Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electron Paramagnetic Resonance (EPR) Study of Gamma-Irradiated Almonds
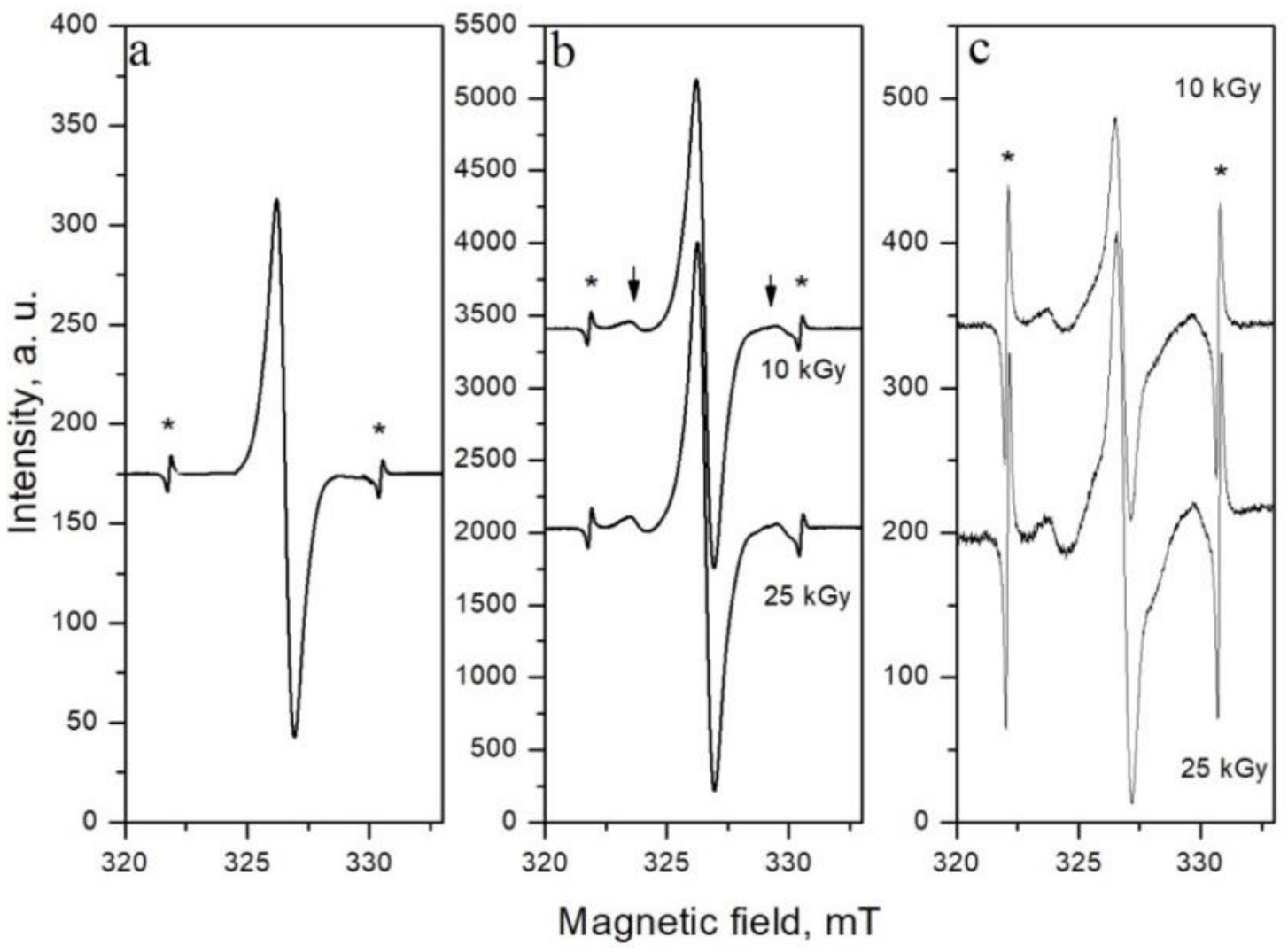
2.1.1. Characteristics of EPR Spectra
2.1.2. Kinetic Study of EPR Spectra
2.1.3. Free Radical Scavenging Activity (FRSA) Assay of Defatted Almonds
2.2. Effect of Gamma Irradiation on Fat Content, Fatty Acids Composition, and Acid Value
2.3. Effect of Gamma Irradiation on Antioxidants and Oxidative Stability of Almond Oil
2.3.1. Tocopherols in Oil from Irradiated Almonds
2.3.2. Polyphenols and Antioxidant Activity of Defatted Almonds
2.3.3. Conjugated Dienes and Trienes in Oil from Irradiated Almonds
2.3.4. Oxidative Stability of Oil from Irradiated Almonds
3. Materials and Methods
3.1. Samples and Reagents
3.2. Gamma Irradiation of Almonds
3.3. Electron Paramagnetic Resonance (EPR) Investigations of Almonds
3.3.1. EPR Measurements
3.3.2. Determination of Antiradical Activity (DPPH Free Radical Scavenging Activity)
Preparation of Extracts
Measurements
3.4. Chemical Characterization of Almonds
3.4.1. Extraction of Oil and Determination of Fat Content
3.4.2. Determination of Fatty Acids Composition
3.4.3. Determination of Tocopherols
3.4.4. Determination of Total Polyphenols and Antioxidant Activity by ORAC and HORAC Methods
3.4.5. Determination of Conjugated Dienes and Trienes Content
3.4.6. Determination of Acid Value, Peroxide Value, Induction Period and Oxidative Stability Index
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gervasi, T.; Barreca, D.; Laganà, G.; Mandalari, G. Health benefits related to tree nut consumption and their bioactive compounds. Int. J. Mol. Sci. 2021, 22, 5960. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Waśkiewicz, A.; Ratajczak, I. The Content of Phenolic Compounds and Mineral Elements in Edible Nuts. Molecules 2022, 27, 4326. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A.; Schneeman, B.A.; Davis, P.A. Almonds and Almond Oil Have Similar Effects on Plasma Lipids and LDL Oxidation in Healthy Men and Women. J. Nutr. 2002, 132, 703–707. [Google Scholar] [CrossRef]
- Dreher, M.L. A comprehensive review of almond clinical trials on weight measures, metabolic health biomarkers and outcomes, and the gut microbiota. Nutrients 2021, 13, 1968. [Google Scholar] [CrossRef] [PubMed]
- Molins, R.A. (Ed.) Introduction. In Food Irradiation: Principles and Applications; Wiley Interscience: New York, NY, USA, 2001; pp. 1–21. [Google Scholar]
- Sánchez-Bravo, P.; Noguera-Artiaga, L.; Gómez-López, V.M.; Carbonell-Barrachina, A.A.; Gabaldón, J.A.; Pérez-López, A.J. Impact of Non-Thermal Technologies on the Quality of Nuts: A Review. Foods 2022, 11, 3891. [Google Scholar] [CrossRef]
- Kwakwa, A.; Prakash, A. Irradiation treatment of nuts. In Food Irradiation: Research and Technology; Sommers, C.H., Fan, X., Eds.; Blackwell Publishing and the Institute of Food Technologists: Boston, MA, USA, 2006; pp. 221–235. [Google Scholar]
- Codex Stan 106-1983, Rev.1-2003; General Standard for Irradiated Foods, Codex Alimentarius Commission. FAO: Rome, Italy, 2003.
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Joseph, R.J.; Khan, A.A.; Rahmani, A.H. Protective effects of ginger extract against glycation and oxidative stress-induced health complications: An In vitro study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Khan, M.A.; Anwar, S.; Aljarbou, A.N.; Al-Orainy, M.; Aldebasi, Y.H.; Islam, S.; Younus, H. Protective effect of thymoquinone on glucose or methylglyoxal-induced glycation of superoxide dismutase. Int. J. Biol. Macromol. 2014, 65, 16–20. [Google Scholar] [CrossRef]
- Gecgel, U.; Gumus, T.; Tasan, M.; Daglioglu, O.; Arici, M. Determination of fatty acid composition of γ-irradiated hazelnuts, walnuts, almonds, and pistachios. Radiat. Phys. Chem. 2011, 80, 578–581. [Google Scholar] [CrossRef]
- Al-Bachir, M. Physicochemical properties of oil extracts from gamma irradiated almond (Prunus amygdalus L.). Innov. Rom. Food Biotechnol. 2014, 14, 37–45. [Google Scholar]
- Di Stefano, V.; Pitonzo, R.; Bartolotta, A.; D’Oca, M.C.; Fuochi, P. Effects of γ-irradiation on the α-tocopherol and fatty acids content of raw unpeeled almond kernels (Prunus dulcis). LWT-Food Sci. Technol. 2014, 59, 572–576. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Iqbal, M.; Anwar, F.; Shahid, S.A.; Shahid, M. Quality characteristics and microbiological safety evaluation of oil extracted from gamma irradiated almond (Prunus dulcis Mill.) seeds. Grasas Aceites 2013, 64, 68–76. [Google Scholar] [CrossRef]
- Mexis, S.F.; Badeka, A.V.; Chouliara, E.; Riganakos, K.A.; Kontominas, M.G. Effect of γ-irradiation on the physico-chemical and sensory properties of raw unpeeled almond kernels (Prunus dulcis). Innov. Food Sci. Emerg. Technol. 2009, 10, 87–92. [Google Scholar] [CrossRef]
- Polovka, M.; Suhaj, M. The effect of irradiation and heat treatment on composition and antioxidant properties of culinary herbs and spices—A review. Food Res. Int. 2010, 26, 138–161. [Google Scholar] [CrossRef]
- Kameya, H.; Ukai, M.; Shimoyama, Y. An ESR study of radiation induced radicals in glucose polymers. Radiat. Phys. Chem. 2013, 84, 232–234. [Google Scholar] [CrossRef]
- EN 1787, 2000; Foodstuffs—Detection of Irradiated Food Containing Cellulose—Method by ESR Spectroscopy. European Committee for Standardization: Brussels, Belgium, 2000.
- Tomaiuolo, M.; Mangiacotti, M.; Trotta, G.; Marchesani, G.; Chiappinelli, A.; Chiaravalle, A.E. Identification of X-ray irradiated walnuts by ESR spectroscopy. Radiat. Phys. Chem. 2018, 150, 35–39. [Google Scholar] [CrossRef]
- Raffi, J.; Yordanov, N.D.; Chabane, S.; Douifi, L.; Gancheva, V.; Ivanova, S. Identification of irradiation treatment of aromatic herbs, spices and fruits by electron paramagnetic resonance and thermoluminescence. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2000, 56, 409–416. [Google Scholar] [CrossRef]
- Yordanov, N.D.; Aleksieva, K. X- and Q-band studies on fine powders of irradiated plants. New approach for detection of their radiation history by using Q-band EPR spectrometry. Radiat. Phys. Chem. 2004, 69, 59–64. [Google Scholar] [CrossRef]
- Moosavi, K.S.; Hosseini, S.; Dehghan, G.; Jahanban-Esfahlan, A. The effect of gamma irradiation on phytochemical content and antioxidant activity of stored and none stored almond (Amygdalus communis L.) hull. Pharm. Sci. 2020, 20, 102–106. [Google Scholar]
- Costa de Camargo, A.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Liu, C.; Tan, J.; Ma, H.; Wang, J. Physiochemical responses of the kernel quality, total phenols and antioxidant enzymes of walnut in different forms to the low-temperature storage. Foods 2021, 10, 2027. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, S.-C.; Hu, C.-C.; Shyu, Y.-S.; Hsu, C.-Y.; Yang, D.-J. Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef]
- Sanchez-Bel, P.; Egea, I.; Romojaro, F.; Martínez-Madrid, M.C. Sensorial and chemical quality of electron beam irradiated almonds (Prunus amygdalus). LWT-Food Sci. Technol. 2008, 41, 442–449. [Google Scholar] [CrossRef]
- Al-Bachir, M. Assessing the Effects of Gamma Irradiation and Storage Time in Quality Properties of Almond (Prunus amygdalus L.). Innov. Rom. Food Biotechnol. 2015, 16, 1–8. [Google Scholar]
- Lundberg, W.O. Autoxidation and Antioxidants; Interscience Publishers: New York, NY, USA, 1962. [Google Scholar]
- Gliszczynska-Swiglo, A.; Sikorska, E.; Khmelinskii, I.; Sikorski, M. Tocopherol content in edible plant oils. Pol. J. Food Nutr. Sci. 2007, 57, 157–161. [Google Scholar]
- Ouzir, M.; El Bernoussi, S.; Tabyaoui, M.; Taghzouti, K. Almond oil: A comprehensive review of chemical composition, extraction methods, preservation conditions, potential health benefits, and safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3344–3387. [Google Scholar] [CrossRef]
- Kodad, O.; Socias i Company, R.; Prats, M.S.; Lopez Ortiz, M.C. Variability in tocopherol concentrations in almond oil and its use as a selection criterion in almond breeding. J. Hortic. Sci. Biotechnol. 2006, 81, 501–507. [Google Scholar] [CrossRef]
- De Toledo, T.C.F.; Canniatti-Brazaca, S.G.; Arthur, V.; Piedade, S.M.S. Effects of gamma radiation on total phenolics, trypsin and tannin inhibitors in soybean grains. Radiat. Phys. Chem. 2007, 76, 1653–1656. [Google Scholar] [CrossRef]
- Alothman, M.R.; Bhat, K.A. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci. Technol. 2009, 20, 201–212. [Google Scholar] [CrossRef]
- Ognyanov, M.; Denev, P.; Teneva, D.; Georgiev, Y.; Taneva, S.; Totseva, I.; Kamenova-Nacheva, M.; Nikolova, Y.; Momchilova, S. Influence of gamma irradiation on different phytochemical constituents of dried rose hip (Rosa canina L.) fruits. Molecules 2022, 27, 1765. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bel, P.; Martinez-Madrid, M.C.; Egea, I.; Romojaro, F. Oil quality and sensory evaluation of almond (Prunus amygdalus) stored after electron beam processing. J. Agric. Food Chem. 2005, 53, 2567–2573. [Google Scholar] [CrossRef]
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2009.
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification, and Structural Analysis of Lipids, 3rd ed.; The Oily Press: Bridgwater, UK, 2003; pp. 205–224. [Google Scholar]
- ISO 9936:2012; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Content by High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2012.
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- AOCS Official Method Ch 5-91. Specific Extinction of Oils and Fats, Ultraviolet Absorption. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 1991. [Google Scholar]
- AOCS Official Method Cd 3d-63. Acid Value of Fats and Oils. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 2017. [Google Scholar]
- Yanishlieva, N.; Popov, A.; Marinova, E. Eine Modifizierte Jodometrische Methode zur Bestimmung der Peroxidzahl in kleinen Lipidproben. Compt. Rend. Acad. Bulg. Sci. 1978, 31, 869–871. [Google Scholar]
- Le Tutour, B.; Guedon, D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]




| Almond Oil | Non-Irradiated | 10 kGy | 25 kGy |
|---|---|---|---|
| Fat content, w/w% | 50.2 ± 0.9 * | 50.8 ± 0.5 | 51.4 ± 0.8 |
| Fatty acids, rel.% | |||
| Palmitic acid (16:0) | 5.9 ± 0.1 | 6.1 ± 0.1 | 6.1 ± 0.1 |
| Palmitoleic acid (16:1) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| Stearic acid (18:0) | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.8 ± 0.1 |
| Oleic acid (18:1) | 64.9 ± 1.0 | 65.6 ± 1.2 | 64.0 ± 0.5 |
| Linoleic acid (18:2) | 26.9 ± 0.8 | 26.1 ± 0.7 | 27.5 ± 1.1 |
| Acid value (mg KOH/g) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.2 |
| α-Tocopherol (mg/kg) | 292 ± 23 a | 215 ± 12 b | 175 ± 19 c |
| Defatted Almonds | Non-Irradiated | 10 kGy | 25 kGy |
|---|---|---|---|
| Total polyphenols (mg/100 g) | 1374 ± 35 a,* | 1379 ± 36 a | 1520 ± 8 b |
| ORAC (µmol TE/g) | 100 ± 3 a | 100 ± 5 a | 156 ± 3 b |
| HORAC (µmol GAE/g) | 61 ± 3 a | 65 ± 2 a | 86 ± 6 b |
| Almond Oil | Non-Irradiated | 10 kGy | 25 kGy |
|---|---|---|---|
| conj. Dienes (A232, 1%) | 1.3 ± 0.1 *,a | 2.4 ± 0.1 b | 3.0 ± 0.01 c |
| conj. Trienes (A268, 1%) | 0.04 ± 0.01 a | 0.09 ± 0.01 b | 1.1 ± 0.01 c |
| OSI at 100 °C (h) | 24.6 ± 0.2 a | 19.5 ± 0.1 b | 18.6 ± 0.1 c |
| OSI at 120 °C (h) | 6.0 ± 0.1 a | 4.6 ± 0.1 b | 4.1 ± 0.1 c |
| Sample | IP, h | R, 10−6 M/s | Effect | Curve in Figure 4 |
|---|---|---|---|---|
| control sample C (0 kGy) | 22 ± 2.0 | 0.30 ± 0.03 | - | C |
| +0.5 mM AscPH *: TOH (1:1) | 32 ± 3.0 | 0.10 ± 0.02 | strong | 5 |
| Irradiated sample (10 kGy) | 13 ± 1.5 | 0.31 ± 0.03 | - | 1 |
| +0.5 mM AscPH | 18 ± 2.0 | 0.12 ± 0.02 | strong | 2 |
| +0.75 mM AscPH | 23 ± 2.0 | 0.11 ± 0.02 | the strongest | 3 |
| +0.5 mM AscPH: TOH (1:1) | 20 ± 2.0 | 0.32 ± 0.03 | strong | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momchilova, S.; Kazakova, A.; Taneva, S.; Aleksieva, K.; Mladenova, R.; Karakirova, Y.; Petkova, Z.; Kamenova-Nacheva, M.; Teneva, D.; Denev, P. Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts. Molecules 2023, 28, 1439. https://doi.org/10.3390/molecules28031439
Momchilova S, Kazakova A, Taneva S, Aleksieva K, Mladenova R, Karakirova Y, Petkova Z, Kamenova-Nacheva M, Teneva D, Denev P. Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts. Molecules. 2023; 28(3):1439. https://doi.org/10.3390/molecules28031439
Chicago/Turabian StyleMomchilova, Svetlana, Adriana Kazakova, Sabina Taneva, Katerina Aleksieva, Ralitsa Mladenova, Yordanka Karakirova, Zhanina Petkova, Mariana Kamenova-Nacheva, Desislava Teneva, and Petko Denev. 2023. "Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts" Molecules 28, no. 3: 1439. https://doi.org/10.3390/molecules28031439
APA StyleMomchilova, S., Kazakova, A., Taneva, S., Aleksieva, K., Mladenova, R., Karakirova, Y., Petkova, Z., Kamenova-Nacheva, M., Teneva, D., & Denev, P. (2023). Effect of Gamma Irradiation on Fat Content, Fatty Acids, Antioxidants and Oxidative Stability of Almonds, and Electron Paramagnetic Resonance (EPR) Study of Treated Nuts. Molecules, 28(3), 1439. https://doi.org/10.3390/molecules28031439









