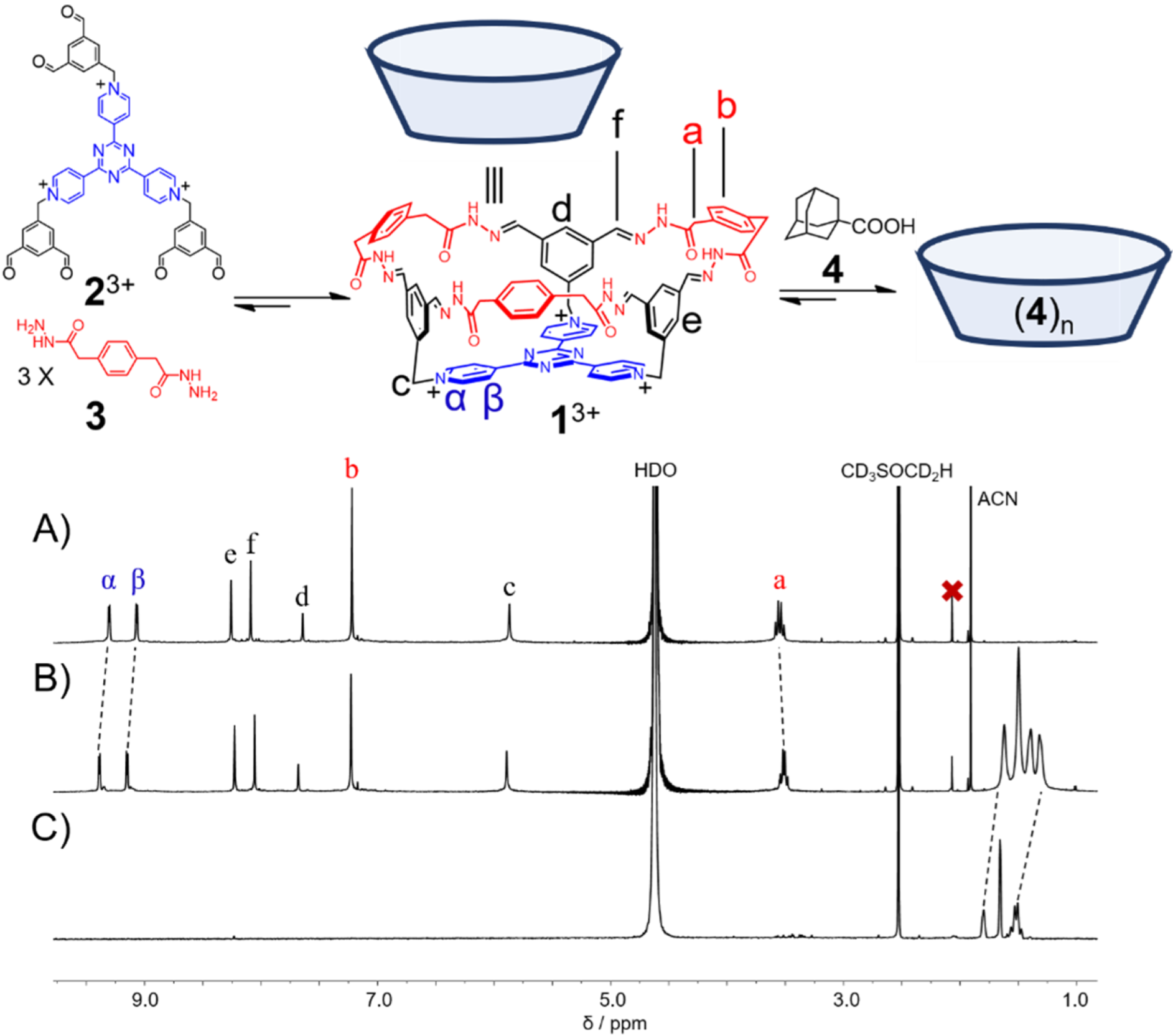
Abstract
A bowl-shaped molecule can be self-assembled by condensing a triscationic hexaaldehyde compound and three equiv. of a dihydrazide linkers in pure water. The molecular bowl is thus composed of a triscationic π-electron deficient platform, as well as a hexagonal rim that contains six acylhydrazone functions. When the counteranions are chloride, the solid-state structure reveals that this molecular bowl undergoes dimerization via N–H···Cl hydrogen bonds, forming a cage-like dimer with a huge inner cavity. This molecular bowl can employ its cavity to accommodate a hydrophobic guest, namely 1-adamantanecarboxylic acid in aqueous media.
1. Introduction
Synthesizing cyclic host molecules and using their pockets or cavities for guest recognition have attracted great attention in the community of host-guest chemistry [1,2]. These hosts are often in the form of rings [3,4], cages [5], and bowls. In the literature, even although a number of cyclic molecules including cyclodextrins(CDs), [6,7,8] calixrenes(CAs) [9], and resorcin[n]arenes [10,11,12,13,14,15], as well as a variety of metallocavitands [16,17,18,19,20,21,22,23,24,25], have been claimed as bowl-shaped hosts, in fact, they are topologically not different from rings, except that the “peripheral walls” of these bowls have two rims with different sizes. A veritable molecule bowl is supposed to contain a bottom platform, on which a macrocyclic peripheral wall is grafted [26]. This host is therefore able to take advantage of both the “platform” and the “peripheral wall” to provide noncovalent interactions to bind guests. Compounds that best fit the definition of a bowl-shape should be curved π-conjugated molecules such as buckybowls, namely corannulene, sumanene, etc. [27,28,29], in which the bottom and the edges of the bowl structure are seamlessly merged. The derivatives of such bowl-shaped molecules have shown moderate affinity towards fullerenes which takes advantage of shape complementarity as well as concave-convex π-π interaction [30,31,32]. The preparation and functionalization of this kind of bowl-shaped conjugated molecules, however, are time-consuming and suffered from low yields. In order to obtain host molecules containing purely organic elements (i.e., C, H, O and N) with decent yields without the need of tedious synthetic procedures, chemists also developed dynamic covalent approaches [33,34,35,36,37,38,39,40] relying on reversible organic reactions. For example, disulfide bond formation was employed by Otto et al. [41,42,43,44,45,46] to accomplish self-assembly in weakly basic aqueous media. Imine [47,48,49,50,51,52,53,54] formation, has been considered as one of more often used dynamic approaches, because its precursors, namely aldehydes and amines are relatively more synthetic accessible, compared to thiol derivatives in disulfide approaches. Unfortunately, this labile bond is apt to undergo hydrolysis in water and therefore not amenable to use in aqueous media. This intrinsic drawback could be overcome by using an α-substituted imine, namely acylhydrazone [55,56,57,58,59]. This more robust dynamic bond has been used in the self-assembly of rings [60,61,62,63,64,65], cages [66,67,68,69,70], catenanes [37,71,72] as well as knots [73]. We thus envision that it might be possible to obtain bowl via acylhydrazone condensation.
Herein, by condensing a triscationic hexaformyl precursor and a bishydrazide in water, a purely organic triscationic bowl was self-assembled in a [1 + 3] manner. In solid state, two bowl molecules form a dimer, driven by the hydrogen bonding interactions between the amide functions of the rim of each bowl and the chloride couteranions. The bowl is capable of accommodating a sparingly soluble guest, namely 1-adamantanecarboxylic acid in water.
2. Results and Discussion
A tricationic hexaformyl compound 23+·3Cl− (1.0 mM) and a bishydrazide 3 (3.0 mM) was combined in D2O at room temperature. After 4 h, the 1H NMR spectrum (Figure 1A) was recorded, in which a set of simple resonances was observed, indicating that a highly symmetrical product, namely a bowl-shaped molecule 13+·3Cl− was obtained in a [1 + 3] condensation manner. The resonance of the methylene unit a splits into two peaks, indicating that within the framework of 13+, the two protons in each of the methylene units become diastereotopic. The successful self-assembly of the bowl 13+ was further convinced by high resolution electrospray ionization mass spectrometry (HR-ESIMS). Two peaks were observed at m/z = 437.1750 and 673.2422, corresponding to the molecular cations of the bowl without and with one counteranion, namely [1]3+ and [1 + Cl]2+, respectively (Figure S7, Supplementary Materials). 13+·3Cl− was isolated in a 30% yield as a pure solid sample by means of counteranion exchange. However, the isolated pure 13+·3Cl− is only sparingly soluble in water, whose solubility may be improved by addition of DMSO.
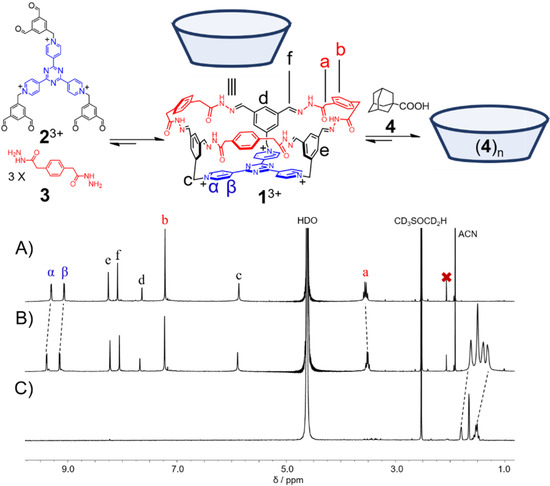
Figure 1.
Partial 1H NMR spectra (500 MHz, 298 K, D2O/DMSO-d6 = 9:1, pD = 3) of 13+·3Cl− (A) before and (B) after addition of guest 4, and (C) guest 4. In both (B,C), excess amount of 4 was suspended in solutions, guaranteeing that it is saturated.
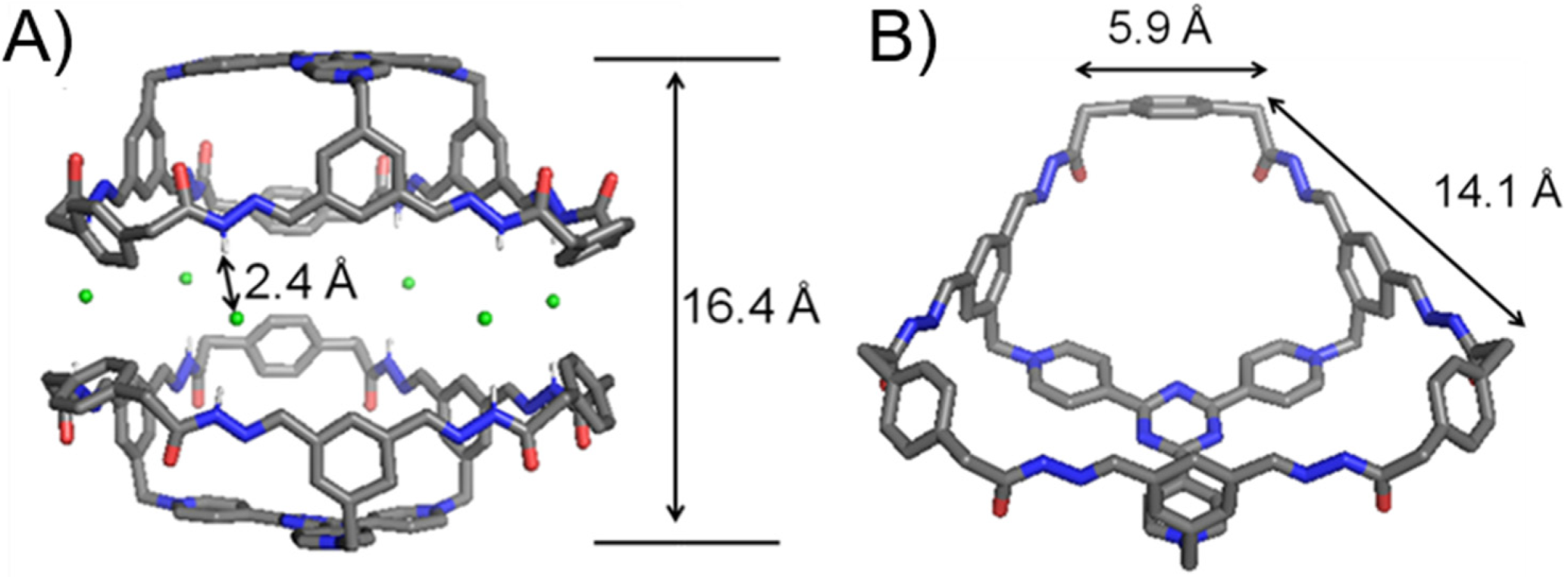
Single crystals of 13+·3Cl− were obtained by slowly diffusing dioxane into an aqueous solution of the self-assembled product. Single-crystal X-ray diffraction analysis unambiguously convinces the formation of the bowl-shaped host 13+ with a C3v symmetry (Figure 2). The plane defined by each of the three phenyl “walls” in the 23+ residue orientates in an almost perpendicular manner, with respect to the tri(4-pyridyl)triazinyl (TPT) “platform”. The three phenyl “walls” are bridged with each other by three 3 residues. The upper rim of the bowl thus forms a large hexagonal opening, in which the longer and shorter edges are 14.1 and 5.9 Å, respectively (Figure 2B). The bowl 13+ features three approximately pentagonal windows. Both the imine and amide protons point to a direction away from the bowl cavity, allowing them to form hydrogen bonds with the Cl− counteranions. Six Cl− counteranions insert into the space between the amide rims of the two bowl molecules. The occurrence of hydrogen bonds is convinced by the corresponding close contacts, i.e., Cl−Hamide distances are around 2.4 Å. Driven by hydrogen bonds, two bowl molecules thus form a triangular prismatic bowl dimer with a D3h symmetry. The two TPT platforms are separated by a distance of 16.4 Å. The volume of cavity of a bowl dimer is estimated to around 2000 Å3 (regard the cavity as a hexagonal prism approximately).
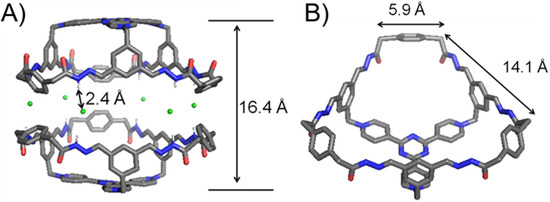
Figure 2.
The (A) side on view and (B) top view of the single-crystal X-ray diffraction structures of 13+·3Cl−. C = grey, H = white, O = red, N = blue, Cl = green. Disordered solvent molecules are omitted for the sake of clarity. Only amide protons are shown because they are engaged in strong hydrogen bonding interactions.
The capability of 13+ to accommodate guests in water was then investigated. Upon addition of a guest 4, namely 1-adamantanecarboxylic acid, the resonances of the bowl 13+ recorded in aqueous DMSO (D2O/DMSO-d6 = 9:1) underwent modest shifts (Figure 1B). NOESY cross peaks (Figure S12, Supplementary Materials) between proton signals of 13+ and 4 unambiguously shows their complexation in solution. Only one set of resonances were observed corresponding to both the bowl and the guest, indicating that the host-guest complex undergoes relatively fast exchange with the their “free” states on the 1H NMR timescale. Since guest 4 (pKa = 4.9) is sensitive to pH changes, we also conducted the titration experiments in acidic (pD = 3) and basic conditions (pD = 9). In pD = 3 aqueous DMSO solution, the changes in the chemical shifts of the bowl 13+ were similar to that of the changes recorded in non-buffered aqueous DMSO solution. For example, the resonances of the pyridinium protons in the TPT base of 13+ were observed to undergo downfield shifts by around 0.1 ppm. In pD = 9 aqueous DMSO solution, however, the chemical shifts of the host remained unchanged upon the titration of 4, and 13+ started to precipitate out when more than 1.5 equiv. of 4 was introduced, as the signals of 13+ gradually decreased during titration. The resonances of the guests underwent upfield shifts in both cases, indicating that the guests were encapsulated within the bowl cavity which provided a shielded magnetic environment (Figure 1B). We envisioned that in basic conditions where 4 exists in its deprotonated form, binding of the first guest is thermodynamically favored due to hydrophobic interaction as well as Coulombic attraction between host and guest. Nevertheless, binding of the second guest is inhibited due to repulsion between negatively charged guest molecules. The mismatch between the host cavity (~1000 Å3) and the deprotonated guest volume (172 Å3) [74] results in a very weak binding, which explains the insignificant change in the resonance of the bowl 13+ in basic conditions. In acidic or unbuffered aqueous solutions where the majority of 4 exists in its neutral form, binding of more than one guest molecule is possible. Due to the insufficient solubility of both host and guest as well as their weak association constant, the attempts to accurately determine the guest/host binding stoichiometry by using Job plot were unsuccessful [75]. However, based on the C3v symmetry of the host cavity and Rebek’s 55% rule [76], we assumed that the bowl-shaped cavity of 13+ can accommodate up to three molecules of guest 4, and such “fully” filled complexation can lead to the observable shifts of the proton signals of the molecular bowl upon addition of 4.
3. Materials and Methods
All reagents and solvents were purchased from commercial sources and used without further purification. Manipulations were performed under a normal laboratory atmosphere unless otherwise noted. Nuclear magnetic resonance (NMR) spectra were recorded at ambient temperature using Bruker AVANCE III 400/500 or Agilent DD2 600 spectrometers, with working frequencies of 400/500/600 and 100/125/150 MHz for 1H and 13C, respectively. Chemical shifts are reported in ppm relative to the residual internal non-deuterated solvent signals (CDCl3: δH = 7.26 ppm, δC = 77.16 ppm, D2O: δH = 4.79 ppm, DMSO-d6: δH = 2.50 ppm, δC = 39.52 ppm). High-resolution mass spectra (HRMS) were measured using a SHIMADZU liquid chromatograph mass spectrometry ion trap time of flight (LCMS-IT-TOF) instrument. X-Ray crystallographic data were collected on a Bruker APEX-II CCD diffractometer.
4. Conclusions
In summary, a bowl-shaped molecule was successfully self-assembled by condensing a tricationic hexaaldehyde and three bishydrazide linker in water. The molecular bowl is composed of a planar triscationic base, on which a triangular rim containing six acylhydrazone functions is grafted. Taking advantage of hydrophobic effect, the cavity of this bowl is able to accommodate a hydrophobic guest in water. Using this bowl-shaped host as a molecular vessel to encapsulate substrates and catalyze their reactions are ongoing in our lab.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28030976/s1. Scheme S1: Synthetic route of 23+·3Cl−; Figure S1: 1H NMR spectrum of 23+·3Cl−; Figure S2–S7: NMR characterizations of 13+·3Cl−; Figure S8: ESI-HRMS of 13+·3Cl−.; Figure S9: 1H NMR spectra of self-assembled products at different precursors concentrations; Figure S10–S11: 1H NMR titration of 13+·3Cl− and 4 in non-buffered solution; Figure S12: NOESY spectrum of the host-guest complex; Figure S13–S16: 1H NMR titration of 13+·3Cl− and 4 in buffer solutions; Figure S17: Different crystallographic views of 13+·3Cl− [77,78,79].
Author Contributions
Synthesis, G.W. and T.J.; compound characterization, G.W.; single crystal characterization, T.J.; writing and revision, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21922108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cram, D.J.; Cram, J.M. Host-Guest Chemistry: Complexes between organic compounds simulate the substrate selectivity of enzymes. Science 1974, 183, 803–809. [Google Scholar] [CrossRef]
- Cram, D.J. The Design of Molecular Hosts, Guests, and Their Complexes (Nobel Lecture). Angew. Chem. Int. Ed. 1988, 27, 1009–1112. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Cram, D.J.; Tanner, M.E.; Thomas, R. The Taming of Cyclobutadiene. Angew. Chem. Int. Ed. 1991, 30, 1024–1027. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef]
- Breslow, R.; Dong, S. Biomimetic Reactions Catalyzed by Cyclodextrins and Their Derivatives. Chem. Rev. 1998, 98, 1997–2011. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Gutsch, C.D. Calixarenes. Acc. Chem. Res. 1983, 16, 161–170. [Google Scholar] [CrossRef]
- Konishi, H.; Ohata, K.; Morikawa, O.; Kobayash, K. Calix[6]resorcinarenes: The first examples of [16]metacyclophanes derived from resorcinols. J. Chem. Soc. Chem. Commun. 1995, 3, 309–310. [Google Scholar] [CrossRef]
- Aoyama, Y.; Tanaka, Y.; Toi, H.; Ogoshi, H. Polar host-guest interaction. Binding of nonionic polar compounds with a resorcinol-aldehyde cyclooligomer as a lipophilic polar host. J. Am. Chem. Soc. 1988, 110, 634–635. [Google Scholar] [CrossRef]
- Ballester, P.; Shivanyuk, A.; Far, A.R.; Rebek, J., Jr. A Synthetic Receptor for Choline and Carnitine. J. Am. Chem. Soc. 2002, 124, 14014–14016. [Google Scholar] [CrossRef] [PubMed]
- Gissot, A.; Rebek, J., Jr. A Functionalized, Deep Cavitand Catalyzes the Aminolysis of a Choline Derivative. J. Am. Chem. Soc. 2004, 126, 7424–7425. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Gibb, C.L.D.; Stevens, E.D.; Gibb, B.C. Deep-cavity cavitands: Synthesis and solid state structure of host molecules possessing large bowl-shaped cavities. Chem. Commun. 1998, 34, 1743–1744. [Google Scholar] [CrossRef]
- Jordan, J.H.; Gibb, B.C. Molecular containers assembled through the hydrophobic effect. Chem. Soc. Rev. 2015, 44, 547–585. [Google Scholar] [CrossRef]
- Lippert, B.; Miguel, P.J.S. Metallatriangles and metallasquares: The diversity behind structurally characterized examples and the crucial role of ligand symmetry. Chem. Soc. Rev. 2011, 40, 4475–4487. [Google Scholar] [CrossRef]
- Frischmann, P.D.; MacLachlan, M.J. Metallocavitands: An emerging class of functional multimetallic host molecules. Chem. Soc. Rev. 2013, 42, 871–890. [Google Scholar] [CrossRef]
- Kulesza, J.; Barrosb, B.S.; Júnior, S.A. Organic–inorganic hybrid materials: Metallacalixarenes. Synthesis and applications. Coord. Chem. Rev. 2013, 257, 2192–2212. [Google Scholar] [CrossRef]
- Thanasekaran, P.; Lee, C.-C.; Lu, K.-L. One-Step Orthogonal-Bonding Approach to the Self-Assembly of Neutral Rhenium-Based Metallacycles: Synthesis, Structures, Photophysics, and Sensing Applications. Acc. Chem. Res. 2012, 45, 1403–1418. [Google Scholar] [CrossRef]
- Severin, K. Supramolecular chemistry with organometallic half-sandwich complexes. Chem. Commun. 2006, 42, 3859–3867. [Google Scholar] [CrossRef]
- Sathiyendiran, M.; Tsai, C.C.; Thanasekaran, P.; Luo, T.T.; Yang, C.I.; Lee, G.H.; Peng, S.M.; Lu, K.L. Organometallic Calixarenes: Syceelike Tetrarhenium(I) Cavitands with Tunable Size, Color, Functionality, and Coin–Slot Complexation. Chem. Eur. J. 2011, 17, 3343–3346. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Yu, S.-Y.; Kusukawa, T.; Funaki, H.; Ogura, K.; Yamaguchi, K. Self-Assembly of Nanometer-Sized Macrotricyclic Complexes from Ten Small Component Molecules. Angew. Chem. Int. Ed. 1998, 37, 2082–2085. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Kusukawa, T.; Biradha, K.; Fujita, M. Hydrophobic Assembling of a Coordination Nanobowl into a Dimeric Capsule Which Can Accommodate up to Six Large Organic Molecules. J. Am. Chem. Soc. 2000, 122, 2665–2666. [Google Scholar] [CrossRef]
- Tashiro, S.; Tominaga, M.; Yamaguchi, Y.; Kato, K.; Fujita, M. Folding a De Novo Designed Peptide into an a Helix through Hydrophobic Binding by a Bowl Shaped Host. Angew. Chem. Int. Ed. 2005, 45, 241–244. [Google Scholar] [CrossRef]
- Tashiro, S.; Tominaga, M.; Yamaguchi, Y.; Kato, K.; Fujita, M. Peptide Recognition: Encapsulation and α-Helical Folding of a Nine-Residue Peptide within a Hydrophobic Dimeric Capsule of a Bowl-Shaped Host. Chem. Eur. J. 2006, 12, 3211–3217. [Google Scholar] [CrossRef]
- Barrett, E.S.; Irwin, J.L.; Edwards, A.J.; Sherburn, M.S. Superbowl Container Molecules. J. Am. Chem. Soc. 2004, 126, 16747–16749. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Shinokubo, H.; Sakurai, H. Figuration of bowl-shaped π-conjugated molecules: Properties and functions. Mater. Chem. Front. 2018, 2, 635–661. [Google Scholar] [CrossRef]
- Krzeszewski, M.; Dobrzycki, L.; Sobolewski, A.L.; Cyranski, M.K.; Gryko, D.T. Bowl-Shaped Pentagon- and Heptagon-Embedded Nanographene Containing a Central Pyrrolo[3,2-b]pyrrole Core. Angew. Chem. Int. Ed. 2021, 60, 14998–15005. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, G. Periphery-Core Strategy to Access a Bowl-Shaped Molecule Bearing Multiple Heteroatoms. Angew. Chem. Int. Ed. 2022, 61, e202208061. [Google Scholar]
- Sygula, A.; Fronczek, F.R.; Sygula, R.; Rabideau, P.W.; Olmstead, M.M. A Double Concave Hydrocarbon Buckycatcher. J. Am. Chem. Soc. 2007, 129, 3842–3843. [Google Scholar] [CrossRef] [PubMed]
- Le, V.H.; Yanney, M.; McGuire, M.; Sygula, A.; Lewis, E.A. Thermodynamics of Host–Guest Interactions between Fullerenes and a Buckycatcher. J. Phys. Chem. B 2014, 118, 11956–11964. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.M.; Garcia-Escudero, L.A.; Garcia-Rodriguez, R.; Martin-Alvarez, J.M.; Miguel, D.; Rayon, V.M. Enhanced association for C70 over C60 with a metal complex with corannulene derivate ligands. Dalton Trans. 2014, 43, 15693–15696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Presly, O.; White, F.; Oppel, I.M.; Mastalerz, M. A Permanent Mesoporous Organic Cage with an Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2014, 53, 1516–1520. [Google Scholar] [CrossRef]
- Klotzbach, S.; Beuerle, F. Shape-Controlled Synthesis and Self-Sorting of Covalent Organic Cage Compounds. Angew. Chem. Int. Ed. 2015, 54, 10356–10360. [Google Scholar] [CrossRef]
- Au-Yeung, H.Y.; Pantos, G.D.; Sanders, J.K.M. Dynamic combinatorial synthesis of a catenane based on donor–acceptor interactions in water. Proc. Natl. Acad. Sci. USA 2009, 106, 10466–10470. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Vysotsky, M.O.; Bogdan, A.; Bolte, M.; Bo, V. Multiple Catenanes Derived from Calix[4]arenes. Science 2004, 304, 1312–1314. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Lammer, A.D.; Wang, M.; Li, X.; Lynch, V.M.; Sessler, J.L. Quantitative self-assembly of a purely organic three-dimensional catenane in water. Nat. Chem. 2015, 7, 1003–1008. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Long, H.; Du, Y.; Jin, Y.; Zhang, W. Solution-Phase Dynamic Assembly of Permanently Interlocked Aryleneethynylene Cages through Alkyne Metathesis. Angew. Chem. Int. Ed. 2015, 54, 7550–7554. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Zhang, C.; Long, H.; Azarnoush, S.; Jin, Y.; Zhang, W. Dynamic covalent synthesis of aryleneethynylene cages through alkyne metathesis: Dimer, tetramer, or interlocked complex? Chem. Sci. 2016, 7, 3370–3376. [Google Scholar] [CrossRef]
- Lee, S.; Yang, A.; Moneypenny, T.P.; Moore, J.S. Kinetically Trapped Tetrahedral Cages via Alkyne Metathesis. J. Am. Chem. Soc. 2016, 138, 2182–2185. [Google Scholar] [CrossRef]
- Li, J.; Carnall, J.M.; Stuart, M.C.; Otto, S. Hydrogel Formation upon Photoinduced Covalent Capture of Macrocycle Stacks from Dynamic Combinatorial Libraries. Angew. Chem. Int. Ed. 2011, 50, 8384–8386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nowak, P.; Otto, S. An Allosteric Receptor by Simultaneous “Casting” and “Molding” in a Dynamic Combinatorial Library. Angew. Chem. Int. Ed. 2015, 54, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nowak, P.; Fanlo-Virgós, H.; Otto, S. Catenanes from catenanes: Quantitative assessment of cooperativity in dynamic combinatorial catenation. Chem. Sci. 2014, 5, 4968–4974. [Google Scholar] [CrossRef]
- Li, J.; Cvrtila, I.; Colomb-Delsuc, M.; Otten, E.; Otto, S. An “Ingredients” Approach to Functional Self-Synthesizing Materials: A Metal-Ion-Selective, Multi-Responsive, Self-Assembled Hydrogel. Chem. Eur. J. 2014, 20, 15709–15714. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nowak, P.; Otto, S. Dynamic Combinatorial Libraries: From Exploring Molecular Recognition to Systems Chemistry. J. Am. Chem. Soc. 2013, 135, 9222–9239. [Google Scholar] [CrossRef]
- Nowak, P.; Colomb-Delsuc, M.; Otto, S.; Li, J. Template-Triggered Emergence of a Self-Replicator from a Dynamic Combinatorial Library. J. Am. Chem. Soc. 2015, 137, 10965–10969. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic Imine Chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Quan, M.L.C.; Cram, D.J. Constrictive binding of large guests by a hemicarcerand containing four portals. J. Am. Chem. Soc. 1991, 113, 2754–2755. [Google Scholar] [CrossRef]
- Berl, V.; Huc, I.; Lehn, J.-M.; DeCian, A.; Fischer, J. Induced Fit Selection of a Barbiturate Receptor from a Dynamic Structural and Conformational/Configurational Library. Eur. J. Org. Chem. 1999, 1999, 3089–3094. [Google Scholar] [CrossRef]
- Godoy-Alcántar, C.; Yatsimirsky, A.K.; Lehn, J.M. Structure-stability correlations for imine formation in aqueous solution. J. Phys. Org. Chem. 2005, 18, 979–985. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Li, G.; Warmuth, R. One-Pot, 18-Component Synthesis of an Octahedral Nanocontainer Molecule. Angew. Chem. Int. Ed. 2006, 45, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rue, N.M.; Sun, J.; Warmuth, R. Polyimine Container Molecules and Nanocapsules. Israel J. Chem. 2011, 51, 743–768. [Google Scholar] [CrossRef]
- Jiao, T.; Chen, L.; Yang, D.; Li, X.; Wu, G.; Zeng, P.; Zhou, A.; Yin, Q.; Pan, Y.; Wu, B.; et al. Trapping White Phosphorus within a Purely Organic Molecular Container Produced by Imine Condensation. Angew. Chem. Int. Ed. 2017, 56, 14545–14550. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, S.; Yu, C.; Li, Z.Y.; Xu, J.; Li, Z.M.; Zou, L.; Leng, X.; Gao, S.; Liu, Z.; et al. De Novo Construction of Catenanes with Dissymmetric Cages by Space-Discriminative Post-Assembly Modification. Angew. Chem. Int. Ed. 2020, 59, 7113–7121. [Google Scholar] [CrossRef] [PubMed]
- Cousins, G.R.L.; Poulsen, S.-A.; Sanders, J.K.M. Dynamic combinatorial libraries of pseudo-peptide hydrazone macrocycles. Chem. Commun. 1999, 35, 1575–1576. [Google Scholar] [CrossRef]
- Furlan, R.L.E.; Ng, Y.-F.; Otto, S.; Sanders, J.K.M. A New Cyclic Pseudopeptide Receptor for Li+ from a Dynamic Combinatorial Library. J. Am. Chem. Soc. 2001, 123, 8876–8877. [Google Scholar] [CrossRef]
- Roberts, S.L.; Furlan, R.L.; Cousins, G.R.; Sanders, J.K. Simultaneous selection, amplification and isolation of a pseudo-peptide receptor by an immobilised N-methyl ammonium ion template. Chem. Commun. 2002, 38, 938–939. [Google Scholar] [CrossRef]
- Nguyen, R.; Huc, I. Optimizing the reversibility of hydrazone formation for dynamic combinatorial chemistry. Chem. Commun. 2003, 39, 942–943. [Google Scholar] [CrossRef]
- Ramstrom, O.; Lohmann, S.; Bunyapaiboonsri, T.; Lehn, J.-M. Dynamic Combinatorial Carbohydrate Libraries: Probing the Binding Site of the Concanavalin A Lectin. Chem. Eur. J. 2004, 10, 1711–1715. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.Y.; Jiao, T.; Zhu, H.; Huang, F.; Li, H. Controllable Self-Assembly of Macrocycles in Water for Isolating Aromatic Hydrocarbon Isomers. J. Am. Chem. Soc. 2018, 140, 5955–5961. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Cao, N.; Yang, C.; Li, H. A Kinetically Stable Macrocycle Self-Assembled in Water. Org. Lett. 2018, 20, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gómez, A.; Fernández-Blanco, Á.; Blanco, V.; Rodríguez, J.; Peinador, C.; García, M.D. Thinking Outside the “Blue Box”: Induced Fit within a Unique Self-Assembled Polycationic Cyclophane. J. Am. Chem. Soc. 2019, 141, 3959–3964. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gomez, A.; Neira, I.; Barriada, J.L.; Melle-Franco, M.; Peinador, C.; Garcia, M.D. Thinking outside the “Blue Box”: From molecular to supramolecular pH-responsiveness. Chem. Sci. 2019, 10, 10680–10686. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, L.; He, C.; Zheng, S.; Reek, J.N.H.; Duan, C. Metal−Organic Capsules with NADH Mimics as Switchable Selectivity Regulators for Photocatalytic Transfer Hydrogenation. J. Am. Chem. Soc. 2019, 141, 12707–12716. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.S.; Li, X.Z.; Hu, S.J.; Yan, D.N.; Zhou, L.P.; Sun, Q.F. Base- and Metal-Dependent Self-Assembly of Lathanide-Organic Coordination Polymers or Macrocycles with Tetradentate Acylhydrazone-based Ditopic Ligands. Chem. Asian J. 2021, 16, 1392–1397. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Yang, Y.; Duan, C. A Metal−Organic Tetrahedron as a Redox Vehicle to Encapsulate Organic Dyes for Photocatalytic Proton Reduction. J. Am. Chem. Soc. 2015, 137, 3967–3974. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Wu, G.; Liu, J.R.; Cao, N.; Wang, L.; Wang, Y.; Li, X.; Hong, X.; Yang, C.; et al. Temperature-dependent self-assembly of a purely organic cage in water. Chem. Commun. 2018, 54, 3138–3141. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Liu, H.K.; Wang, Z.K.; Song, B.; Zhang, D.W.; Wang, H.; Li, Z.; Li, X.; Li, Z.T. Olive-Shaped Organic Cages: Synthesis and Remarkable Promotion of Hydrazone Condensation through Encapsulation in Water. J. Org. Chem. 2021, 86, 3943–3951. [Google Scholar] [CrossRef]
- Wu, G.; Chen, Y.; Fang, S.; Tong, L.; Shen, L.; Ge, C.; Pan, Y.; Shi, X.; Li, H. A Self-Assembled Cage for Wide-Scope Chiral Recognition in Water. Angew. Chem. Int. Ed. 2021, 60, 16594–16599. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, F.; El-Sayed, M.E.-S.; Wang, W.; Du, S.; Su, K.; Yuan, D. Water-stable hydrazone-linked porous organic cages. Chem. Sci. 2021, 12, 13307–13315. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, G.; Jiao, T.; Shen, L.; Ma, G.; Pan, Y.; Li, H. Precursor control over the self-assembly of [2]catenanes via hydrazone condensation in water. Chem. Commun. 2018, 54, 5106–5109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, L.; Wang, C.-Y.; Jiao, T.; Pan, Y.; Li, H. Ultramacrocyclization via selective catenation in water. Chem. Commun. 2019, 55, 13108–13111. [Google Scholar] [CrossRef]
- Cougnon, F.B.L.; Caprice, K.; Pupier, M.; Bauza, A.; Frontera, A. A Strategy to Synthesize Molecular Knots and Links Using the Hydrophobic Effect. J. Am. Chem. Soc. 2018, 140, 12442–12450. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Cho, Y.; Lee, M.; Laskowski, R.A.; Ryu, S.E.; Sugihara, K.; Kim, D.S. BetaCavityWeb: A webserver for molecular voids and channels. Nucleic Acids Res. 2015, 43, W413–W418. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, S.; Rebek, J., Jr. The 55% Solution: A Formula for Molecular Recognition in the Liquid State. Chem. Eur. J. 1998, 4, 1016–1022. [Google Scholar] [CrossRef]
- Newkome, G.R.; Cho, T.J.; Moorefield, C.N.; Cush, R.; Russo, P.S.; GodÌnez, L.A.; Saunders, M.J.; Mohapatra, P. Hexagonal Terpyridine–Ruthenium and –Iron Macrocyclic Complexes by Stepwise and Self-Assembly Procedures. Chem. Eur. J. 2002, 8, 2946–2954. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta. Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).