Munaut, F.; El Jaziri, M.; Urveg-Ratsimamanga, S.; Marchand-Brynaert, J.; Corbisier, A.-M.; Declerck, S.; et al. Characterization of an endophytic whorl-forming Streptomyces from Catharanthus roseus stems producing polyene macrolide antibiotic. Can. J. Microbiol. 2012, 58, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification, and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Tapias, E.C.; Kim, H.K.; Lefeber, A.W.; Erkelens, C.; Verhoeven, J.T.; Brzin, J.; Zel, J.; Verpoorte, R. Metabolic Discrimination of Catharanthus roseus Leaves Infected by Phytoplasma Using 1H-NMR Spectroscopy and Multivariate Data Analysis. Plant Physiol. 2004, 135, 2398–2410. [Google Scholar] [CrossRef]
- Tiwari, R.; Awasthi, A.; Mall, M.; Shukla, A.K.; Srinivas, K.S.; Syamasundar, K.; Kalra, A. Bacterial endophyte-mediated enhancement of in planta content of key terpenoid indole alkaloids and growth parameters of Catharanthus roseus. Ind. Crop. Prod. 2013, 43, 306–310. [Google Scholar] [CrossRef]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, R.; Jacobs, D.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus Alkaloids: Pharmacognosy and Biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [PubMed]
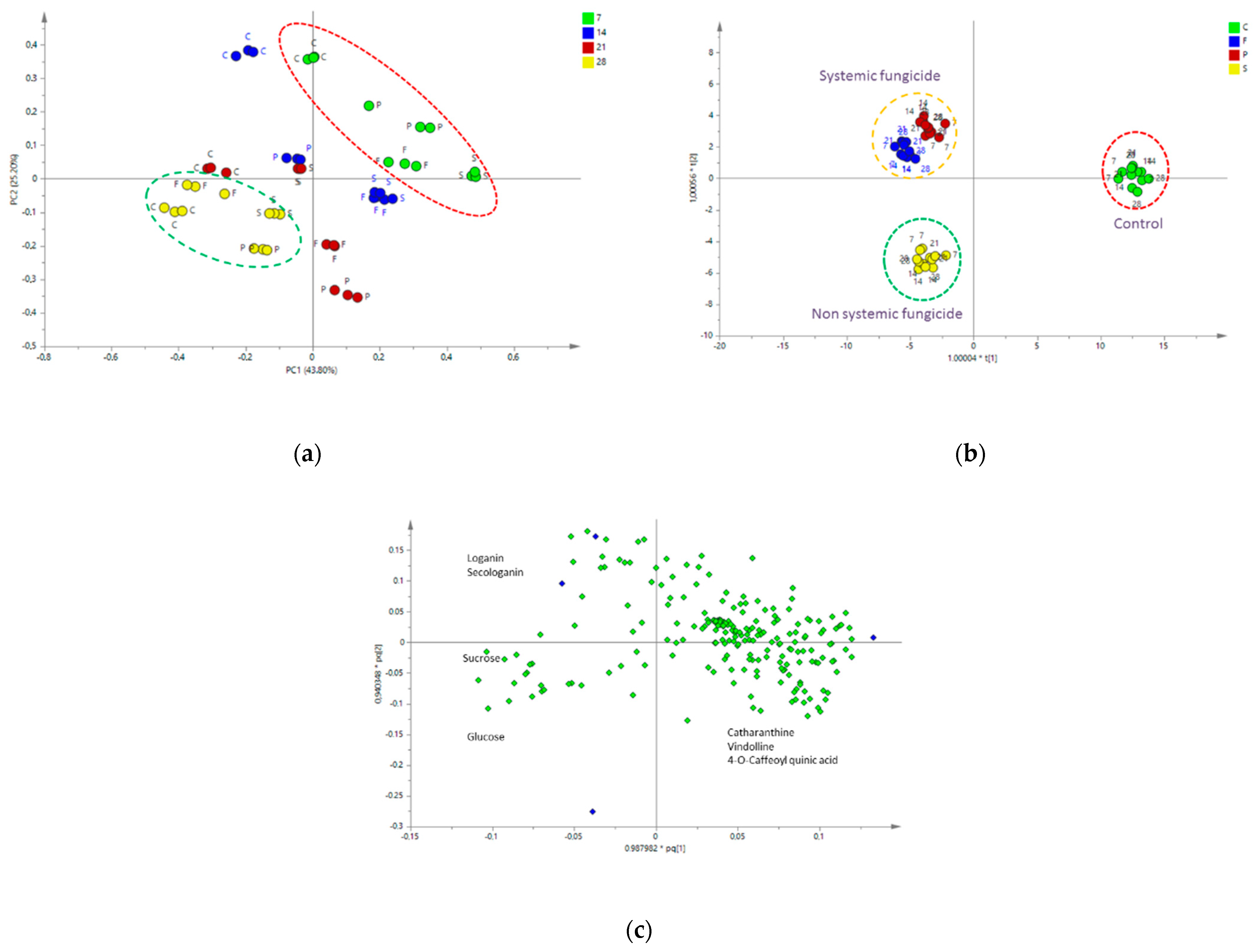
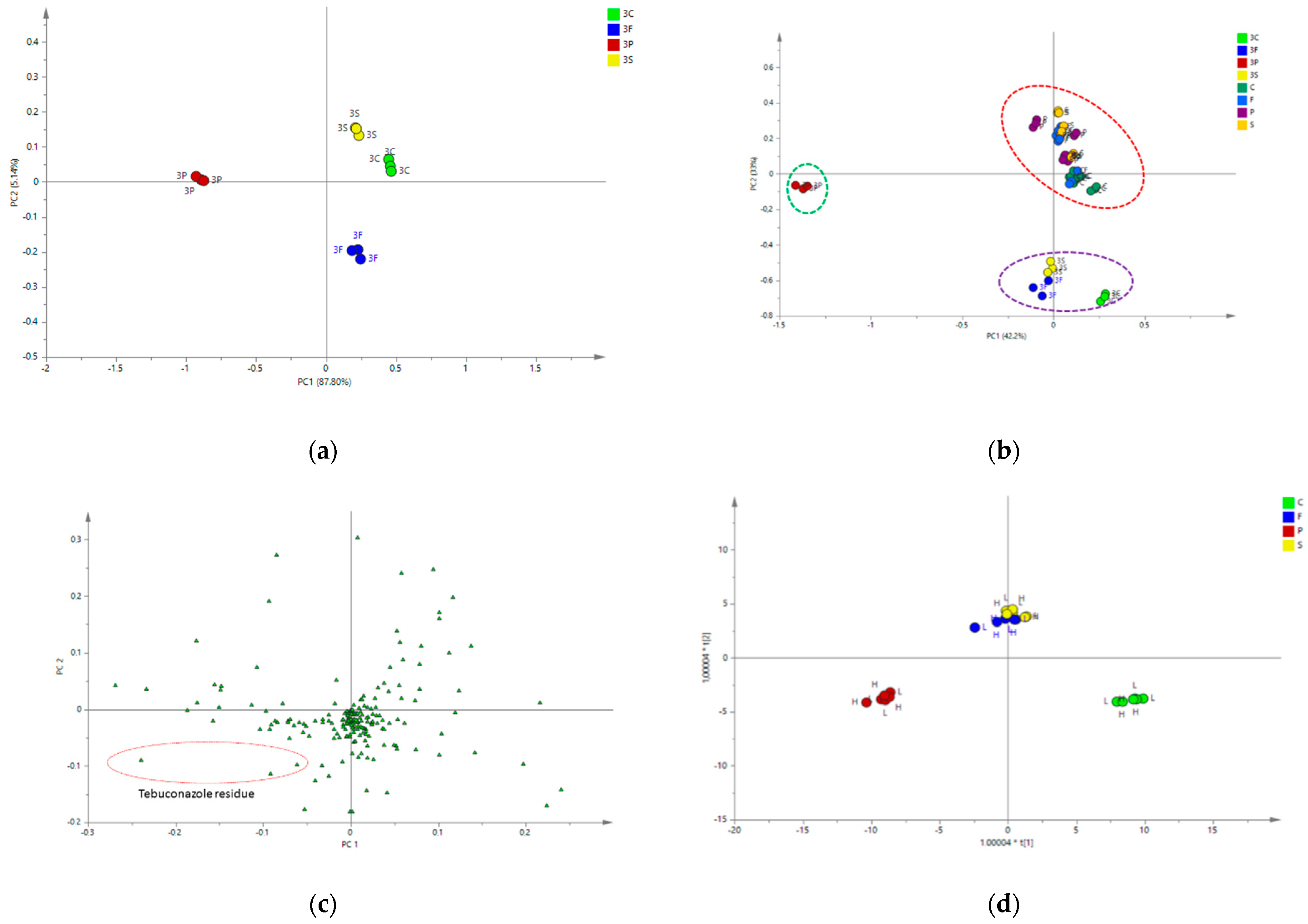


| No. | Metabolite | Chemical Shifts (δ) and Coupling Constants (Hz) |
|---|---|---|
| 1 | Isoleucine | δ 0.95 (t, J = 7. Hz), δ 1.03 (d, J = 6.8) |
| 2 | Leucine | δ 0.98 (d, J = 6.2 Hz), δ 0.97 (d, J = 6.1 Hz) |
| 3 | Valine | δ 1.00 (d, J = 7.0 Hz), δ1.03 (d, J = 7.0 Hz), δ 2.28 (m) |
| 4 | Threonine | δ 1.34 (d, J = 6.6 Hz) |
| 5 | Alanine | δ 1.48 (d, J = 7.2 Hz) |
| 6 | Arginine | δ 1.67 (m), δ 1.75 (m), δ 1.92 (m) |
| 7 | Choline | δ 3.22 (s) |
| 8 | Proline | δ 4.07 (dd, J = 8.6 Hz, 6.4 Hz), δ 2.31 (m), |
| 9 | Glutamine | δ 2.45 (m), δ 2.13 (m) |
| 10 | Aspartic acid | δ 2.82 (dd, J = 16.9 Hz, 3.9 Hz), δ 2.95 (dd, J = 17.0 Hz, 8.2 Hz) |
| 11 | Asparagine | δ 2.82 (dd, J = 16.9 Hz, 8.2 Hz), δ 2.96 (dd, J = 16.9 Hz, 3.9 Hz) |
| 12 | Acetic acid | δ 1.94 (s) |
| 13 | Myoinositol | δ 3.47 (dd, J = 10.0 Hz, 2.9 Hz), δ 3.62 (t, J = 9.7 Hz) |
| 14 | Sucrose | δ 5.41 (d, J = 3.8), δ 4.17 (d, J =8.6) |
| 15 | Glucose | δ 5.18 (d, J = 3.8), δ 4.58 (d, J = 7.9) |
| 16 | Chlorogenic acid | δ 7.62 (d, J = 15.9 Hz), δ 7.14 (d, J = 2.1 Hz), δ 7.05 (dd, J = 8.4 Hz, 2.2 Hz) |
| 17 | 4-o-Caffeoyl quinic acid | δ 7.67 (d, J = 15.9), δ 7.17 (d, J = 2.1), δ 7.08 (dd, J = 8.3, 2.0), 2.1), δ 6.44 (d, J = 15.9), 2.09 (m) |
| 18 | Loganin | δ 7.42 (d, J = 1.2), δ 5.34 (d, J = 4.0), δ 4.73 (d, J = 8.0), δ 1.08 (d, J = 7.2). |
| 19 | Secologanin | δ 7.44 (dd, J = 11.7, 0.9), δ 7.56 (d, J = 1.9), δ 9.65 (d, J = 1.4) |
| 20 | Catharanthine | δ 1.10 (t, J = 7.3), δ 7.36 (d, J = 8), δ 7.55 (d, J = 8) |
| 21 | Vindoline | δ 0.51 (t, J = 7.4), δ 2.00 (s), δ 2.66 (s), δ 5.93 (m), δ 6.22 (d, J = 2.3), δ 7.11 (d, J = 8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purnama, P.C.; Hernandez, L.C.; Verpoorte, R. Do Fungicides Affect Alkaloid Production in Catharanthus roseus (L.) G. Don. Seedlings? Molecules 2023, 28, 1405. https://doi.org/10.3390/molecules28031405
Purnama PC, Hernandez LC, Verpoorte R. Do Fungicides Affect Alkaloid Production in Catharanthus roseus (L.) G. Don. Seedlings? Molecules. 2023; 28(3):1405. https://doi.org/10.3390/molecules28031405
Chicago/Turabian StylePurnama, Purin Candra, Leonardo Castellanos Hernandez, and Robert Verpoorte. 2023. "Do Fungicides Affect Alkaloid Production in Catharanthus roseus (L.) G. Don. Seedlings?" Molecules 28, no. 3: 1405. https://doi.org/10.3390/molecules28031405
APA StylePurnama, P. C., Hernandez, L. C., & Verpoorte, R. (2023). Do Fungicides Affect Alkaloid Production in Catharanthus roseus (L.) G. Don. Seedlings? Molecules, 28(3), 1405. https://doi.org/10.3390/molecules28031405







