Saikosaponin A and Its Epimers Alleviate LPS-Induced Acute Lung Injury in Mice
Abstract
:1. Introduction
2. Results
2.1. Analysis of Bupleurum chinense DC. Methanol Extracts
2.2. SSs Effectively Inhibited Pulmonary Edema in LPS-Induced ALI Mice
2.3. SSs Reduced Proinflammatory Cytokine Levels in Serum and Lung Tissue
2.4. SSs Relieved the Histological Damage Induced by LPS in Mouse Lungs
2.5. SSs Restrained the Gene Levels of Inflammatory Cytokines in the Lungs of ALI Mice
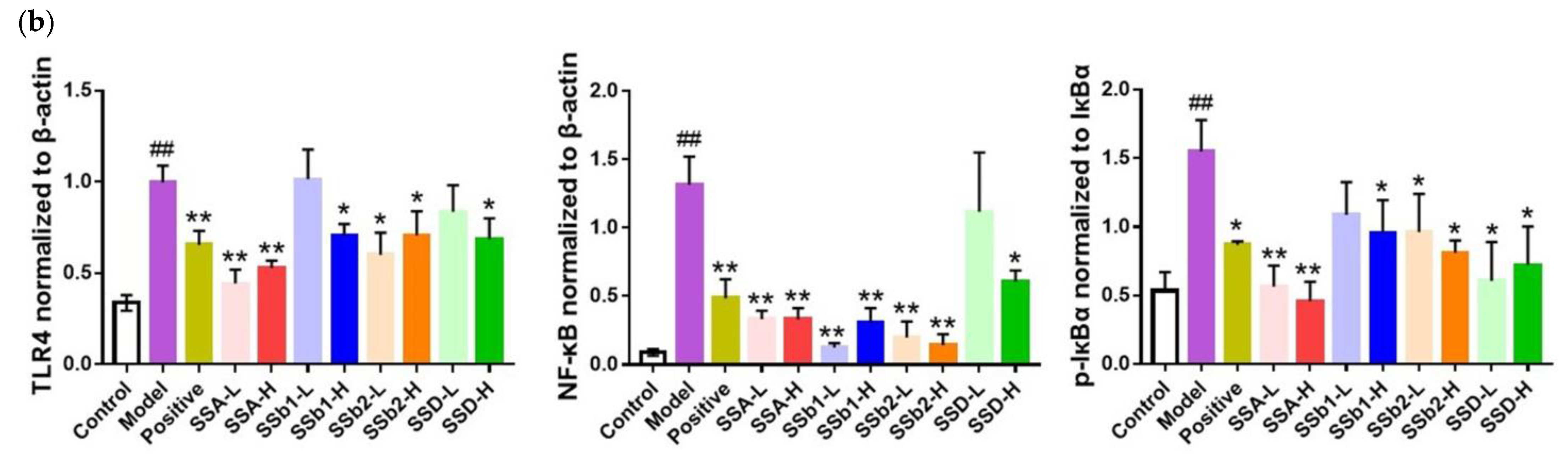
2.6. SSs Decreased the Expression of TLR4 and NF-κB in the Lungs of ALI Mice
2.7. Statistical Analysis of SS Anti-ALI Indices
+ 0.255x11 + 0.225x12 + 0.233x13 + 0.299x14,
+ 0.457x11 − 0.008x12 + 0.303x13 + 0.145x14
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation and Chemical Analysis of Bupleurum chinense DC.
4.3. Establishment of ALI and Treatment
4.4. The Lung W/D Ratio Analysis
4.5. Proinflammatory Cytokine Levels Analysis
4.6. Histopathological Analysis
4.7. Real-Time Quantitative PCR Analysis
4.8. Western Blotting Analysis
4.9. Principal Component Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute lung injury: A clinical and molecular review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perl, M.; Lomas-Neira, J.; Venet, F.; Chung, C.S.; Ayala, A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev. Respir. Med. 2011, 5, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Li, H.; Li, Y.; Dai, M.; Zhang, L.; Liu, S.; Tan, H.; Deng, P.; Liu, J.; Mao, Z.; et al. Nets promote ali/ards inflammation by regulating alveolar macrophage polarization. Exp. Cell Res. 2019, 382, 111486. [Google Scholar] [CrossRef]
- Tang, M.; Tian, Y.; Li, D.; Lv, J.; Li, Q.; Kuang, C.; Hu, P.; Wang, Y.; Wang, J.; Su, K.; et al. Tnf-alpha mediated increase of hif-1alpha inhibits vasp expression, which reduces alveolar-capillary barrier function during acute lung injury (ali). PLoS ONE 2014, 9, e102967. [Google Scholar]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of tnfalpha in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of nf-kappab pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Y.; Xu, Y.; Jia, J.; Xi, W.; Deng, H.; Tu, W. Dexmedetomidine resists intestinal ischemia-reperfusion injury by inhibiting tlr4/myd88/nf-kappab signaling. J. Surg. Res. 2021, 260, 350–358. [Google Scholar] [CrossRef]
- Fan, Z.; Yao, J.; Li, Y.; Hu, X.; Shao, H.; Tian, X. Anti-inflammatory and antioxidant effects of curcumin on acute lung injury in a rodent model of intestinal ischemia reperfusion by inhibiting the pathway of nf-kb. Int. J. Clin. Exp. Pathol. 2015, 8, 3451–3459. [Google Scholar]
- Jiang, H.; Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Zheng, S.; Yu, H.; Wang, X.; Yang, B.; et al. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of bupleuri radix: A systematic review. Biomed. Pharmacother. 2020, 131, 110679. [Google Scholar] [CrossRef]
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix bupleuri: A review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed Res. Int. 2017, 2017, 7597596. [Google Scholar] [CrossRef]
- Li, Z.Y.; Sun, H.M.; Xing, J.; Qin, X.M.; Du, G.H. Chemical and biological comparison of raw and vinegar-baked radix bupleuri. J. Ethnopharmacol. 2015, 165, 20–28. [Google Scholar] [CrossRef]
- Liu, P.P.; Shan, G.S.; Zhang, F.; Chen, J.N.; Jia, T.Z. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed astragali radix by uplc-qtof-ms combined with novel informatics unifi platform. Chin. J. Nat. Med. 2018, 16, 714–720. [Google Scholar] [CrossRef]
- Cui, L.H.; Li, C.X.; Zhuo, Y.Z.; Yang, L.; Cui, N.Q.; Zhang, S.K. Saikosaponin d ameliorates pancreatic fibrosis by inhibiting autophagy of pancreatic stellate cells via pi3k/akt/mtor pathway. Chem. Biol. Interact. 2019, 300, 18–26. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Pan, R.; Xu, Y.; Wang, Q.; Song, M. Total saikosaponins of bupleurum yinchowense reduces depressive, anxiety-like behavior and increases synaptic proteins expression in chronic corticosterine-treated mice. BMC Complement. Altern. Med. 2018, 18, 117. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Deng, N.; Jin, H.N.; Xuan, Z.Z.; Qian, Y.X.; Wu, Z.Y.; Xie, W. Saikosaponin a modulates remodeling of kv4.2-mediated a-type voltage-gated potassium currents in rat chronic temporal lobe epilepsy. Drug Des. Dev. Ther. 2018, 12, 2945–2958. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Zhang, H.; Ou, Y.; Jiang, Y.; Zhong, D.; Qi, H.; Dang, Q. Saikosaponin-d suppresses cell growth in renal cell carcinoma through egfr/p38 signaling pathway. Neoplasma 2017, 64, 518–525. [Google Scholar] [CrossRef]
- Chen, J.; Duan, M.; Zhao, Y.; Ling, F.; Xiao, K.; Li, Q.; Li, B.; Lu, C.; Qi, W.; Zeng, Z.; et al. Saikosaponin a inhibits influenza a virus replication and lung immunopathology. Oncotarget 2015, 6, 42541–42556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.Z.; Guo, X.T.; Chen, J.W.; Zhao, Y.; Cong, X.; Jiang, Z.L.; Cao, R.F.; Cui, K.; Gao, S.S.; Tian, W.R. Saikosaponin-d attenuates heat stress-induced oxidative damage in llc-pk1 cells by increasing the expression of anti-oxidant enzymes and hsp72. Am. J. Chin. Med. 2014, 42, 1261–1277. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin a mediates the inflammatory response by inhibiting the mapk and nf-kappab pathways in lps-stimulated raw 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.A.; Sun, M.N.; Hu, Z.S. Saikosaponin a ameliorates lps-induced acute lung injury in mice. Inflammation 2018, 41, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Liu, M.; Zhong, T.D.; Fang, X.M. Saikosaponin-d attenuates ventilator-induced lung injury in rats. Int. J. Clin. Exp. Med. 2015, 8, 15137–15145. [Google Scholar] [PubMed]
- Ren, D.; Luo, J.; Li, Y.; Zhang, J.; Yang, J.; Liu, J.; Zhang, X.; Cheng, N.; Xin, H. Saikosaponin b2 attenuates kidney fibrosis via inhibiting the hedgehog pathway. Phytomedicine 2020, 67, 153163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, L.; Liu, L.; Zhao, R. Saikosaponin b2 enhances the hepatotargeting effect of anticancer drugs through inhibition of multidrug resistance-associated drug transporters. Life Sci. 2019, 231, 116557. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, F.F.; He, X.; Li, K.; Gao, Y.; Xu, X.L.; Jiang, N.H.; Ding, L.; Song, W.J.; He, Y.Q.; et al. Antitumor effects of saikosaponin b2 on breast cancer cell proliferation and migration. Mol. Med. Rep. 2019, 20, 1943–1951. [Google Scholar] [CrossRef]
- Shin, J.S.; Im, H.T.; Lee, K.T. Saikosaponin b2 suppresses inflammatory responses through ikk/ikappabalpha/nf-kappab signaling inactivation in lps-induced raw 264.7 macrophages. Inflammation 2019, 42, 342–353. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, X.; Kang, R.; Wang, Y.; Guo, X.; Jing, W.; Wei, F.; Ma, S. Systematic characterization and identification of saikosaponins in extracts from bupleurum marginatum var. Stenophyllum using uplc-pda-q/tof-ms. Front. Chem. 2021, 9, 747987. [Google Scholar] [CrossRef]
- Lei, T.; Chen, S.; Wang, K.; Zhang, D.; Dong, L.; Lv, C.; Wang, J.; Lu, J. Characterization and discrimination of raw and vinegar-baked bupleuri radix based on uhplc-q-tof-ms coupled with multivariate statistical analysis. Biomed. Chromatogr. 2018, 32, e4044. [Google Scholar] [CrossRef]
- Du, T.; Zeng, M.; Chen, L.; Cao, Z.; Cai, H.; Yang, G. Chemical and absorption signatures of xiao chai hu tang. Rapid Commun. Mass Spectrom. 2018, 32, 1107–1125. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, H.; Tu, S.; Duan, Y.; Pei, K.; Xu, Y.; Liu, J.; Niu, M.; Zhang, Y.; Shen, L.; et al. Identification and analysis of compound profiles of sinisan based on ‘individual herb, herb-pair, herbal formula’ before and after processing using uhplc-q-tof/ms coupled with multiple statistical strategy. Molecules 2018, 23, 3128. [Google Scholar] [CrossRef] [Green Version]
- Kou, W.M. Effect of processing on pharmacodynamics of Bupleurum chinense and its clinical application. Lishizhen Med. Mater. Med. Res. 2006, 17, 142–143. [Google Scholar]
- Zhou, Z.; Chen, H.; Tang, X.; He, B.; Gu, L.; Feng, H. Total saikosaponins attenuates depression-like behaviors induced by chronic unpredictable mild stress in rats by regulating the pi3k/akt/nf-kappab signaling axis. Evid. Based Complement Altern. Med. 2022, 2022, 4950414. [Google Scholar] [CrossRef]
- Liu, A.; Tanaka, N.; Sun, L.; Guo, B.; Kim, J.H.; Krausz, K.W.; Fang, Z.; Jiang, C.; Yang, J.; Gonzalez, F.J. Saikosaponin d protects against acetaminophen-induced hepatotoxicity by inhibiting nf-kappab and stat3 signaling. Chem. Biol. Interact. 2014, 223, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.Y.; Di, H.Y.; Li, H.; Cheng, X.Q.; Zhang, Y.Y.; Chen, D.F. Bupleurum chinense dc polysaccharides attenuates lipopolysaccharide-induced acute lung injury in mice. Phytomedicine 2012, 19, 130–137. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, X.; Cao, M.; Zheng, S.; Ma, Y.; Huang, Q. Nf-kappab and ampk-nrf2 pathways support the protective effect of polysaccharides from polygonatum cyrtonema hua in lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 2022, 291, 115153. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Duan, H.; Sivakumar, R.; Li, X. Chronic exposure of nanomolar mc-lr caused oxidative stress and inflammatory responses in hepg2 cells. Chemosphere 2018, 192, 305–317. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Chan, L.P.; Liu, C.; Chiang, F.Y.; Wang, L.F.; Lee, K.W.; Chen, W.T.; Kuo, P.L.; Liang, C.H. Il-8 promotes inflammatory mediators and stimulates activation of p38 mapk/erk-nf-kappab pathway and reduction of jnk in hnscc. Oncotarget 2017, 8, 56375–56388. [Google Scholar] [CrossRef] [Green Version]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Iqbal, M.; Anwer, M.K.; Al, H.A.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of t cell receptors, pro-inflammatory cytokines and nf-kappab activation against lps-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Xu, Y. Effect of alpha-tocopherol in alleviating the lipopolysaccharide-induced acute lung injury via inhibiting nuclear factor kappa-b signaling pathways. Bioengineered 2022, 13, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Yang, Q.; Cui, M.; Ci, Z.; Li, N.; Peng, W. Baicalin liposome alleviates lipopolysaccharide-induced acute lung injury in mice via inhibiting tlr4/jnk/erk/nf-kappab pathway. Mediat. Inflamm. 2020, 2020, 8414062. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.X.; Zhang, Y.J.; Yang, Y.H.; Lu, M.L.; Zhang, J.; Li, S.T.; Zhang, S.P.; Li, G. Astragaloside iv attenuates inflammatory cytokines by inhibiting tlr4/nf-small ka, cyrillicb signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013, 150, 1062–1070. [Google Scholar] [CrossRef]
- Noreen, M.; Shah, M.A.; Mall, S.M.; Choudhary, S.; Hussain, T.; Ahmed, I.; Jalil, S.F.; Raza, M.I. Tlr4 polymorphisms and disease susceptibility. Inflamm. Res. 2012, 61, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.; Cong, P.; Cicala, M.; Alloni, R.; Carotti, S.; Behar, J. Ursodeoxycholic acid improves muscle contractility and inflammation in symptomatic gallbladders with cholesterol gallstones. Gut 2007, 56, 815–820. [Google Scholar] [CrossRef]
- Kwok, H.H.; Guo, G.L.; Lau, J.K.; Cheng, Y.K.; Wang, J.R.; Jiang, Z.H.; Keung, M.H.; Mak, N.K.; Yue, P.Y.; Wong, R.N. Stereoisomers ginsenosides-20(s)-rg(3) and -20(r)-rg(3) differentially induce angiogenesis through peroxisome proliferator-activated receptor-gamma. Biochem. Pharmacol. 2012, 83, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Yuan, Z.; Zhang, X.; Yan, R.; Zhao, Y.; Liao, M.; Chen, J. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of nf-κb signaling pathway. Int. Immunopharmacol. 2012, 14, 121–126. [Google Scholar] [CrossRef]
- Committee, C.P. Pharmacopoeia of the People’s Republic of China; Committee, C.P., Ed.; China Pharmaceutical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Yang, Y.; Zhong, Z.T.; Xiao, Y.G.; Chen, H.B. The activation of ampk/nrf2 pathway in lung epithelial cells is involved in the protective effects of kinsenoside on lipopolysaccharide-induced acute lung injury. Oxidative Med. Cell. Longev. 2022, 2022, 3589277. [Google Scholar] [CrossRef]
- Han, S.; Yuan, R.; Cui, Y.; He, J.; Wang, Q.Q.; Zhuo, Y.; Yang, S.; Gao, H. Hederasaponin c alleviates lipopolysaccharide-induced acute lung injury in vivo and in vitro through the pip2/nf-kappab/nlrp3 signaling pathway. Front. Immunol. 2022, 13, 846384. [Google Scholar] [CrossRef]
- Kang, J.Y.; Xu, M.M.; Sun, Y.; Ding, Z.X.; Wei, Y.Y.; Zhang, D.W.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin attenuates lps-induced pyroptosis in acute lung injury by inhibiting nlrp3-gsdmd pathway via activating nrf2/ho-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Wu, X.; Kong, Q.; Xia, Z.; Zhan, L.; Duan, W.; Song, X. Penehyclidine hydrochloride alleviates lipopolysaccharide-induced acute lung injury in rats: Potential role of caveolin-1 expression upregulation. Int. J. Mol. Med. 2019, 43, 2064–2074. [Google Scholar] [CrossRef]

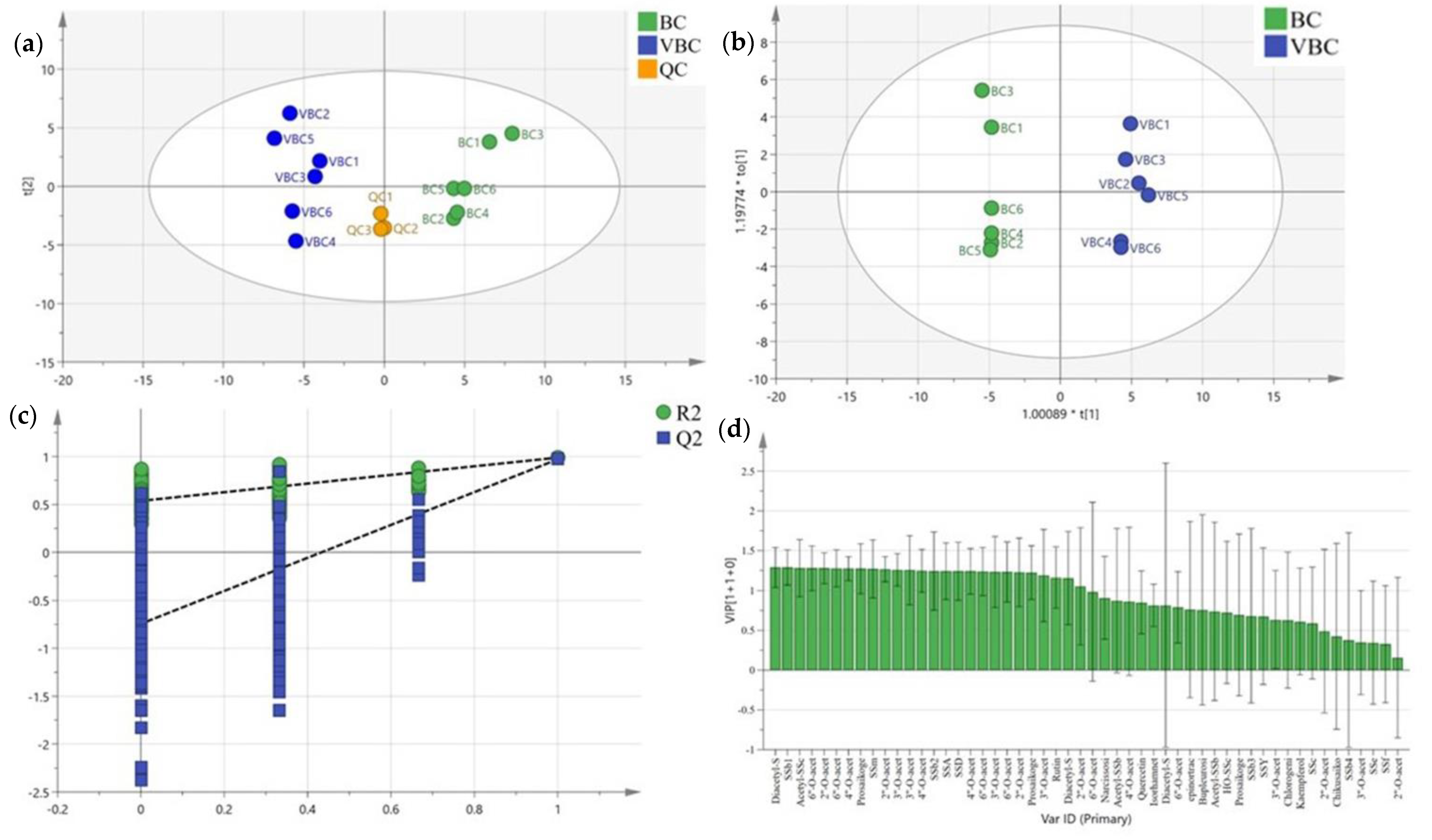






| Number | Identification | RT (min) | Molecular Formula | Theoretical [M-H]− | Experimental [M-H]− | Error (ppm) | MS/MS |
|---|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 2.42 | C16H18O9 | 353.08671 | 353.08444 | 6.425 | 353.08444, 191.05335 |
| 2 | Rutin | 3.27 | C27H30O16 | 609.14501 | 609.13983 | 8.506 | 609.13983, 300.02368 |
| 3 | Narcissoside | 4.07 | C28H32O16 | 623.16066 | 623.15674 | 6.292 | 623.15674, 315.04791, 314.04306 |
| 4 | Bupleuroside V | 4.74 | C42H66O15 | 809.43180 | 809.42487 | 8.559 | 855.42505, 809.42487, 779.41461, 617.36328, 471.30539 |
| 5 | epinortrachelogenin | 5.99 | C20H22O7 | 373.12818 | 373.12521 | 7.958 | 373.12521, 179.06854, 164.04540 |
| 6 | Quercetin | 6.01 | C15H10O7 | 301.03428 | 301.03262 | 5.511 | 301.03262, 151.00133 |
| 7 | Chikusaikoside II | 6.39 | C48H78O18 | 941.51044 | 941.50360 | 7.267 | 987.50659, 941.50360, 795.44507, 779.45148, 617.40125 |
| 8 | Kaempferol | 6.86 | C15H10O6 | 285.03936 | 285.03812 | 4.366 | 285.03812, 151.07874 |
| 9 | HO-SSc | 7.08 | C48H80O18 | 943.52609 | 943.52014 | 6.308 | 989.51270, 943.52014, 797.44751 |
| 10 | SSf | 7.10 | C48H79O17 | 927.53118 | 927.52325 | 8.547 | 973.52899, 927.52325, 781.46655, 765.47333, 619.41663 |
| 11 | Isorhamnetin | 7.11 | C16H12O7 | 315.05775 | 315.04709 | 9.012 | 315.047089, 300.02390, 151.00102 |
| 12 | SSc | 7.26 | C48H78O17 | 925.51553 | 925.50879 | 7.279 | 971.51337, 925.50879, 779.45245, 763.45630, 617.40051 |
| 13 | SSb3 | 7.68 | C43H72O14 | 811.48383 | 811.47858 | 6.474 | 857.48779, 811.47858, 649.42609, 471.34198 |
| 14 | SSb4 | 7.80 | C43H72O14 | 811.48383 | 811.47821 | 6.929 | 857.48645, 811.47821, 649.42554 |
| 15 | Acetyl-SSc | 7.84 | C50H80O18 | 967.52609 | 967.51984 | 6.462 | 1013.52161, 967.51984, 925.50836, 907.49945, 779.44580, 761.43976 |
| 16 | Acetyl-SSb3 | 8.21 | C45H74O15 | 853.49440 | 853.48871 | 6.664 | 899.49365, 853.48871, 811.47876, 793.46765, 649.42651 |
| 17 | SSA | 8.39 | C42H68O13 | 779.45762 | 779.45154 | 7.798 | 825.45807, 779.45154, 617.40009, 471.34628 |
| 18 | SSb2 | 8.75 | C42H68O13 | 779.45762 | 779.45184 | 7.413 | 825.45422, 779.45184, 617.39990, 471.34164 |
| 19 | 2″-O-acetyl-SSA | 8.79 | C44H70O14 | 821.46818 | 821.46210 | 7.405 | 821.46210, 779.45355, 761.44025, 617.39899 |
| 20 | SSb1 | 8.87 | C42H68O13 | 779.45762 | 779.45215 | 7.016 | 825.45758, 779.45215, 617.40082, 471.34387 |
| 21 | 2″-O-acetyl-SSb2 | 8.91 | C44H70O14 | 821.46818 | 821.46442 | 4.581 | 821.46442, 779.45215, 761.45129, 617.40057 |
| 22 | Acetyl-SSb4 | 8.96 | C45H74O15 | 853.49440 | 853.48908 | 6.242 | 899.49561, 853.48908, 811.47858, 793.46942, 649.42645 |
| 23 | 3″-O-acetyl-SSA | 9.03 | C44H70O14 | 821.46818 | 821.46625 | 2.353 | 821.46625, 779.45239, 761.43872 |
| 24 | Prosaikogenin H | 9.28 | C36H58O8 | 617.40480 | 617.40076 | 6.536 | 663.40533, 617.40076 |
| 25 | Prosaikogenin F | 9.52 | C36H58O8 | 617.40480 | 617.40125 | 5.742 | 663.40643, 617.40125 |
| 26 | SSe | 9.54 | C42H68O12 | 763.46270 | 763.45752 | 6.790 | 809.46307, 763.45752, 601.40729 |
| 27 | SSm | 9.67 | C42H68O12 | 763.46270 | 763.45691 | 7.589 | 809.46191, 763.45691, 601.40564 |
| 28 | 3″-O-acetyl-SSb2 | 9.68 | C44H70O14 | 821.46818 | 821.46161 | 8.002 | 821.46161, 779.45032, 617.39728 |
| 29 | 2″-O-acetyl-SSb1 | 9.78 | C44H70O14 | 821.46818 | 821.46204 | 7.478 | 821.46204, 779.46057, 617.40015 |
| 30 | 3″-O-acetyl-SSb1 | 9.88 | C44H70O14 | 821.46818 | 821.46167 | 7.929 | 821.46167, 779.44995, 617.40662 |
| 31 | 4″-O-acetyl-SSb1 | 10.33 | C44H70O14 | 821.46818 | 821.46649 | 2.061 | 821.46649, 617.39783 |
| 32 | Diacetyl-SSb2 | 10.40 | C46H72O15 | 863.47875 | 863.47522 | 4.086 | 909.48029, 863.47522, 821.45264, 761.44074 |
| 33 | 2″-O-acetyl-SSe | 10.57 | C44H70O13 | 805.47327 | 805.46771 | 6.901 | 851.47345, 805.46771, 763.45740, 745.44678, 601.40863 |
| 34 | 6″-O-acetyl-SSb1 | 10.60 | C44H70O14 | 821.46818 | 821.46393 | 5.177 | 821.46393, 779.45471 |
| 35 | SSD | 10.66 | C42H68O13 | 779.45762 | 779.45258 | 6.464 | 825.45654, 779.45258, 617.40076 |
| 36 | 2″-O-acetyl-SSm | 10.93 | C44H70O13 | 805.47327 | 805.46710 | 7.658 | 851.47400, 805.46710, 763.45471, 745.44263 |
| 37 | SSY | 11.08 | C42H66O13 | 777.44197 | 777.43640 | 7.162 | 823.44617, 777.43640, 615.38428 |
| 38 | 4″-O-acetyl-SSA | 11.20 | C44H70O14 | 821.46818 | 821.46240 | 7.040 | 821.46240, 779.45435, 761.44324, 617.39819 |
| 39 | 4″-O-acetyl-SSb2 | 11.32 | C44H70O14 | 821.46818 | 821.46619 | 2.426 | 821.46619, 779.44183, 761.45038, 617.40247 |
| 40 | 6″-O-acetyl-SSA | 11.44 | C44H70O14 | 821.46818 | 821.46100 | 8.744 | 821.46100, 779.44879 |
| 41 | 6″-O-acetyl-SSb2 | 11.58 | C44H70O14 | 821.46818 | 821.46167 | 7.929 | 821.46167, 779.44751 |
| 42 | 3″-O-acetyl-SSe | 11.66 | C44H70O13 | 805.47327 | 805.46698 | 7.807 | 851.47260, 805.46698, 763.45648, 745.44745, 601.40564 |
| 43 | 2″-O-acetyl-SSD | 11.70 | C44H70O14 | 821.46818 | 821.46289 | 6.443 | 821.46289, 779.45270, 761.44043, 617.39905 |
| 44 | 3″-O-acetyl-SSD | 11.82 | C44H70O14 | 821.46818 | 821.46228 | 7.186 | 821.46228, 779.45050, 761.44135 |
| 45 | 3″-O-acetyl-SSm | 12.04 | C44H70O13 | 805.47327 | 805.46649 | 8.416 | 851.47028, 805.46649, 763.45490, 745.44727 |
| 46 | Prosaikogenin G | 12.47 | C36H58O8 | 617.40480 | 617.40000 | 7.831 | 663.40503, 617.40000 |
| 47 | 4″-O-acetyl-SSD | 12.59 | C44H70O14 | 821.46818 | 821.45367 | 7.668 | 821.45367, 779.45801, 617.39990 |
| 48 | 6″-O-acetyl-SSD | 12.70 | C44H70O14 | 821.46818 | 821.46143 | 8.221 | 821.46143, 617.40204 |
| 49 | Diacetyl-SSA | 12.93 | C46H72O15 | 863.47875 | 863.47205 | 7.757 | 909.47742, 863.47205, 821.46124, 779.45111, 761.43970, 617.39887 |
| 50 | 6″-O-acetyl-SSe | 13.96 | C44H70O13 | 805.47327 | 805.46552 | 9.620 | 851.47376, 805.46552, 763.45001, 745.44611 |
| 51 | Diacetyl-SSD | 13.68 | C46H72O15 | 863.47875 | 863.47089 | 9.100 | 909.47644, 863.47089, 821.46143, 761.43646, 617.39813 |
| 52 | 6″-O-acetyl-SSm | 14.90 | C44H70O13 | 805.47327 | 805.46491 | 6.010 | 851.47363, 805.46491, 763.45136, 745.44421 |
| Number | Identification | VIP | p-Value | Change Trend after Processing |
|---|---|---|---|---|
| 1 | Diacetyl-SSb2 | 1.28867 | <0.001 | ↑ |
| 2 | SSb1 | 1.2881 | <0.001 | ↑ |
| 3 | Acetyl-SSc | 1.27938 | <0.001 | ↑ |
| 4 | 6″-O-acetyl-SSb1 | 1.27844 | <0.001 | ↑ |
| 5 | 2″-O-acetyl-SSD | 1.27832 | <0.001 | ↓ |
| 6 | 6″-O-acetyl-SSb2 | 1.27645 | <0.001 | ↓ |
| 7 | 4″-O-acetyl-SSA | 1.27386 | <0.001 | ↓ |
| 8 | Prosaikogenin H | 1.27353 | <0.001 | ↑ |
| 9 | SSm | 1.27284 | <0.001 | ↑ |
| 10 | 2″-O-acetyl-SSA | 1.26759 | <0.001 | ↓ |
| 11 | 3″-O-acetyl-SSA | 1.25863 | <0.001 | ↓ |
| 12 | 3″-O-acetyl-SSe | 1.25478 | <0.001 | ↑ |
| 13 | 4″-O-acetyl-SSb2 | 1.24614 | <0.001 | ↓ |
| 14 | SSb2 | 1.24421 | <0.001 | ↑ |
| 15 | SSA | 1.24241 | <0.001 | ↓ |
| 16 | SSD | 1.24104 | <0.001 | ↓ |
| 17 | 4″-O-acetyl-SSb1 | 1.24072 | <0.001 | ↑ |
| 18 | 6″-O-acetyl-SSA | 1.23792 | <0.001 | ↓ |
| 19 | 3″-O-acetyl-SSm | 1.23202 | <0.001 | ↑ |
| 20 | 6″-O-acetyl-SSe | 1.23135 | <0.001 | ↓ |
| 21 | 2″-O-acetyl-SSb1 | 1.22735 | <0.001 | ↑ |
| 22 | Prosaikogenin G | 1.22414 | <0.001 | ↓ |
| 23 | 3″-O-acetyl-SSb1 | 1.18866 | <0.001 | ↑ |
| 24 | Rutin | 1.16195 | <0.001 | ↓ |
| 25 | Diacetyl-SSA | 1.15538 | <0.001 | ↓ |
| 26 | 2″-O-acetyl-SSe | 1.05265 | 0.01 < p < 0.001 | ↓ |
| Ingredient | Initial Eigenvalue | Extract Square Sum Load | ||||
|---|---|---|---|---|---|---|
| Total | Variance Contribution (%) | Cumulative Variance Contribution (%) | Total | Variance Contribution (%) | Cumulative Variance Contribution (%) | |
| 1 | 10.529 | 75.205 | 75.205 | 10.529 | 75.205 | 75.205 |
| 2 | 1.135 | 8.104 | 83.309 | 1.135 | 8.104 | 83.309 |
| Group | Dosage (mg/kg) | Score of Principal Components Y1 | Score of Principal Components Y2 | Comprehensive Score | Number |
|---|---|---|---|---|---|
| Control | -- | −6.00 | 1.52 | −4.39 | 1 |
| Model | -- | 7.69 | 1.45 | 5.90 | 11 |
| Positive | 5.00 | −0.36 | 1.06 | −0.19 | 6 |
| SSA-L | 2.50 | 0.76 | −0.63 | 0.52 | 8 |
| SSA-H | 10.00 | −1.33 | −0.03 | −1.00 | 3 |
| SSb1-L | 2.50 | 0.97 | −0.50 | 0.69 | 9 |
| SSb1-H | 10.00 | −1.03 | 0.12 | −0.76 | 4 |
| SSb2-L | 2.50 | 0.29 | −2.13 | 0.05 | 7 |
| SSb2-H | 10.00 | −1.55 | −0.59 | −1.22 | 2 |
| SSD-L | 2.50 | 1.53 | −0.47 | 1.12 | 10 |
| SSD-H | 10.00 | −0.97 | 0.20 | −0.72 | 5 |
| Gene Name | Forward Primers (5′–3′) | Reverse Primers (5′–3′) |
|---|---|---|
| TNF-α | ATGTCTCAGCCTCTTCTCATTC | GCTTGTCACTCGAATTTTGAGA |
| IL-1β | TGGTGTGTGACGTTCCCATT | CAGCACGAGGGTTTTTTGTTG |
| IL-6 | CCGGAGAGGAGACTTCACAG | CAGAATTGCCATTGCACAAC |
| 18S | AGTTCCAGCACATTTTGCGAG | TCATCCTCCGTGAGTTCTCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, D.; Chen, Y.; Sun, Y.; Zhang, Z.; Cui, N.; Zhang, W.; Qi, Y.; Zeng, Y.; Hu, B.; Yang, B.; et al. Saikosaponin A and Its Epimers Alleviate LPS-Induced Acute Lung Injury in Mice. Molecules 2023, 28, 967. https://doi.org/10.3390/molecules28030967
Peng D, Chen Y, Sun Y, Zhang Z, Cui N, Zhang W, Qi Y, Zeng Y, Hu B, Yang B, et al. Saikosaponin A and Its Epimers Alleviate LPS-Induced Acute Lung Injury in Mice. Molecules. 2023; 28(3):967. https://doi.org/10.3390/molecules28030967
Chicago/Turabian StylePeng, Donghui, Yuchan Chen, Yanping Sun, Zhihong Zhang, Na Cui, Wensen Zhang, Ying Qi, Yuanning Zeng, Bin Hu, Bingyou Yang, and et al. 2023. "Saikosaponin A and Its Epimers Alleviate LPS-Induced Acute Lung Injury in Mice" Molecules 28, no. 3: 967. https://doi.org/10.3390/molecules28030967






