Characterization of Biological Properties of Individual Phenolamides and Phenolamide-Enriched Leaf Tomato Extracts
Abstract
1. Introduction
2. Results
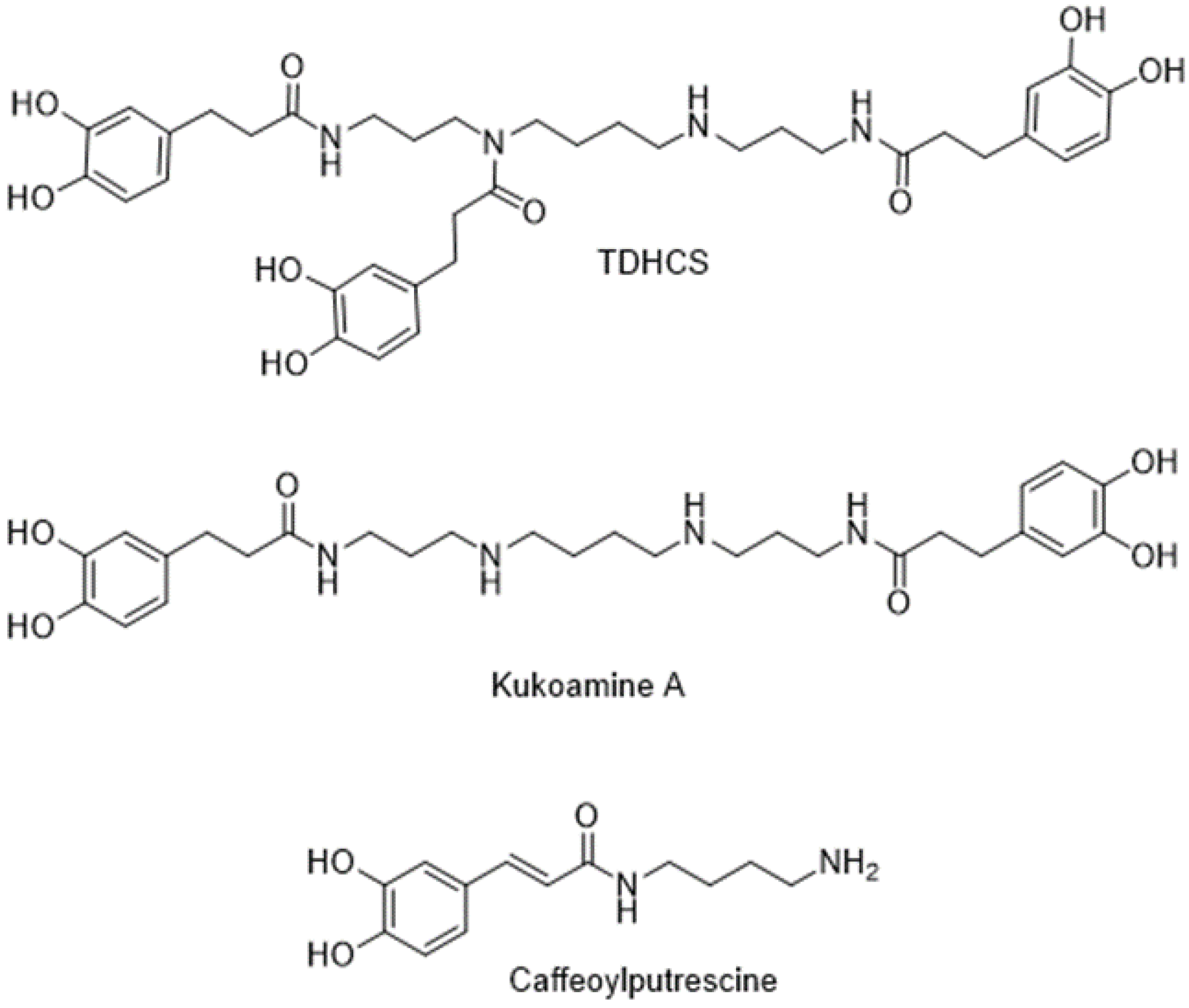
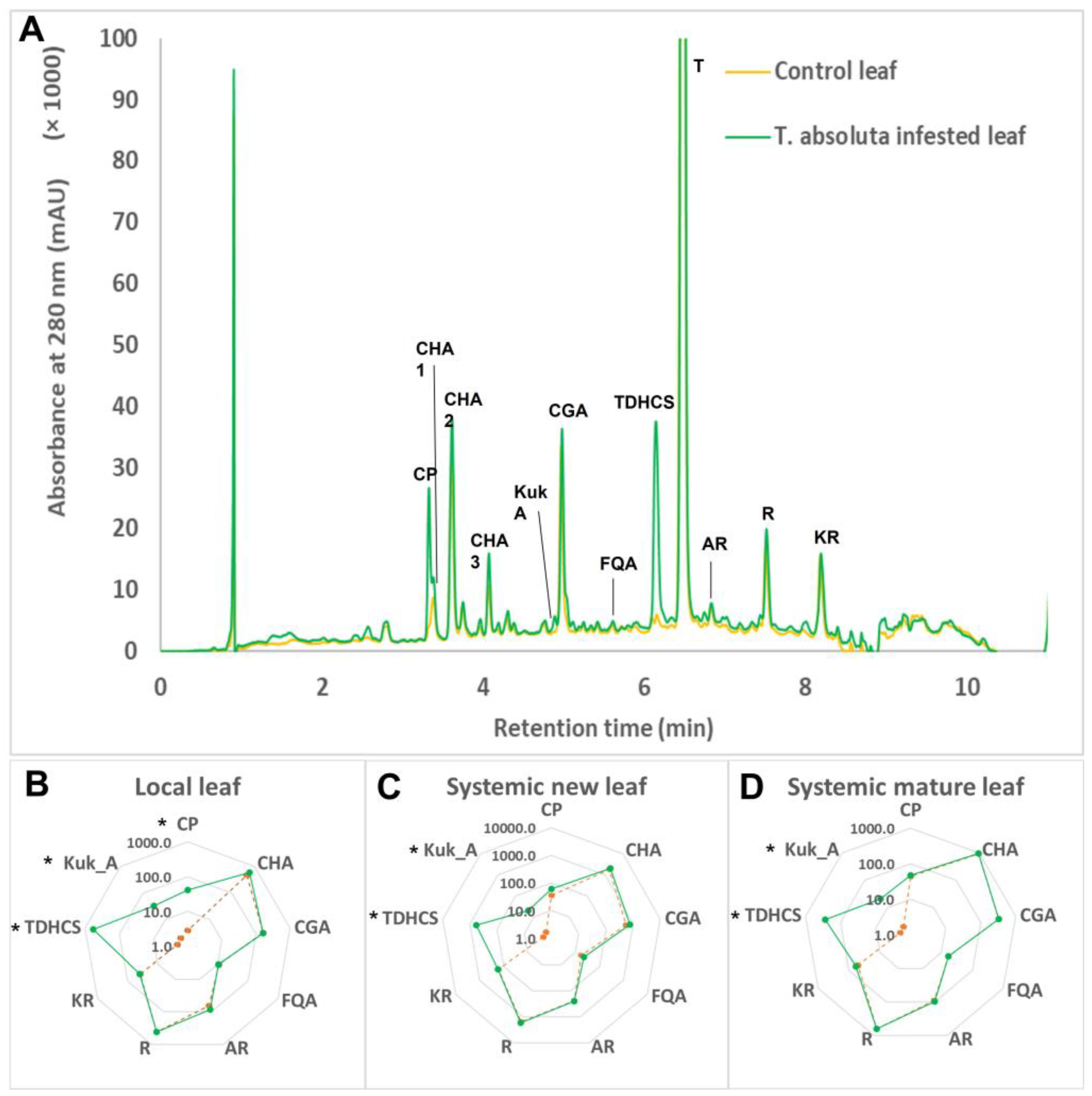
2.1. Phenolic Composition of Tomato Leaves under T. absoluta Herbivory
2.2. Innovative Synthesis of N1,N5,N14-tris(dihydrocaffeoyl)spermine (TDHCS)
2.3. Antibacterial Activity of the Three Phenolamides
2.4. Anticancer Activity of TDHCS on MCF-7 and Caco-2 Cells
2.5. Cytotoxicity and Anti-Inflammatory Activity of Three Phenolamides
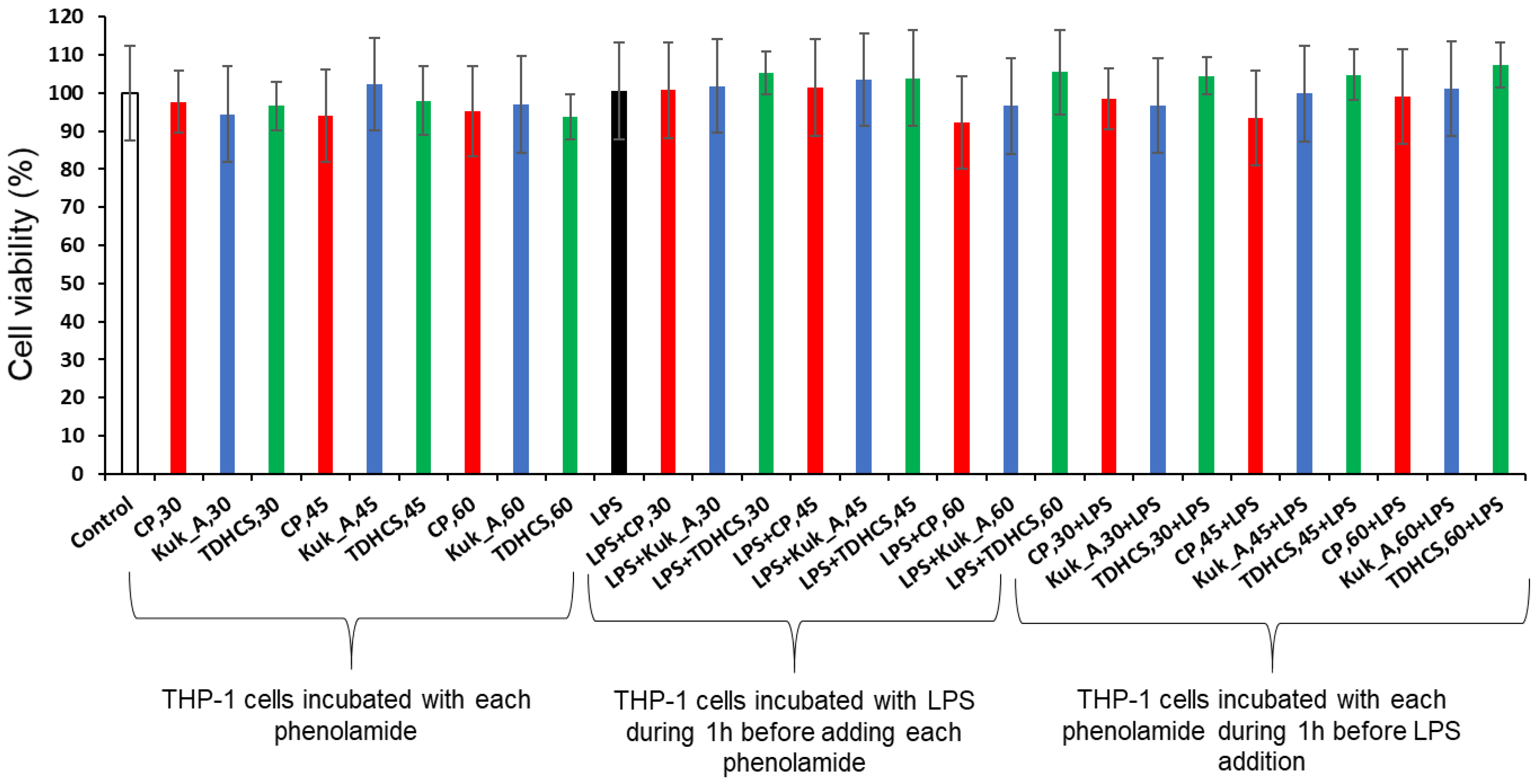
2.5.1. Cytotoxicity of the Three Phenolamides on Differentiated THP-1 Cells
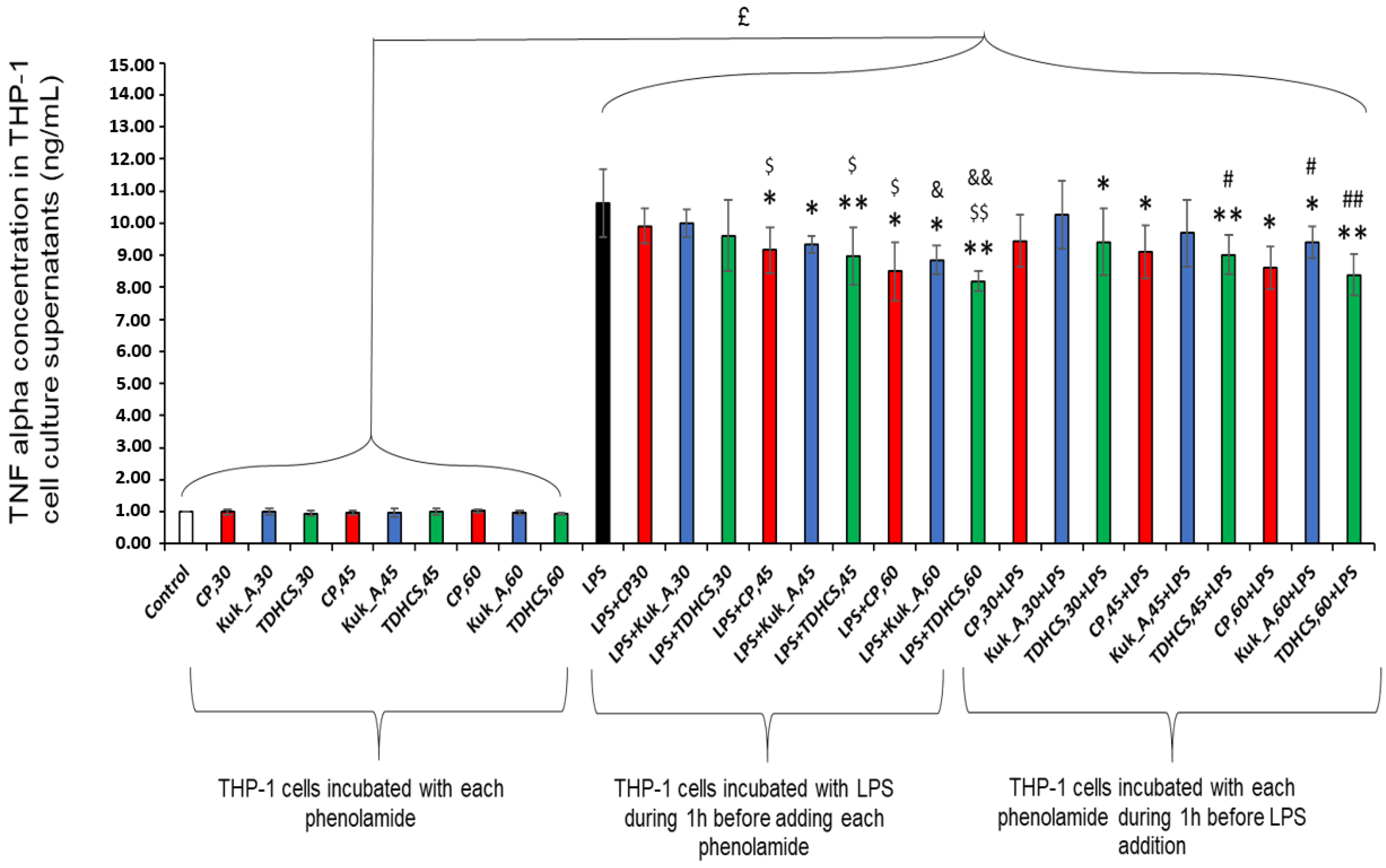
2.5.2. Anti-Inflammatory Activity of the Three Phenolamides on Differentiated THP-1 Cells
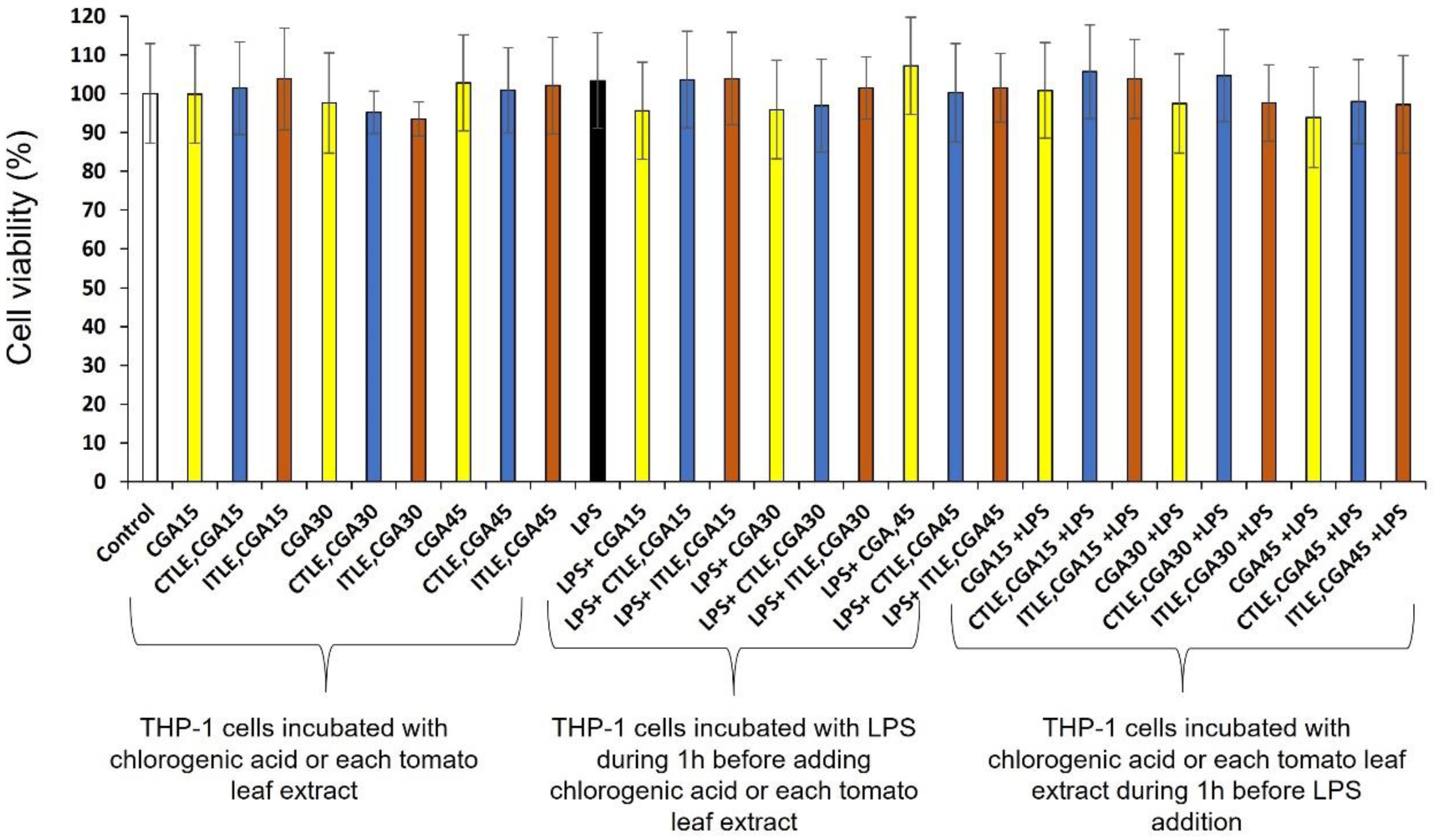
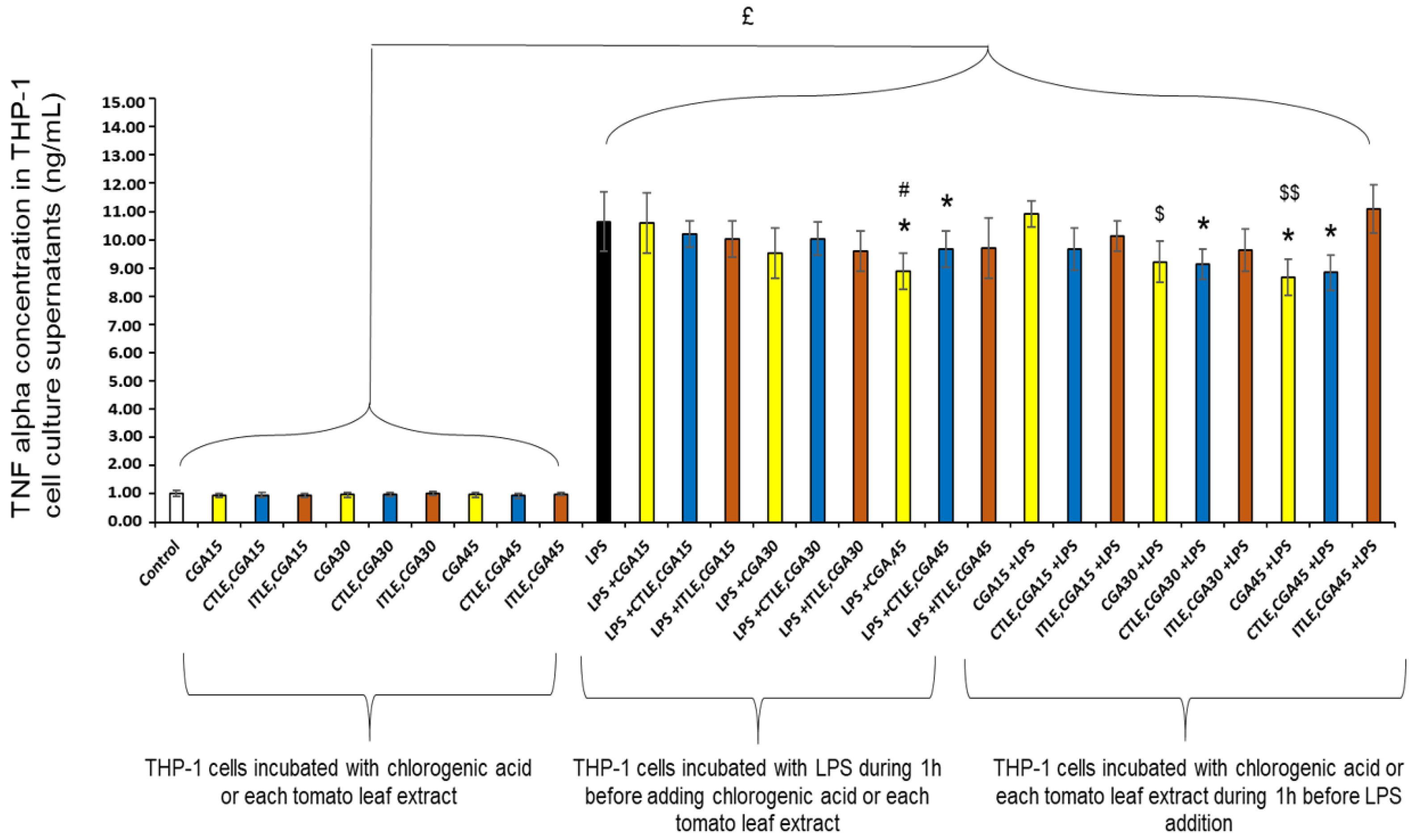
2.6. Cytotoxicity and Anti-Inflammatory Activity of Tomato Leaf Extracts on Differentiated THP-1 Cells
2.6.1. Cytotoxicity of Tomato Leaf Extracts on Differentiated THP-1 Cells
2.6.2. Anti-Inflammatory Activity of Tomato Leaf Extracts on Differentiated THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Obtention and Growth Conditions
4.2. Metabolite Extraction from Tomato Leaves
4.3. Phenolamides
4.3.1. General Information
4.3.2. Synthesis of N1,N5,N14-tris(dihydrocaffeoyl)spermine
4.4. Analysis of Phenolamides Content by UPLC Analysis
4.5. Antibacterial Activity
4.5.1. Bacteria Strains and Culture Conditions
4.5.2. MIC Determination
4.6. Anticancer Activity
4.6.1. Cell Culture and Treatments
4.6.2. Cell Proliferation Assay
4.7. Anti-Inflammatory Activity
4.7.1. Cell Culture and Treatments of THP-1 Cells
4.7.2. THP-1 Cell Viability Measurement
4.7.3. TNF-α Quantification from THP-1 Cell Culture Supernatants
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is Early-Onset Cancer an Emerging Global Epidemic? Current Evidence and Future Implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate Change Increases Cross-Species Viral Transmission Risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S. Special Issue: Emerging Wildlife Viral Diseases. Viruses 2022, 14, 807. [Google Scholar] [CrossRef]
- Rafei, A.; Elliott, M.R.; Jones, R.E.; Riosmena, F.; Cunningham, S.A.; Mehta, N.K. Obesity Incidence in U.S. Children and Young Adults: A Pooled Analysis. Am. J. Prev. Med. 2022, 63, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-First Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Grant, T.L.; Wood, R.A. The Influence of Urban Exposures and Residence on Childhood Asthma. Pediatr. Allergy Immunol. 2022, 33, e13784. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol. Front. Oncol. 2021, 11, 617937. [Google Scholar] [CrossRef]
- Scheijen, B. Molecular Mechanisms Contributing to Glucocorticoid Resistance in Lymphoid Malignancies. Cancer Drug Resist. 2019, 2, 647–664. [Google Scholar] [CrossRef]
- De Iudicibus, S.; Franca, R.; Martelossi, S.; Ventura, A.; Decorti, G. Molecular Mechanism of Glucocorticoid Resistance in Inflammatory Bowel Disease. World J. Gastroenterol. 2011, 17, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Bombaywala, S.; Mandpe, A.; Paliya, S.; Kumar, S. Antibiotic Resistance in the Environment: A Critical Insight on Its Occurrence, Fate, and Eco-Toxicity. Environ. Sci. Pollut. Res. Int. 2021, 28, 24889–24916. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic Resistance Increases with Local Temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Home Page-Medicinal Plant Names Services. Available online: https://mpns.science.kew.org/ (accessed on 14 December 2022).
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Behl, T.; Mehta, K.; Sehgal, A.; Singh, S.; Sharma, N.; Ahmadi, A.; Arora, S.; Bungau, S. Exploring the Role of Polyphenols in Rheumatoid Arthritis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5372–5393. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-Cancer Effects of Dietary Polyphenols via ROS-Mediated Pathway with Their Modulation of MicroRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Iqbal, M.O.; Javaid, U.; Tareen, M.B.K.; Amna, D.; Ramzan, A.; Piracha, S.; Naeem, M. Inhibitory Effect of Polyphenols (Phenolic Acids, Lignans, and Stilbenes) on Cancer by Regulating Signal Transduction Pathways: A Review. Clin. Transl. Oncol. 2022, 24, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Roumani, M.; Le Bot, J.; Boisbrun, M.; Magot, F.; Péré, A.; Robin, C.; Hilliou, F.; Larbat, R. Transcriptomics and Metabolomics Analyses Reveal High Induction of the Phenolamide Pathway in Tomato Plants Attacked by the Leafminer Tuta Absoluta. Metabolites 2022, 12, 484. [Google Scholar] [CrossRef]
- Roumani, M.; Besseau, S.; Gagneul, D.; Robin, C.; Larbat, R. Phenolamides in Plants: An Update on Their Function, Regulation, and Origin of Their Biosynthetic Enzymes. J. Exp. Bot. 2021, 72, 2334–2355. [Google Scholar] [CrossRef] [PubMed]
- Bassard, J.-E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging Polyamines to the Phenolic Metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef]
- Liu, H.; Ma, S.; Xia, H.; Lou, H.; Zhu, F.; Sun, L. Anti-Inflammatory Activities and Potential Mechanisms of Phenolic Acids Isolated from Salvia Miltiorrhiza f. Alba Roots in THP-1 Macrophages. J. Ethnopharmacol. 2018, 222, 201–207. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Wang, D.; Tao, M.; Xu, W.; Olatunji, O.J. Anti-Inflammatory Activities of Kukoamine A From the Root Bark of Lycium chinense Miller. Nat. Prod. Commun. 2020, 15, 1934578X20912088. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant Specialized Metabolites with a Wide Range of Promising Pharmacological and Health-Promoting Interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Chu, H.; Zhao, Q.; Ding, H.; Cai, C. Neuroprotective Effects of Kukoamine A on 6-OHDA-Induced Parkinson’s Model through Apoptosis and Iron Accumulation Inhibition. Chin. Herb. Med. 2020, 13, 105–115. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Sun, Z.; Dong, L.; Gao, L.; Liu, C.; Wang, X. Kukoamine A Inhibits Human Glioblastoma Cell Growth and Migration through Apoptosis Induction and Epithelial-Mesenchymal Transition Attenuation. Sci. Rep. 2016, 6, 36543. [Google Scholar] [CrossRef] [PubMed]
- Tebayashi, S.; Horibata, Y.; Mikagi, E.; Kashiwagi, T.; Mekuria, D.B.; Dekebo, A.; Ishihara, A.; Kim, C.-S. Induction of Resistance against the Leafminer, Liriomyza Trifolii, by Jasmonic Acid in Sweet Pepper. Biosci. Biotechnol. Biochem. 2007, 71, 1521–1526. [Google Scholar] [CrossRef]
- Bandoly, M.; Hilker, M.; Steppuhn, A. Oviposition by Spodoptera Exigua on Nicotiana Attenuata Primes Induced Plant Defence against Larval Herbivory. Plant J. 2015, 83, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Rocha, C.; Halitschke, R.; Baldwin, I.T.; Pandey, S.P. MicroRNA390 Modulates Nicotiana Attenuata’s Tolerance Response to Manduca Sexta Herbivory. Plant Direct 2021, 5, e350. [Google Scholar] [CrossRef] [PubMed]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic Characterization and Variability in Leaves, Stems and Roots of Micro-Tom and Patio Tomatoes, in Response to Nitrogen Limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Yingyongnarongkul, B.; Apiratikul, N.; Aroonrerk, N.; Suksamrarn, A. Synthesis of Bis, Tris and Tetra(Dihydrocaffeoyl)Polyamine Conjugates as Antibacterial Agents against VRSA. Arch. Pharm. Res. 2008, 31, 698–704. [Google Scholar] [CrossRef]
- Bourne, G.T.; Golding, S.W.; McGeary, R.P.; Meutermans, W.D.; Jones, A.; Marshall, G.R.; Alewood, P.F.; Smythe, M.L. The Development and Application of a Novel Safety-Catch Linker for BOC-Based Assembly of Libraries of Cyclic Peptides. J. Org. Chem. 2001, 66, 7706–7713. [Google Scholar] [CrossRef]
- Wilms, V.S.; Bauer, H.; Tonhauser, C.; Schilmann, A.-M.; Müller, M.-C.; Tremel, W.; Frey, H. Catechol-Initiated Polyethers: Multifunctional Hydrophilic Ligands for PEGylation and Functionalization of Metal Oxide Nanoparticles. Biomacromolecules 2013, 14, 193–199. [Google Scholar] [CrossRef]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia Oblonga Miller) Peel Polyphenols Modulate LPS-Induced Inflammation in Human THP-1-Derived Macrophages through NF-ΚB, P38MAPK and Akt Inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef]
- Le, T.T.; Ropars, A.; Aymes, A.; Frippiat, J.-P.; Kapel, R. Multicriteria Optimization of Phenolic Compounds Capture from a Sunflower Protein Isolate Production Process By-Product by Adsorption Column and Assessment of Their Antioxidant and Anti-Inflammatory Effects. Foods 2021, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Kyselka, J.; Bleha, R.; Dragoun, M.; Bialasová, K.; Horáčková, Š.; Schätz, M.; Sluková, M.; Filip, V.; Synytsya, A. Antifungal Polyamides of Hydroxycinnamic Acids from Sunflower Bee Pollen. J. Agric. Food Chem. 2018, 66, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Fernández-Fernández, R.; Juárez-Fernández, G.; Martínez-Álvarez, S.; Eguizábal, P.; Zarazaga, M.; Lozano, C.; Torres, C. Wild Animals Are Reservoirs and Sentinels of Staphylococcus Aureus and MRSA Clones: A Problem with “One Health” Concern. Antibiotics 2021, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 18. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Song, Y.; Zhang, C.; Jiang, Y.; Li, W.; Xu, B.; Jiang, Z. Kukoamine A Inhibits C-C Motif Chemokine Receptor 5 to Attenuate Lipopolysaccharide-Induced Lung Injury. Drug Dev. Res. 2022, 83, 1455–1466. [Google Scholar] [CrossRef]
- Wang, D.; Qu, H.; Kang, H.; Xu, F.; Huang, W.; Cai, X. Kukoamine A Attenuates Lipopolysaccharide-Induced Apoptosis, Extracellular Matrix Degradation, and Inflammation in Nucleus Pulposus Cells by Activating the P13K/Akt Pathway. Bioengineered 2022, 13, 8772–8784. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in Inflammation, Repair and Regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the Maintenance of Homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. Macrophage Polarity and Disease Control. Int. J. Mol. Sci. 2021, 23, 144. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Zhang, M.; Borovikova, L.V.; Wang, H.; Metz, C.; Tracey, K.J. Spermine Inhibition of Monocyte Activation and Inflammation. Mol. Med. 1999, 5, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Lamers, W.H.; Koehler, E.S.; Geuns, J.M.C.; Alhonen, L.; Uimari, A.; Pirnes-Karhu, S.; Van Overmeire, E.; Morias, Y.; Brys, L.; et al. Pivotal Advance: Arginase-1-Independent Polyamine Production Stimulates the Expression of IL-4-Induced Alternatively Activated Macrophage Markers While Inhibiting LPS-Induced Expression of Inflammatory Genes. J. Leukoc. Biol. 2012, 91, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Latour, Y.L.; Gobert, A.P.; Wilson, K.T. The Role of Polyamines in the Regulation of Macrophage Polarization and Function. Amino Acids 2020, 52, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Hammoud Mahdi, D.; Jankuhn, S.; Lipowicz, B.; Vissiennon, C. Bioactive Plant Compounds in Coffee Charcoal (Coffeae Carbo) Extract Inhibit Cytokine Release from Activated Human THP-1 Macrophages. Molecules 2019, 24, 4263. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Parr, A.; Mellon, F.A.; Colquhoun, I.J. Dihydrocaffeoyl polyamines (kukoamine and allies) in potato (Solanum tuberosum) tuber detected during metabolite profiling. J. Agr. Food Chem. 2005, 53, 5461–5466. [Google Scholar] [CrossRef]
- Li, Y.Y.; Di, R.; Hsu, W.L.; Huang, Y.Q.; Cheung, H.N. Quality control of Lycium chinense and Lycium barbarum cortex (Digupi) by HPLC using kukoamines as markers. Chin. Med. 2017, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Back, K. Production of phenylpropanoid amides in recombinant Escherichia coli. Metab. Eng. 2009, 11, 64–68. [Google Scholar] [CrossRef]
- Sim, G.Y.; Yang, S.-M.; Kim, B.G.; Ahn, J.-H. Bacterial synthesis of N-hydroxycinnamoyl phenethylamines andtyramines, Microb. Cell Factories 2015, 14, 162. [Google Scholar] [CrossRef]
- Kang, K.; Lee, K.; Sohn, S.-O.; Park, S.; Lee, S.; Kim, S.Y.; Kim, Y.S.; Back, K. Ectopic expression of serotonin N-hydroxycinnamoyltransferase and differential production of phenylpropanoid amides in transgenic tomato tissues. Sci. Hortic. 2009, 120, 504–510. [Google Scholar] [CrossRef]
- Perrin, J.; Kulagina, N.; Unlubayir, M.; Munsch, T.; Carqueijeiro, I.; Dugé de Bernonville, T.; De Craene, J.-O.; Clastre, M.; St-Pierre, B.; Giglioli-Guivarc’h, N.; et al. Exploiting Spermidine N-Hydroxycinnamoyltransferase Diversity and Substrate Promiscuity to Produce Various Trihydroxycinnamoyl Spermidines and Analogues in Engineered Yeast. ACS Synth. Biol. 2021, 10, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Royer, M.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Robin, C. Is the C:N Ratio a Reliable Indicator of C Allocation to Primary and Defence-Related Metabolisms in Tomato? Phytochemistry 2013, 88, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility Test Methods. In Manual of Clinical Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1253–1273. ISBN 978-1-68367-280-7. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Grare, M.; Mourer, M.; Fontanay, S.; Regnouf-de-Vains, J.-B.; Finance, C.; Duval, R. In vitro activity of para-guanidinoethylcalix[4]arene against susceptible and anti-biotic-resistant Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother. 2007, 60, 575–581. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumani, M.; Ropars, A.; Robin, C.; Duval, R.E.; Frippiat, J.-P.; Boisbrun, M.; Larbat, R. Characterization of Biological Properties of Individual Phenolamides and Phenolamide-Enriched Leaf Tomato Extracts. Molecules 2023, 28, 1552. https://doi.org/10.3390/molecules28041552
Roumani M, Ropars A, Robin C, Duval RE, Frippiat J-P, Boisbrun M, Larbat R. Characterization of Biological Properties of Individual Phenolamides and Phenolamide-Enriched Leaf Tomato Extracts. Molecules. 2023; 28(4):1552. https://doi.org/10.3390/molecules28041552
Chicago/Turabian StyleRoumani, Marwa, Armelle Ropars, Christophe Robin, Raphaël E. Duval, Jean-Pol Frippiat, Michel Boisbrun, and Romain Larbat. 2023. "Characterization of Biological Properties of Individual Phenolamides and Phenolamide-Enriched Leaf Tomato Extracts" Molecules 28, no. 4: 1552. https://doi.org/10.3390/molecules28041552
APA StyleRoumani, M., Ropars, A., Robin, C., Duval, R. E., Frippiat, J.-P., Boisbrun, M., & Larbat, R. (2023). Characterization of Biological Properties of Individual Phenolamides and Phenolamide-Enriched Leaf Tomato Extracts. Molecules, 28(4), 1552. https://doi.org/10.3390/molecules28041552







