Green Extractants in Assisting Recovery of REEs: A Case Study
Abstract
:1. Introduction
2. Results and Discussion
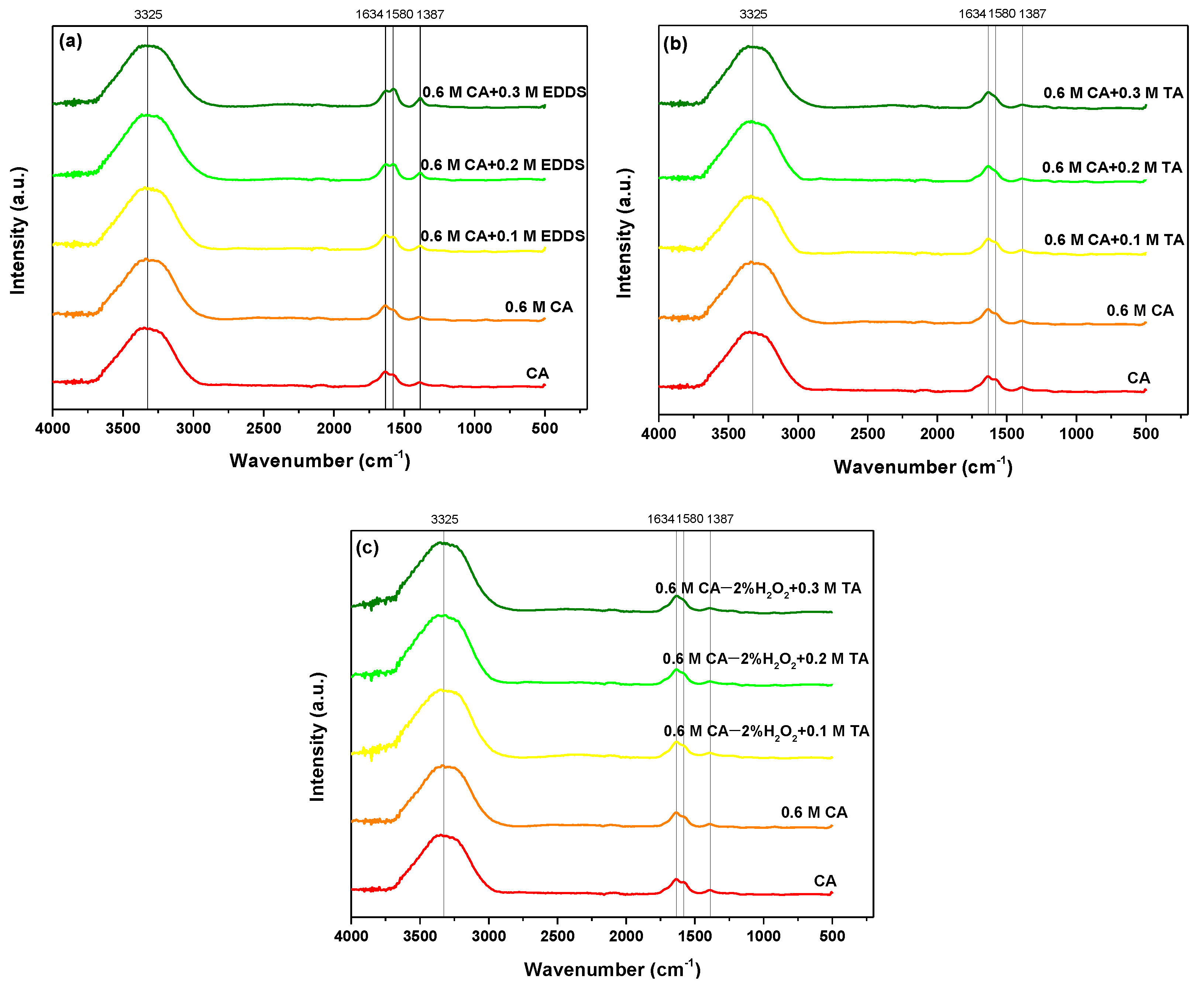
2.1. Physicochemical Characterization of Materials
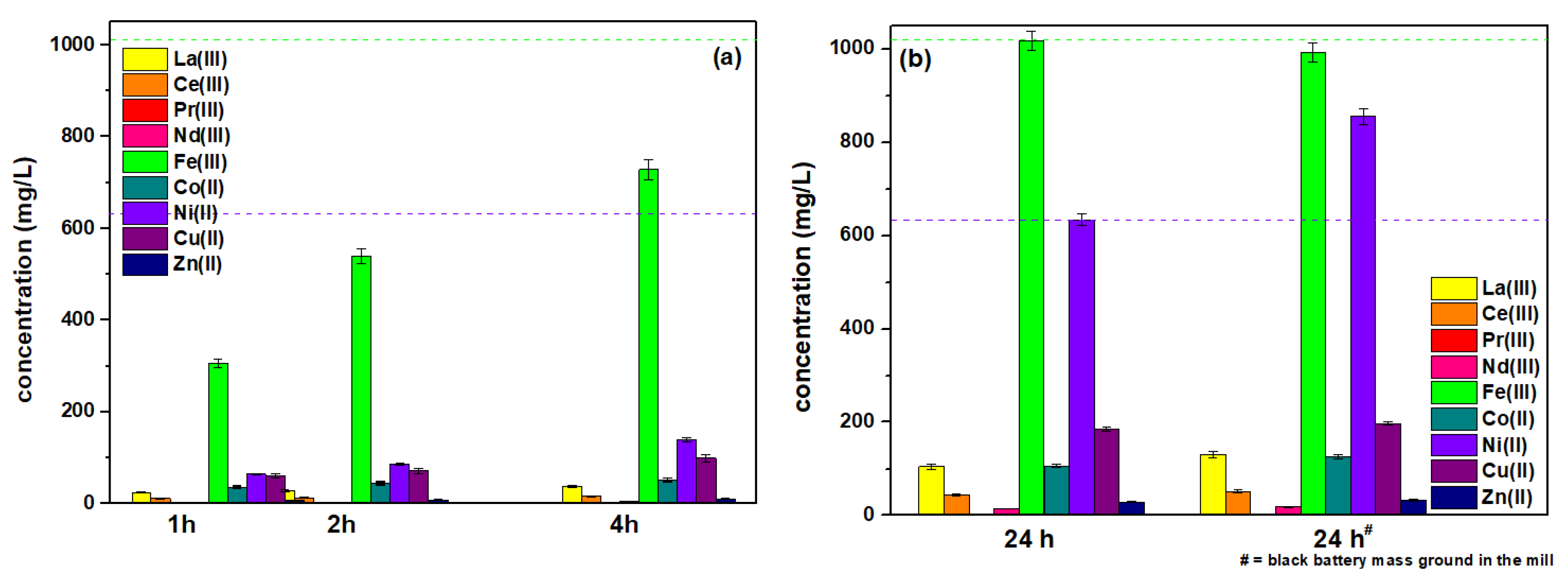
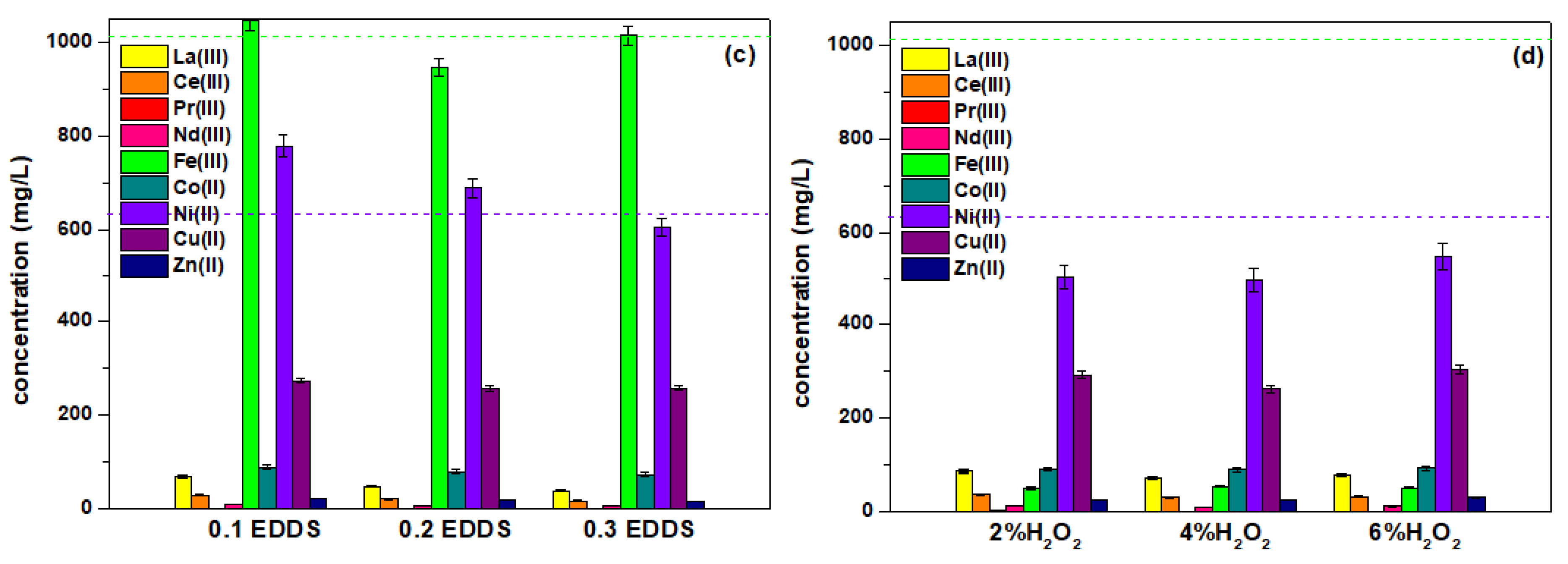
2.2. Leaching Parameters
2.3. Separation Steps—Summary
3. Materials and Methods
3.1. Materials
3.2. Leaching Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Balassone, G.; Manfredi, C.; Vasca, E.; Bianco, M.; Boni, M.; Di Nunzio, A.; Lombardo, F.; Mozzillo, R.; Marino, A.; Mormone, A.; et al. Recycling REEs from the waste products of silius mine (SE Sardinia, Italy): A preliminary study. Sustainability 2021, 13, 14000. [Google Scholar] [CrossRef]
- European Commission Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474 (accessed on 7 December 2022).
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of LREE, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, P. Rare earth export market statistics and analysis in 2015. Rare Earth Inf. 2015, 2016, 12–15. [Google Scholar]
- Habib, K.; Parajuly, K.; Wenzel, H. Tracking the flow of resources in electronic waste—The case of end-of-life computer hard disk drives. Environ. Sci. Technol. 2015, 49, 12441–12449. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Moomy, R.; Eggert, R.G. China’s public policies toward rare earths, 1975–2018. Miner. Econ. 2020, 33, 127–151. [Google Scholar] [CrossRef] [Green Version]
- Habashi, F. Extractive metallurgy of rare earths. Can. Metall. Q. 2013, 52, 224–233. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Wang, B.; Wang, K.; Wu, R.; Ekberg, C.; Volinsky, A.A. Multiscale recycling rare earth elements from real waste trichromatic phosphors containing glass. J. Clean. Prod. 2019, 238, 117998. [Google Scholar] [CrossRef]
- Shukla, N.; Dhawan, N. Rapid microwave processing of discarded tubular lights for extraction of rare earth values. Process Saf. Environ. Prot. 2020, 142, 238–249. [Google Scholar] [CrossRef]
- Janoš, P.; Kuráň, P.; Ederer, J.; Š͗astný, M.; Vrtoch, L.; Pšenička, M.; Henych, J.; Mazanec, K.; Skoumal, M. Recovery of cerium dioxide from spent glass-polishing slurry and its utilization as a reactive sorbent for fast degradation of toxic organophosphates. Adv. Mater. Sci. Eng. 2015, 2015, 241421. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, U.S.; Byeon, M.S.; Kang, W.K.; Hwang, K.T.; Cho, W.S. Recovery of cerium from glass polishing slurry. J. Rare Earths 2011, 29, 1075–1078. [Google Scholar] [CrossRef]
- Borra, C.R.; Vlugt, T.J.H.; Yang, Y.; Offerman, S.E. Recovery of cerium from glass polishing waste: A critical review. Metals 2018, 8, 801. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Hassas, B.V.; Rezaee, M.; Zhou, C.; Pisupati, S.V. Recovery of rare earth elements from coal fly ash through sequential chemical roasting, water leaching, and acid leaching processes. J. Clean. Prod. 2021, 284, 124725. [Google Scholar] [CrossRef]
- Rozelle, P.L.; Khadilkar, A.B.; Pulati, N.; Soundarrajan, N.; Klima, M.S.; Mosser, M.M.; Miller, C.E.; Pisupati, S.V. A Study on Removal of Rare Earth Elements from U.S. Coal Byproducts by Ion Exchange. Metall. Mater. Trans. E 2016, 3, 6–17. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, C.; Pan, J.; Zhang, N.; Liu, C.; Cao, S.; Hu, T.; Ji, W. Study on extraction of rare earth elements from coal fly ash through alkali fusion—Acid leaching. Miner. Eng. 2019, 136, 36–42. [Google Scholar] [CrossRef]
- Wu, F.; Liu, X.; Qu, G.; Ning, P. A critical review on extraction of valuable metals from solid waste. Sep. Purif. Technol. 2022, 301, 122043. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Barthen, R.; Puhakka, J.A.; Lakaniemi, A.M. Leaching of rare earth elements and base metals from spent NiMH batteries using gluconate and its potential bio-oxidation products. J. Hazard. Mater. 2021, 414, 125564. [Google Scholar] [CrossRef]
- Schmitz, A.M.; Pian, B.; Medin, S.; Reid, M.C.; Wu, M.; Gazel, E.; Barstow, B. Generation of a Gluconobacter oxydans knockout collection for improved extraction of rare earth elements. Nat. Commun. 2021, 12, 6693. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Bernardes, A.M.; Tenório, J.A.S. Spent NiMH batteries: Characterization and metal recovery through mechanical processing. J. Power Sources 2006, 160, 1465–1470. [Google Scholar] [CrossRef]
- Skorik, N.; Kumok, V.; Perov, E.; Avgustan, K.; Serebrennikov, V. Complexes of rare earth citrates in acid solutions. Zhurnal Neorg. Khimii 1965, 10, 653–656. [Google Scholar]
- Su, H.F.; Tan, F.; Lin, J.F. An integrated approach combines hydrothermal chemical and biological treatment to enhance recycle of rare metals from coal fly ash. Chem. Eng. J. 2020, 395, 124640. [Google Scholar] [CrossRef]
- Agarwal, V.; Khalid, M.K.; Porvali, A.; Wilson, B.P.; Lundström, M. Recycling of spent NiMH batteries: Integration of battery leach solution into primary Ni production using solvent extraction. Sustain. Mater. Technol. 2019, 22, e00121. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Wang, P.; Na, D.; Yang, Z.; Zhang, J. Solvent extraction of Ce(III) and Pr(III) with P507 using SiC foam as a static mixer. J. Rare Earths 2022, 40, 676–686. [Google Scholar] [CrossRef]
- Zhang, D.R.; Chen, H.R.; Nie, Z.Y.; Xia, J.L.; Li, E.P.; Fan, X.L.; Zheng, L. Extraction of Al and rare earths (Ce, Gd, Sc, Y) from red mud by aerobic and anaerobic bi-stage bioleaching. Chem. Eng. J. 2020, 401, 125914. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Aurich, A.; Förster, A.; Mauersberger, S.; Barth, G.; Stottmeister, U. Citric acid production from renewable resources by Yarrowia lipolytica. Biotechnol. Adv. 2003, 21, 454–455. [Google Scholar]
- Becker, M.Y.; Kohlheb, N.; Hunger, S.; Eschrich, S.; Müller, R.; Aurich, A. Early-stage sustainability assessment of biotechnological processes: A case study of citric acid production. Eng. Life Sci. 2020, 20, 90–103. [Google Scholar] [CrossRef] [Green Version]
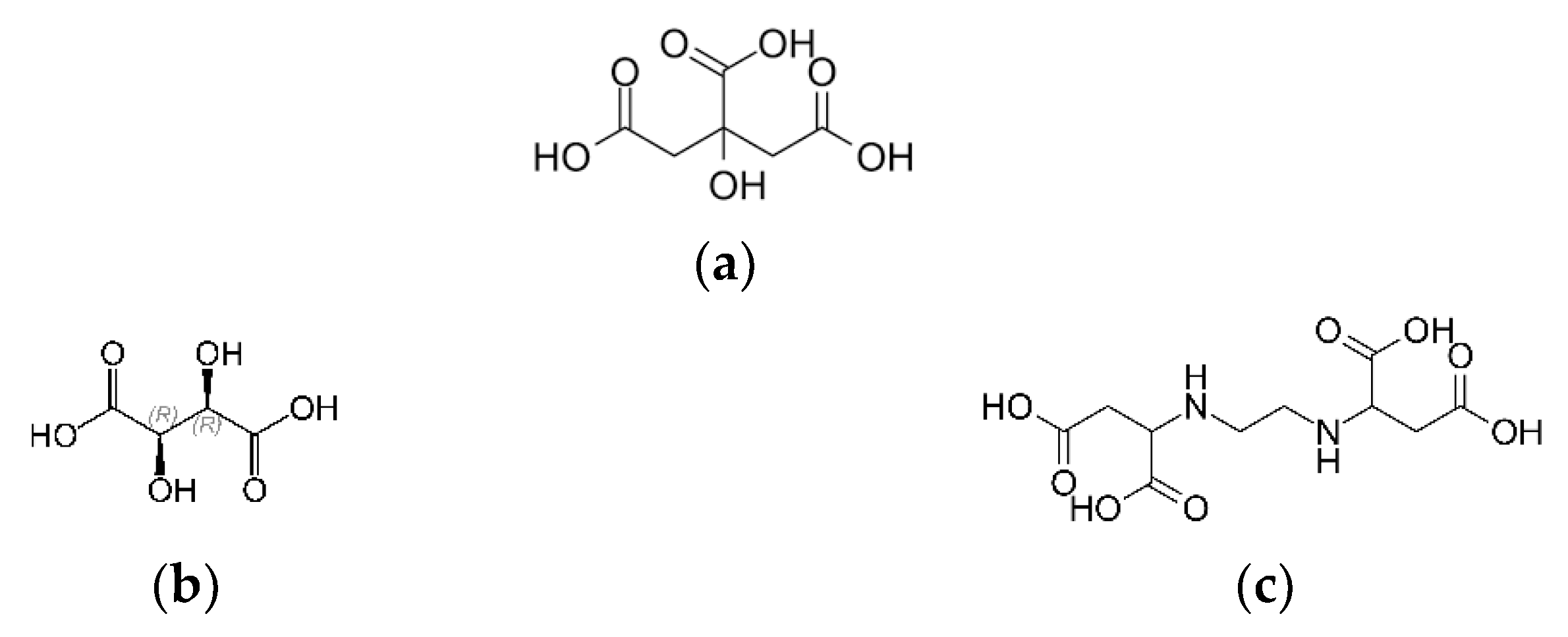
- Burdzy, K.; Aurich, A.; Hunger, S.; Jastrząb, R.; Zabiszak, M.; Kołodyńska, D. Green citric acid in the sorption process of rare earth elements. Chem. Eng. J. 2022, 437, 135366. [Google Scholar] [CrossRef]
- Apelblat, A.; Apelblat, A. Physicochemical properties of inorganic citrates. In Citric Acid; Springer: Cham, Switzerland, 2014; pp. 267–357. ISBN 9783319112336. [Google Scholar]
- Bastug, A.S.; Göktürk, S.; Sismanoglu, T. 1:1 Binary complexes of citric acid with some metal ions: Stability and thermodynamic parameters. Asian J. Chem. 2008, 20, 1269–1278. [Google Scholar] [CrossRef]
- Howard, W.L.; Wilson, D. Chelating agents. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-48508-7. [Google Scholar]
- Maślowska, J.; Bielawski, M.; Baranowska, A. Thermoanalytical investigation of citric acid and complexe salts of transition metals with citric acid. Thermochim. Acta 1985, 92, 235–239. [Google Scholar] [CrossRef]
- Wu, S.; Chang, Z.; Wang, K.; Xiong, W. Preparation and thermal behaviour of rare earth citrate hydrates. J. Therm. Anal. 1995, 45, 199–206. [Google Scholar] [CrossRef]
- da Silva, M.F.P.; Matos, J.R.; Isolani, P.C. Synthesis characterization and thermal analysis of 1:1 and 2:3 lanthanide(II) citrates. J. Therm. Anal. Calorim. 2008, 94, 305–311. [Google Scholar] [CrossRef]
- Popa, M.; Kakihana, M. Synthesis and thermoanalytical investigation of an amorphous praseodymium citrate. J. Therm. Anal. Calorim. 2001, 65, 281–293. [Google Scholar] [CrossRef]
- Hubicki, Z.; Gȩca, M.; Kołodyńska, D. Sorption of heavy metal metatartrate complexes on polystyrene anion exchangers. Environ. Technol. 2011, 32, 569–582. [Google Scholar] [CrossRef]
- Hubicki, Z.; Gȩca, M.; Kołodynska, D. The effect of the presence of metatartaric acid on removal effectiveness of heavy metal ions on chelating ion exchangers. Environ. Technol. 2011, 32, 805–816. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Jaworska, J.S.; Schowanek, D.; Feijtel, T.C.J. Environmental risk assessment for trisodium [S,S]-ethylene diamine disuccinate, a biodegradable chelator used in detergent applications. Chemosphere 1999, 38, 3597–3625. [Google Scholar] [CrossRef]
- Tandy, S.; Ammann, A.; Schulin, R.; Nowack, B. Biodegradation and speciation of residual SS-ethylenediaminedisuccinic acid (EDDS) in soil solution left after soil washing. Environ. Pollut. 2006, 142, 191–199. [Google Scholar] [CrossRef]
- Sturza, C.M.; Boscencu, R.; Nacea, V. The lanthanides: Physico-chemical properties relevant for their biomedical applications. Farmacia 2008, LVI, 326–338. [Google Scholar]
- Kołodyńska, D.; Hubicki, Z. Investigation of sorption and separation of lanthanides on the ion exchangers of various types. In Ion Exchange Technologies; Kilislioğlu, A., Ed.; InTech Publishers: Rijeka, Croatia, 2012; pp. 101–154. ISBN 980-953-307-139-3. [Google Scholar] [CrossRef] [Green Version]
- Klinger, J.M. A historical geography of rare earth elements: From discovery to the atomic age. Extr. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Kostromina, N.A.; Trunova, E.K.; Tananaeva, N.N. Complex formation of Fe(III) with tartaric acid by the method of nuclear magnetic relaxation. Theor. Exp. Chem. 1988, 23, 462–466. [Google Scholar] [CrossRef]
- Thompson, V.S.; Gupta, M.; Jin, H.; Vahidi, E.; Yim, M.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; et al. Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Bernardes, A.M.; Tenório, J.A.S. Spent NiMH batteries-The role of selective precipitation in the recovery of valuable metals. J. Power Sources 2009, 193, 914–923. [Google Scholar] [CrossRef]
- Rao, M.; Zhang, T.; Li, G.; Zhou, Q.; Luo, J.; Zhang, X.; Zhu, Z.; Peng, Z.; Jiang, T. Solvent extraction of Ni and Co from the phosphoric acid leaching solution of laterite ore by P204 and P507. Metals 2020, 10, 545. [Google Scholar] [CrossRef] [Green Version]
- Petranikova, M.; Ebin, B.; Tunsu, C. Selective recovery of cobalt from the secondary streams after NiMH batteries processing using Cyanex 301. Waste Manag. 2019, 83, 194–201. [Google Scholar] [CrossRef]
- Li, D.Q.; Wang, Z.H.; Zeng, G.F.; Xue, Z. Extraction mechanism of cerium (Ⅳ) from sulphuric acid solution with HEHEHP. J. Chin. Soc. Rare Earths 1984, 2, 9. [Google Scholar]
- Larsson, K.; Ekberg, C.; Ødegaard-Jensen, A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste: Part II: Mixer-settler experiments. Hydrometallurgy 2013, 133, 168–175. [Google Scholar] [CrossRef]
- Liao, C.F.; Jiao, Y.F.; Liang, Y.; Jiang, P.G.; Nie, H.P. Adsorption-extraction mechanism of heavy rare earth by Cyanex272-P507 impregnated resin. Trans. Nonferrous Met. Soc. China 2010, 20, 1511–1516. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, Y. Solvent extraction of erbium(Ⅲ) with P507 in nitric acid solution and its separation from calcium(Ⅱ) and ferrum(Ⅲ). CIESC J. 2014, 65, 258–263. [Google Scholar] [CrossRef]
- LI, D. A review on yttrium solvent extraction chemistry and separation process. J. Rare Earths 2017, 35, 107. [Google Scholar] [CrossRef]
- Yun, C.Y.; Lee, C.; Lee, G.G.; Jo, S.; Sung, S.W. Modeling and simulation of multicomponent solvent extraction processes to purify rare earth metals. Hydrometallurgy 2016, 159, 40–45. [Google Scholar] [CrossRef]
- Mohammadi, M.; Forsberg, K.; Kloo, L.; Martinez De La Cruz, J.; Rasmuson, Å. Separation of Nd(III), Dy(III) and Y(III) by solvent extraction using D2EHPA and EHEHPA. Hydrometallurgy 2015, 156, 215–224. [Google Scholar] [CrossRef]
- Sato, T. Liquid-liquid extraction of rare-earth elements from aqueous acid solutions by acid organophosphorous compounds. Hydrometallurgy 1989, 22, 121–140. [Google Scholar] [CrossRef]



| Composition | Fe | Ni | Co | Cu | Zn | La | Ce | Pr | Nd |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 11.293 | 30.769 | 3.414 | 3.268 | 0.787 | 4.306 | 1.839 | 0.128 | 0.551 |
| Composition | O | Al | Si | K | Mn | Ca | Cl | Mg | Y |
| Content (%) | 26.015 | 5.035 | 2.602 | 1.589 | 1.046 | 1.036 | 0.553 | 0.190 | 0.122 |
| Parameter | CA | CA-TA | CA-EDDS |
|---|---|---|---|
| leaching solution (M) | 0.6 | 0.1, 0.2, 0.3 | 0.1, 0.2, 0.3 |
| with H2O2 addition | 2, 4, 6% | 2% | - |
| pH | 4.45–4.47 | 3.72–4.21 | 4.81–5.50 |
| time (h) #with black battery mass ground in the mill | 1, 2, 4, 24, 24# | 1, 2, 4, 24, 24# | 1, 2, 4, 24, 24# |
| temperature (K) Pregnant leach solution (PLS) | 293 and 333 | 293 | 293 |
 |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodyńska, D.; Burdzy, K.; Hunger, S.; Aurich, A.; Ju, Y. Green Extractants in Assisting Recovery of REEs: A Case Study. Molecules 2023, 28, 965. https://doi.org/10.3390/molecules28030965
Kołodyńska D, Burdzy K, Hunger S, Aurich A, Ju Y. Green Extractants in Assisting Recovery of REEs: A Case Study. Molecules. 2023; 28(3):965. https://doi.org/10.3390/molecules28030965
Chicago/Turabian StyleKołodyńska, Dorota, Katarzyna Burdzy, Steffi Hunger, Andreas Aurich, and Yongming Ju. 2023. "Green Extractants in Assisting Recovery of REEs: A Case Study" Molecules 28, no. 3: 965. https://doi.org/10.3390/molecules28030965







