In Vitro, In Vivo, and In Silico Analyses of Molecular Anti-Pigmentation Mechanisms of Selected Thai Rejuvenating Remedy and Bioactive Metabolites
Abstract
1. Introduction
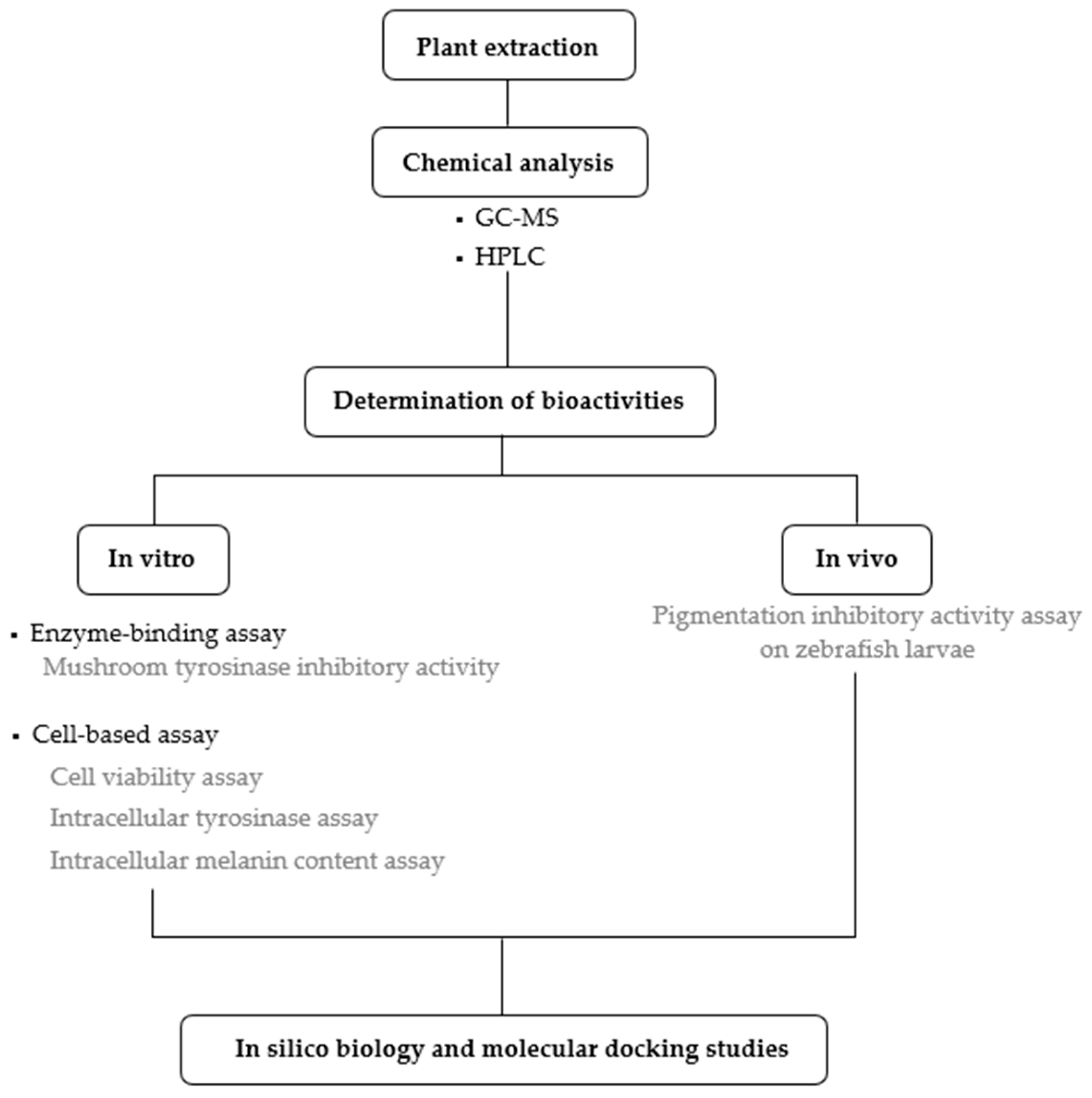
2. Results
2.1. Anti-Mushroom Tyrosinase Activity of the Selected Remedy
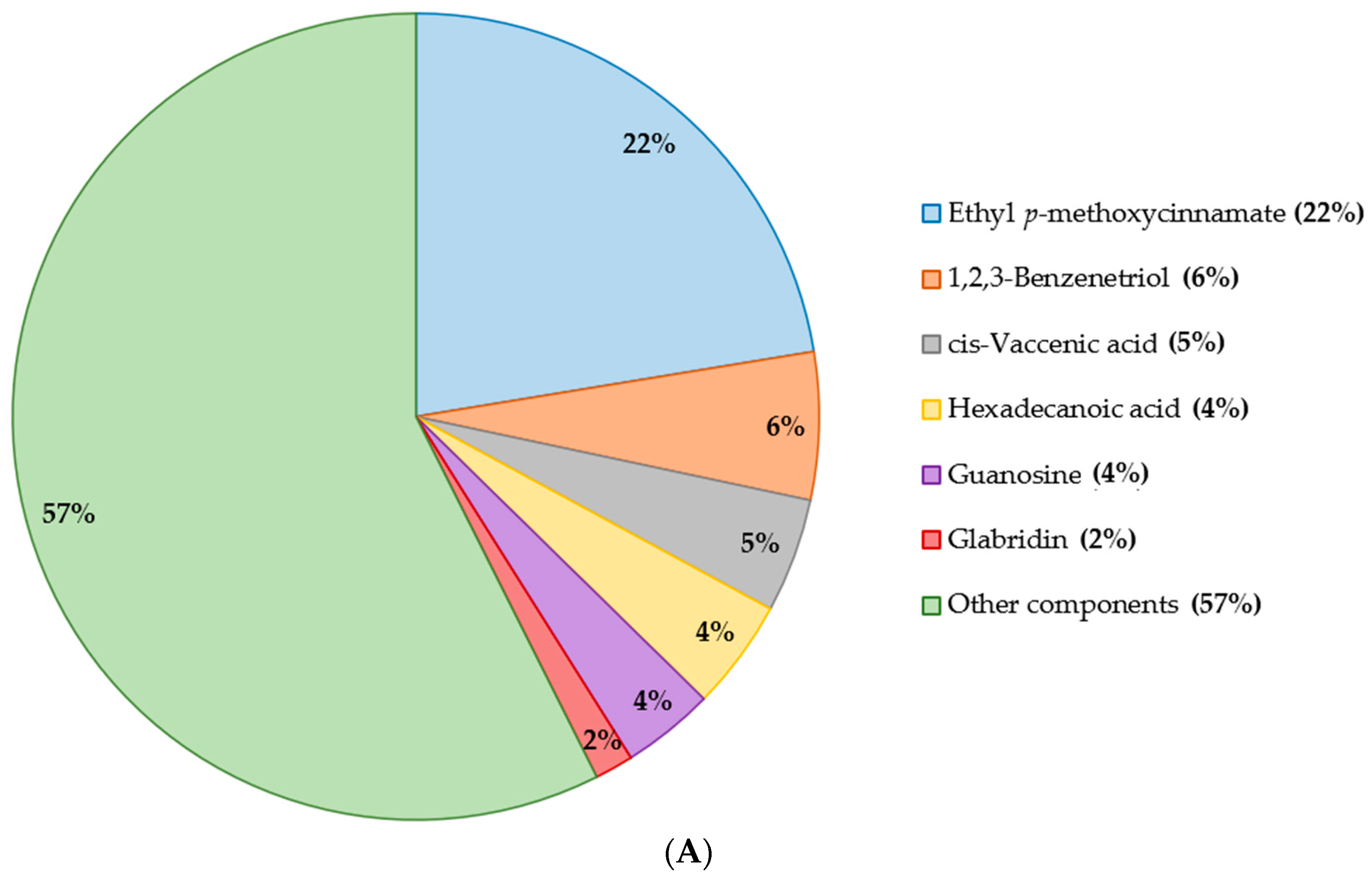
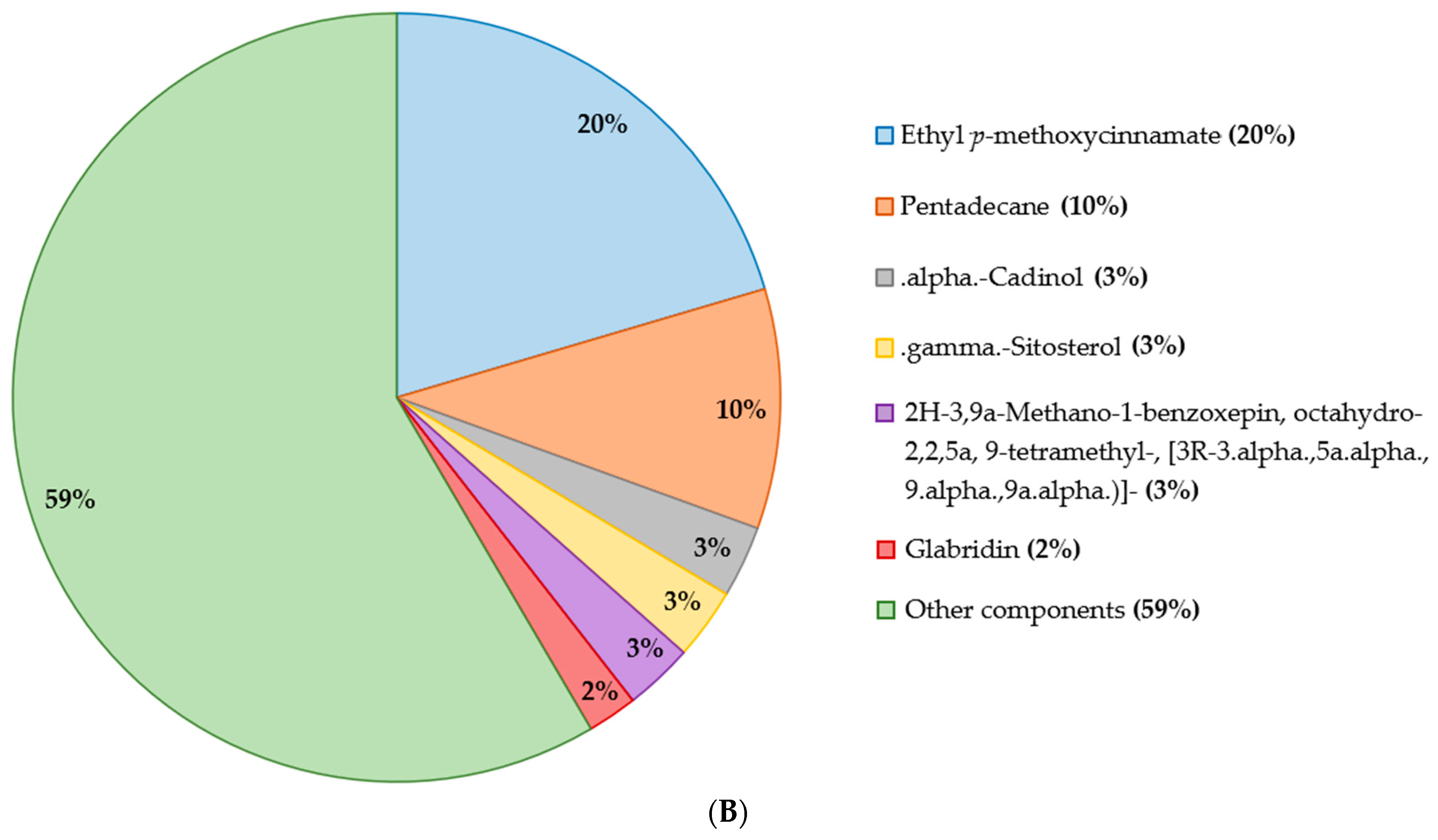
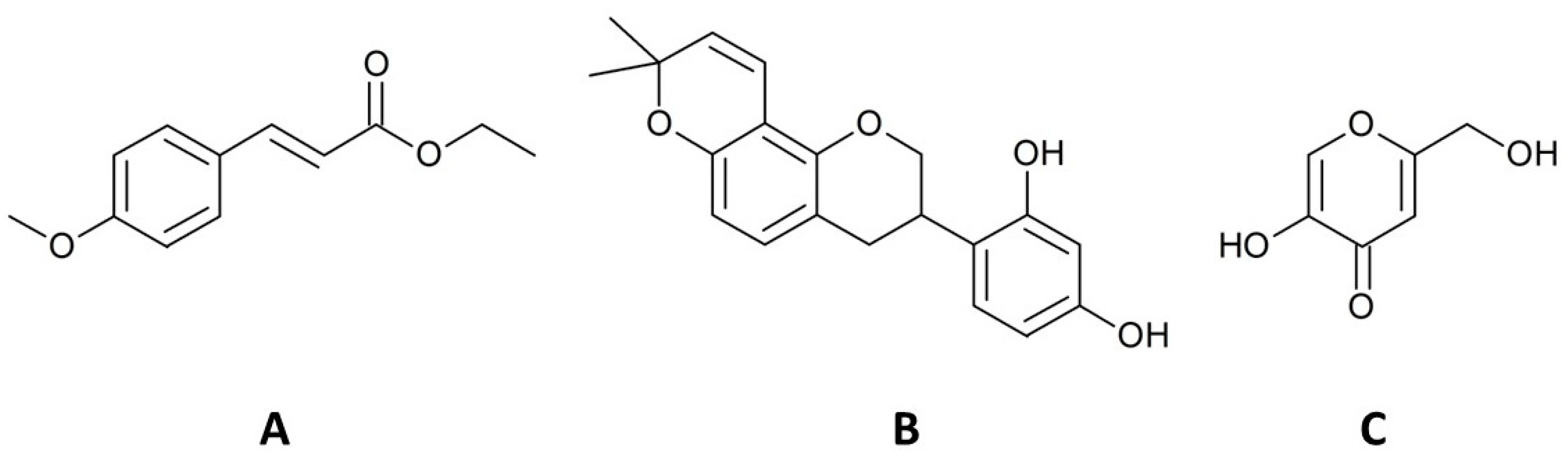
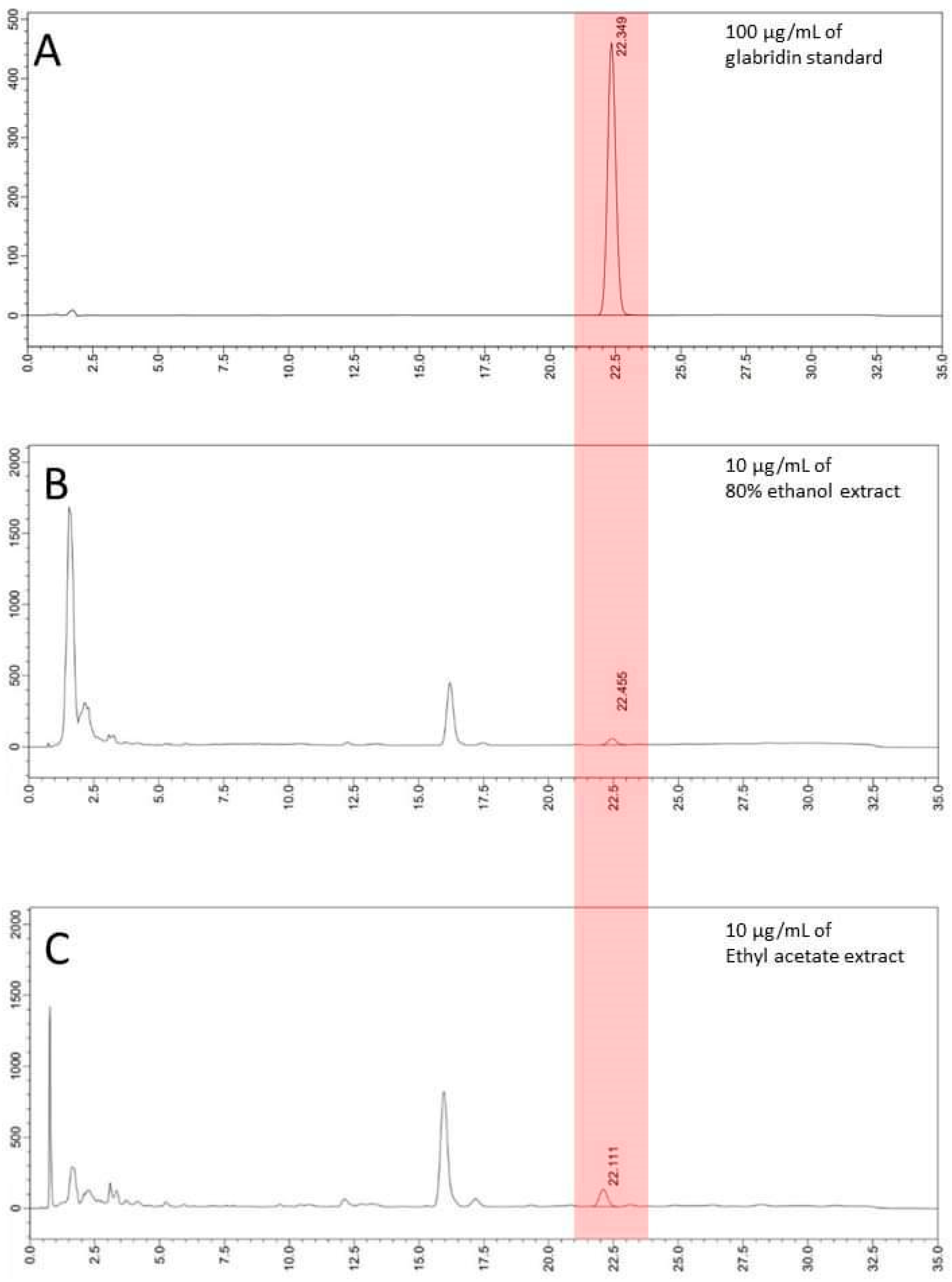
2.2. Chemical Analysis and Metabolites Determination
2.2.1. Gas Chromatography Coupled with Mass Spectroscopy (GC–MS)
2.2.2. HPLC Analysis
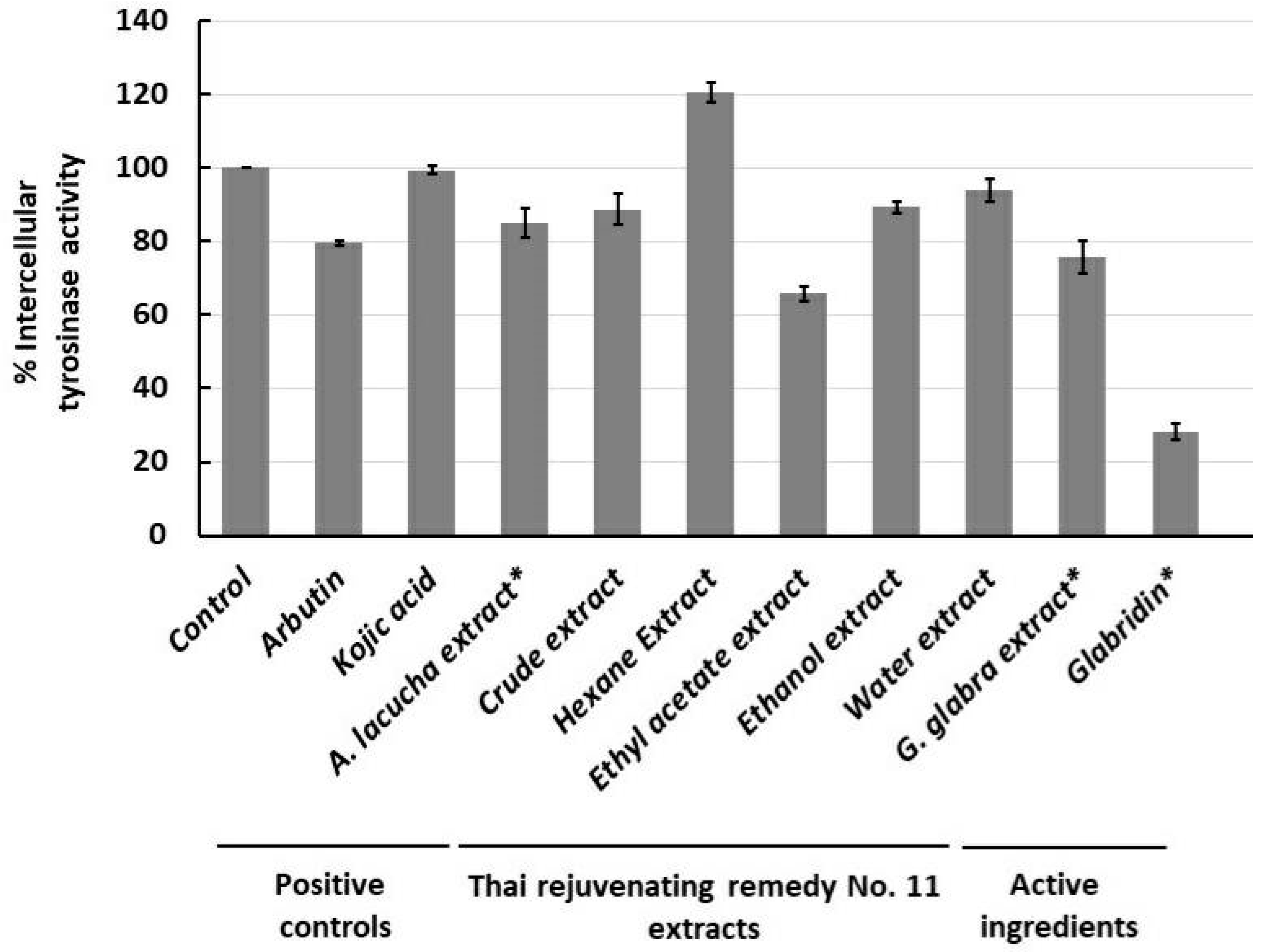
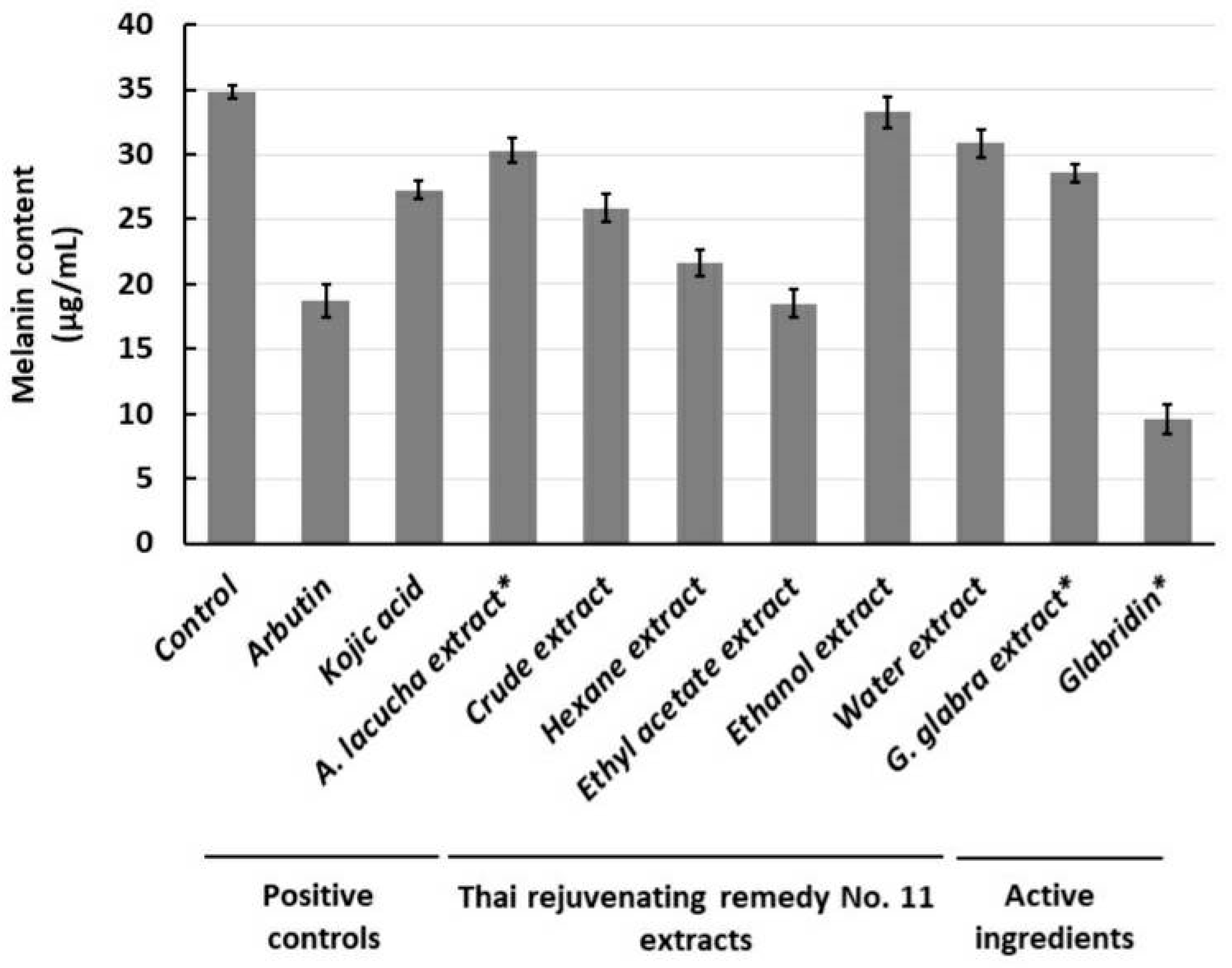
2.3. Anti-Murine Melanoma (B16F1) Intracellular Tyrosinase Activity and Melanin Content
2.3.1. Cytotoxicity Evaluation
2.3.2. Anti-Murine Melanoma (B16F1) Intracellular Tyrosinase Activity
2.3.3. Murine Melanoma (B16F1) Intracellular Melanin Content
2.3.4. Correlation between Anti-Murine Melanoma (B16F1) Intracellular Tyrosinase Activity and Melanin Content
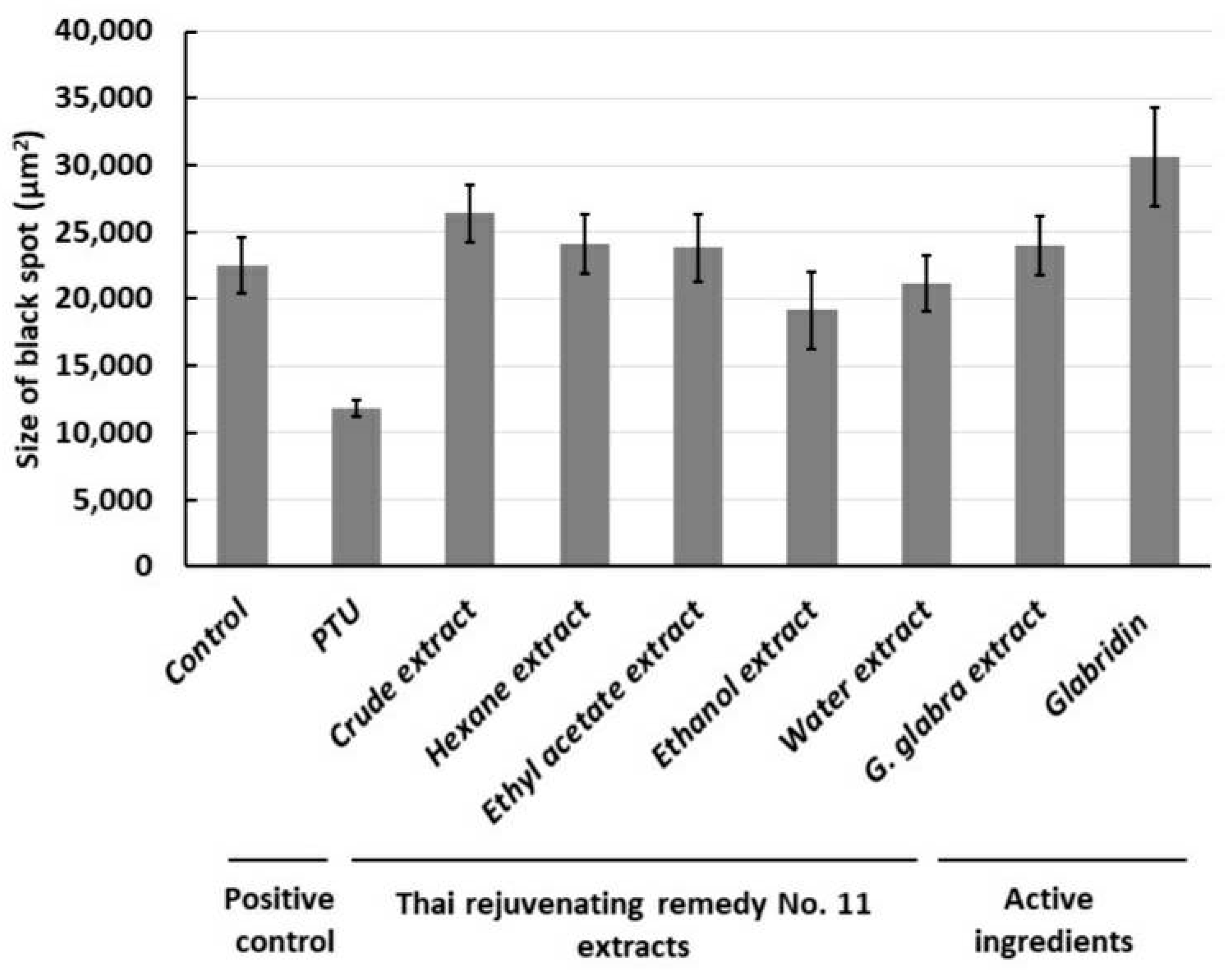
2.4. Anti-Pigmentation in Zebrafish Larvae
2.5. In Silico Biology and Molecular Docking Studies
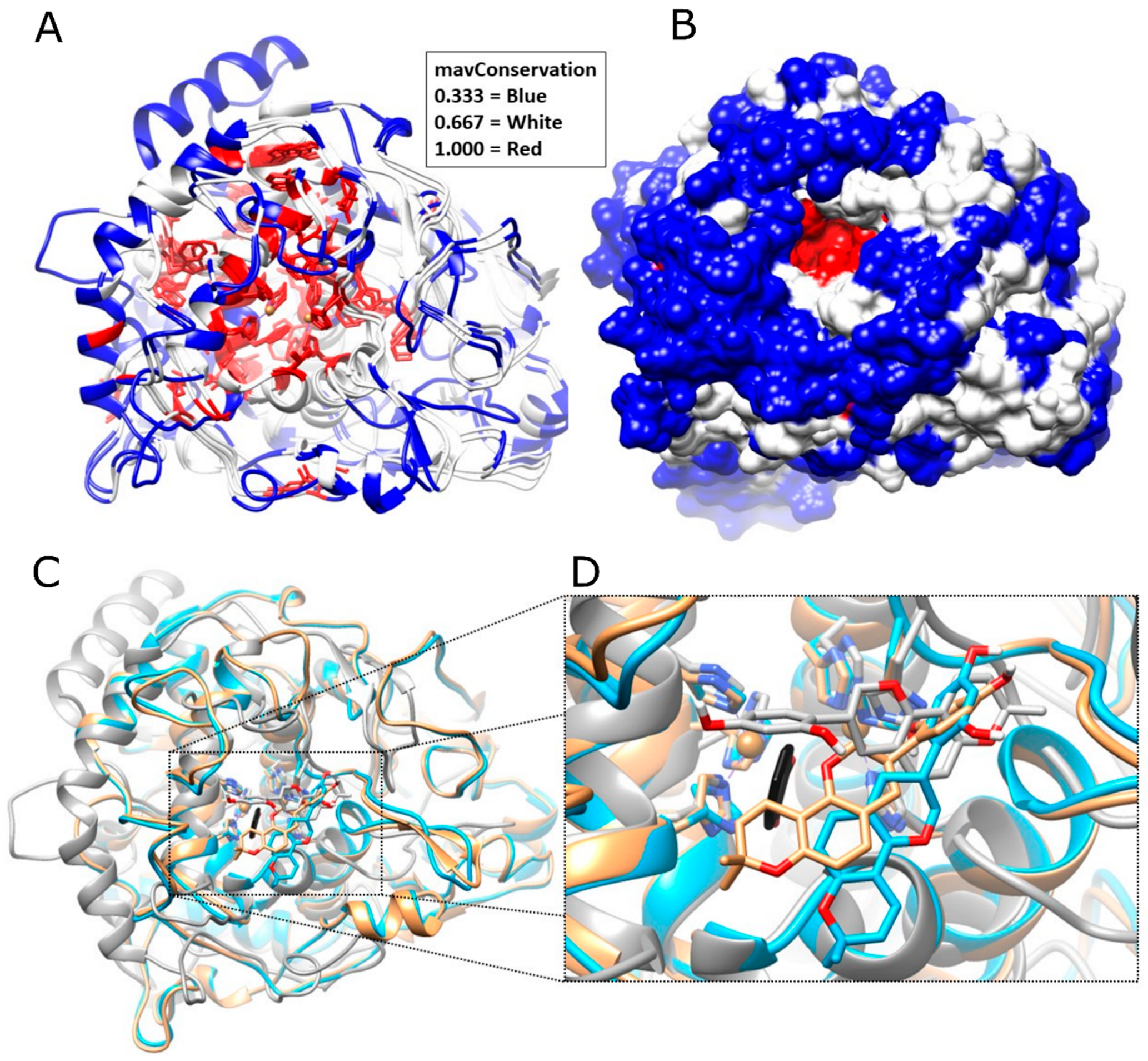
2.5.1. Theoretical Interspecies Glabridin–Tyrosinase Interactions
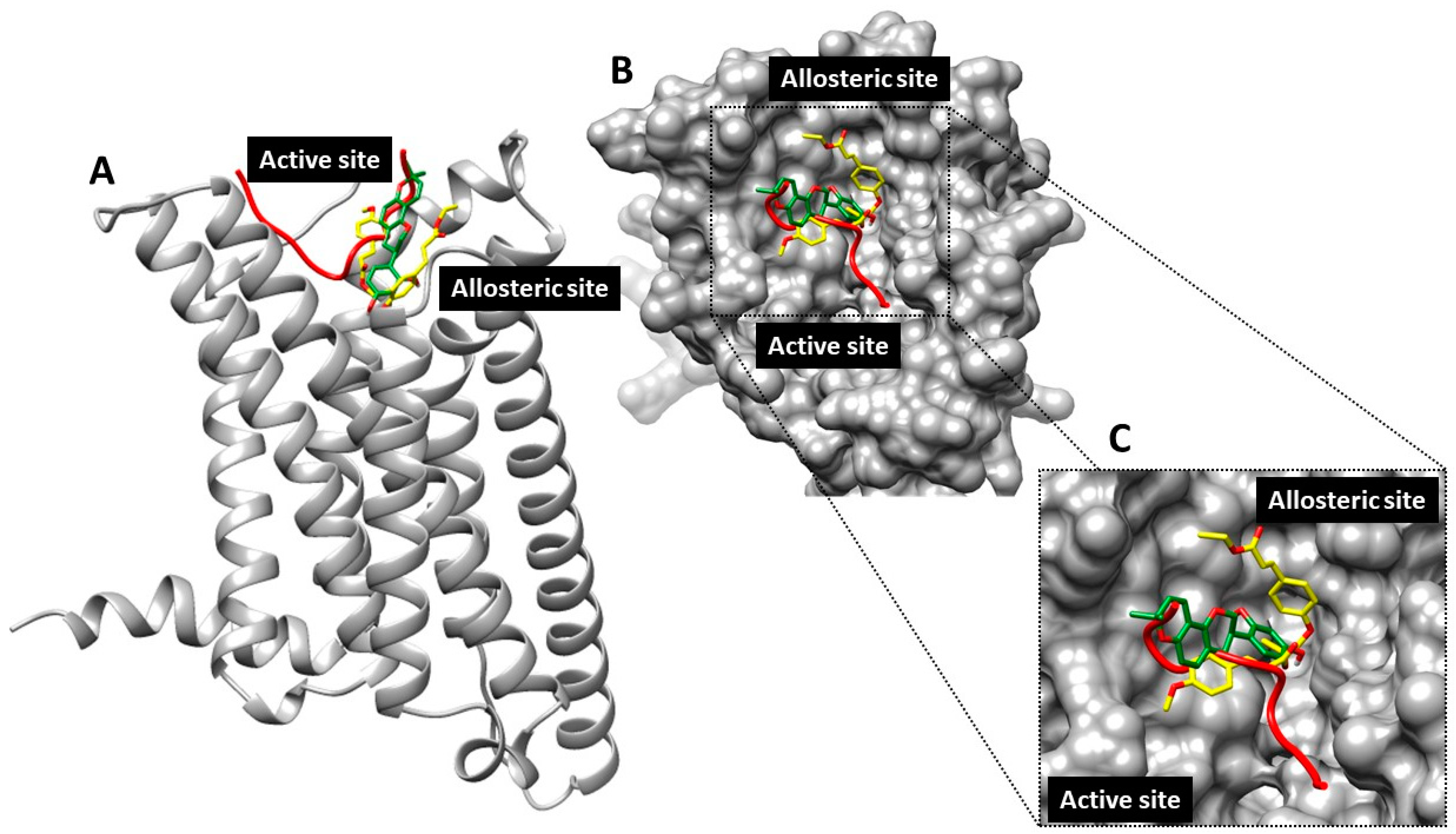
2.5.2. Proposed Molecular Interplay between Glabridin and Ethyl p-methoxycinnamate in Melanin Synthesis via Melanocortin 1 Receptor (MC1R)
3. Discussion
4. Materials and Methods
4.1. Chemical Agents
4.2. Plant Materials
Preparation of Crude Extracts
4.3. Chemical Analysis
4.3.1. Gas Chromatography–Mass Spectrometry Analysis (GC–MS Analysis)
4.3.2. High-Performance Liquid Chromatography Analysis (HPLC Analysis)
4.4. Determination of Bioactivities
4.4.1. Determination of Mushroom Tyrosinase Inhibitory Activity (Enzyme-Binding Assay)
4.4.2. Determination of Intracellular Tyrosinase Inhibitory Activity and Melanin Content (Cell-Based Assay)
Cell Culture Procedure
Cell Viability Assay
Intracellular Tyrosinase Assay
Intracellular Melanin Content
4.4.3. Pigmentation Inhibitory Activity Assay on Zebrafish Larvae
4.5. In Silico Biology and Molecular Docking Studies
4.5.1. 2D Multiple Sequences Alignment and Phylogenetic Analysis
4.5.2. 3D Structural Alignments of Used Tyrosinases in This Study
4.5.3. Ligand–Protein Docking via Autodock Vina
4.5.4. Vina Post-Docking Analysis
4.5.5. Protein–Protein Docking
4.6. Statistic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Prasansuklab, A.; Brimson, J.M.; Tencomnao, T. Potential Thai Medicinal Plants for Neurodegenerative Diseases: A Review Focusing on the Anti-Glutamate Toxicity Effect. Tradit. Complement. Med. 2020, 10, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ruangchuay, S.; Wang, Q.; Wang, L.; Lin, J.; Wang, Y.; Zhong, G.; Maneenoon, K.; Huang, Z.; Chusri, S. Antioxidant and Antiaging Effect of Traditional Thai Rejuvenation Medicines in Caenorhabditis Elegans. J. Integr. Med. 2021, 19, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Nootim, P.; Kapol, N.; Bunchuailua, W.; Poompruek, P.; Tungsukruthai, P. Current State of Cancer Patient Care Incorporating Thai Traditional Medicine in Thailand: A Qualitative Study. J. Integr. Med. 2020, 18, 41–45. [Google Scholar] [CrossRef]
- Favela, L.H. Cognitive Science as Complexity Science. Wiley Interdiscip. Rev. Cogn. Sci. 2020, 11, e1525. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging. 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability. 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Scarcella, G.; Dethlefsen, M.W.; Nielsen, M.C.E. Treatment of Solar Lentigines Using a Combination of Picosecond Laser and Biophotonic Treatment. Clin. Case Rep. 2018, 6, 1868–1870. [Google Scholar] [CrossRef]
- Armenta, A.M.; Henkel, E.D.; Ahmed, A.M. Pigmentation Disorders in the Elderly. Drugs Aging. 2019, 36, 235–245. [Google Scholar] [CrossRef]
- Iraji, F.; Mousavi, A.; Poostiyan, N.; Saber, M. Q-Switched Frequency-Doubled Nd:YAG (532 Nm) Laser versus Trichloroacetic Acid 35% Peeling in the Treatment of Dorsal Hand Solar Lentigo: An Assessor-Blind Split-Hand Randomized Controlled Trial. J. Cosmet. Dermatol. 2022, 21, 6776–6782. [Google Scholar] [CrossRef]
- Menzies, S.W.; Liyanarachchi, S.; Coates, E.; Smith, A.; Cooke-Yarborough, C.; Lo, S.; Armstrong, B.; Scolyer, R.A.; Guitera, P. Estimated Risk of Progression of Lentigo Maligna to Lentigo Maligna Melanoma. Melanoma Res. 2020, 30, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Krueger, L.; Saizan, A.; Stein, J.A.; Elbuluk, N. Dermoscopy of Acquired Pigmentary Disorders: A Comprehensive Review. Int. J. Dermatol. 2022, 61, 7–19. [Google Scholar] [CrossRef]
- Desai, S.R. Hyperpigmentation Therapy: A Review. J. Clin. Aesthet. Dermatol. 2014, 7, 13–17. [Google Scholar] [PubMed]
- Nautiyal, A.; Wairkar, S. Management of Hyperpigmentation: Current Treatments and Emerging Therapies. Pigment Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase Inhibition by Some Flavonoids: Inhibitory Activity, Mechanism by in Vitro and in Silico Studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef]
- Arroo, R.R.J.; Sari, S.; Barut, B.; Özel, A.; Ruparelia, K.C.; Şöhretoğlu, D. Flavones as Tyrosinase Inhibitors: Kinetic Studies in Vitro and in Silico. Phytochem. Anal. 2020, 31, 314–321. [Google Scholar] [CrossRef]
- Bonesi, M.; Xiao, J.; Tundis, R.; Aiello, F.; Sicari, V.; Loizzo, M.R. Advances in the Tyrosinase Inhibitors from Plant Source. Curr. Med. Chem. 2019, 26, 3279–3299. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Gao, J. Production of a Mutant of Large-Scale Loach Paramisgurnus Dabryanus with Skin Pigmentation Loss by Genome Editing with CRISPR/Cas9 System. Transgenic Res. 2019, 28, 341–356. [Google Scholar] [CrossRef]
- Square, T.; Romášek, M.; Jandzik, D.; Cattell, M.V.; Klymkowsky, M.; Medeiros, D.M. CRISPR/Cas9-Mediated Mutagenesis in the Sea Lamprey Petromyzon Marinus: A Powerful Tool for Understanding Ancestral Gene Functions in Vertebrates. Development 2015, 142, 4180–4187. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory Mechanisms of Glabridin on Tyrosinase. Spectrochim. Acta A Mol. 2016, 168, 111–117. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Sharma, P.; Tripathi, A.; Tripathi, P.N.; Srivastava, P.; Seth, A.; Shrivastava, S.K. Computational Exploration and Experimental Validation to Identify a Dual Inhibitor of Cholinesterase and Amyloid-Beta for the Treatment of Alzheimer’s Disease. J. Comput. Aided Mol. Des. 2020, 34, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, D.S.F.; Siharis, F.; Abdurrahman, S.; Isrul, M.; Fakih, T.M. In Silico Analysis of Marine Natural Product from Sponge (Clathria Sp.) for Their Activity as Inhibitor of SARS-CoV-2 Main Protease. J. Biomol. Struct. Dyn. 2021, 40, 11526–11532. [Google Scholar] [CrossRef]
- Ko, H.-J.; Kim, H.J.; Kim, S.Y.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Whang, W.K.; Choi, H.-R.; Park, K.-C.; Kim, D.-S. Hypopigmentary Effects of Ethyl P-Methoxycinnamate Isolated from Kaempferia Galanga. Phytother. Res. 2014, 28, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, Y.; Dai, A.; Yin, W.; Guo, J.; Yang, D.; Zhou, F.; Jiang, Y.; Wang, M.-W.; Xu, H.E. Structural Mechanism of Calcium-Mediated Hormone Recognition and Gβ Interaction by the Human Melanocortin-1 Receptor. Cell Res. 2021, 31, 1061–1071. [Google Scholar] [CrossRef]
- Jiménez-García, B.; Pons, C.; Fernández-Recio, J. PyDockWEB: A Web Server for Rigid-Body Protein–Protein Docking Using Electrostatics and Desolvation Scoring. Bioinformatics 2013, 29, 1698–1699. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Singh, N.; Villoutreix, B.O.; Ecker, G.F. Rigorous Sampling of Docking Poses Unveils Binding Hypothesis for the Halogenated Ligands of L-Type Amino Acid Transporter 1 (LAT1). Sci. Rep. 2019, 9, 15061. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C. Determination of Phytochemical Compounds, and Tyrosinase Inhibitory and Antimicrobial Activities of Bioactive Compounds from Streblus Ilicifolius (S Vidal) Corner. Trop. J. Pharm. Res. 2016, 15, 497–506. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C.; Nuankaew, W.; Kang, T.H. Effects of Selected Moraceae Plants on Tyrosinase Enzyme and Melanin Content. Pharmacogn. Mag. 2019, 15, 708. [Google Scholar] [CrossRef]
- Dej-adisai, S.; Parndaeng, K.; Wattanapiromsakul, C.; Hwang, J.S. Three New Isoprenylated Flavones from Artocarpus Chama Stem and Their Bioactivities. Molecules 2022, 27, 3. [Google Scholar] [CrossRef]
- Kamal, Y.T.; Singh, M.; Tamboli, E.T.; Parveen, R.; Zaidi, S.M.A.; Ahmad, S. Rapid RP-HPLC Method for the Quantification of Glabridin in Crude Drug and in Polyherbal Formulation. J. Chromatogr. Sci. 2012, 50, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Seyedarabi, A.; Ariaeenejad, S.; Moosavi-Movahedi, A.A.; Rayati, S.; Poursasan, N.; Asiaie, N.; Seraj, Z.; Mehraban, F.; Seyedarabi, S.E. Novel X-Ray Sequences and Crystal Structures of Persian and Starry Sturgeon Methemoglobins: Highlighting the Role of Heme Pocket Waters in Causing Autoxidation. Biochim Biophys Acta Proteins Proteom. 2019, 1867, 586–594. [Google Scholar] [CrossRef]
- Mena, E.L.; Jevtić, P.; Greber, B.J.; Gee, C.L.; Lew, B.G.; Akopian, D.; Nogales, E.; Kuriyan, J.; Rape, M. Structural Basis for Dimerization Quality Control. Nature 2020, 586, 452–456. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminformatics. 2012, 4, 17. [Google Scholar] [CrossRef]
- Sakulkeo, O.; Wattanapiromsakul, C.; Pitakbut, T.; Dej-adisai, S. Alpha-Glucosidase Inhibition and Molecular Docking of Isolated Compounds from Traditional Thai Medicinal Plant, Neuropeltis Racemosa Wall. Molecules 2022, 27, 639. [Google Scholar] [CrossRef]
- Pitakbut, T. The Antiviral Activity of Andrographolide, the Active Metabolite from Andrographis Paniculata (Burm. f.) Wall. Ex Nees. against SARS-CoV-2 by Using Bio- and Chemoinformatic Tools. Walailak J. Sci. Tech. 2020, 17, 851–866. [Google Scholar] [CrossRef]
- Phoopha, S.; Wattanapiromsakul, C.; Pitakbut, T.; Dej-Adisai, S. A New Stilbene Derivative and Isolated Compounds from Bauhinia Pottsii Var. Pottsii with Their Anti-Alpha-Glucosidase Activity. Pharmacogn. Mag. 2020, 16, 161. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus Bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Pitakbut, T.; Nguyen, G.-N.; Kayser, O. Activity of THC, CBD, and CBN on Human ACE2 and SARS-CoV1/2 Main Protease to Understand Antiviral Defense Mechanism. Planta Med. 2021, 88, 1047–1059. [Google Scholar] [CrossRef]
- Carr, N.T. Using Microsoft Excel® to Calculate Descriptive Statistics and Create Graphs. Lang Assess Q. 2008, 5, 43–62. [Google Scholar] [CrossRef]
- Papautsky, E.L.; Hamlish, T. Patient-Reported Treatment Delays in Breast Cancer Care during the COVID-19 Pandemic. Breast Cancer Res. Treat. 2020, 184, 249–254. [Google Scholar] [CrossRef]

| Crude Extracts | Tyrosinase Inhibitory Activity | |
|---|---|---|
| Tyrosinase Inhibition at 20 µg/mL (%) | IC50 Values (µg/mL) | |
| Thai rejuvenating remedy No. 11 | ||
| 1. Hexane | 6.09 ± 1.89 | 243.91 |
| 2. Ethyl acetate | 71.06 ± 1.38 | 9.98 |
| 3. Ethanol | 63.37 ± 0.92 | 12.74 |
| 4. Water | 13.64 ± 1.24 | 102.32 |
| 5. 80% Ethanol | 70.44 ± 0.23 | 9.41 |
| Crude Extracts | Tyrosinase Inhibitory Activity | |
|---|---|---|
| Tyrosinase Inhibition at 20 µg/mL (%) | IC50 Values (µg/mL) | |
| Ingredients in the remedy No. 11 | ||
| 1. Ardisia elliptica | 7.67 ± 0.58 | - |
| 2. Cymbopogon citratus | 11.38 ± 2.31 | - |
| 3. Entada rheedii | 23.98 ± 0.62 | - |
| 4. Glycyrrhiza glabra | 80.07 ± 1.04 | 3.74 |
| 5. Kaempferia galanga | 10.88 ± 0.97 | - |
| 6. Phyllanthus emblica | 5.03 ± 0.59 | - |
| 7. Piper sarmentosum | 6.11 ± 2.69 | - |
| 8. Plumbago indica | 12.05 ± 2.28 | - |
| 9. Terminalia chebula | 6.06 ± 1.79 | - |
| Standard metabolite 1. Glabridin | - | 0.08 |
| Positive control | ||
| 1. Kojic acid | 85.93 ± 0.24 | 3.92 |
| 2. Artocarpus lacucha, wood water extract | 96.02 ± 0.36 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dej-adisai, S.; Koyphokaisawan, N.; Wattanapiromsakul, C.; Nuankaew, W.; Kang, T.H.; Pitakbut, T. In Vitro, In Vivo, and In Silico Analyses of Molecular Anti-Pigmentation Mechanisms of Selected Thai Rejuvenating Remedy and Bioactive Metabolites. Molecules 2023, 28, 958. https://doi.org/10.3390/molecules28030958
Dej-adisai S, Koyphokaisawan N, Wattanapiromsakul C, Nuankaew W, Kang TH, Pitakbut T. In Vitro, In Vivo, and In Silico Analyses of Molecular Anti-Pigmentation Mechanisms of Selected Thai Rejuvenating Remedy and Bioactive Metabolites. Molecules. 2023; 28(3):958. https://doi.org/10.3390/molecules28030958
Chicago/Turabian StyleDej-adisai, Sukanya, Nitinant Koyphokaisawan, Chatchai Wattanapiromsakul, Wanlapa Nuankaew, Tong Ho Kang, and Thanet Pitakbut. 2023. "In Vitro, In Vivo, and In Silico Analyses of Molecular Anti-Pigmentation Mechanisms of Selected Thai Rejuvenating Remedy and Bioactive Metabolites" Molecules 28, no. 3: 958. https://doi.org/10.3390/molecules28030958
APA StyleDej-adisai, S., Koyphokaisawan, N., Wattanapiromsakul, C., Nuankaew, W., Kang, T. H., & Pitakbut, T. (2023). In Vitro, In Vivo, and In Silico Analyses of Molecular Anti-Pigmentation Mechanisms of Selected Thai Rejuvenating Remedy and Bioactive Metabolites. Molecules, 28(3), 958. https://doi.org/10.3390/molecules28030958