Effects of Apigenin on Pharmacokinetics of Dasatinib and Probable Interaction Mechanism
Abstract
:1. Introduction
2. Results
2.1. Pharmacokinetic Interaction
2.2. Effect of Apigenin on Hepatic and Intestinal CYP3A2 Protein Expression
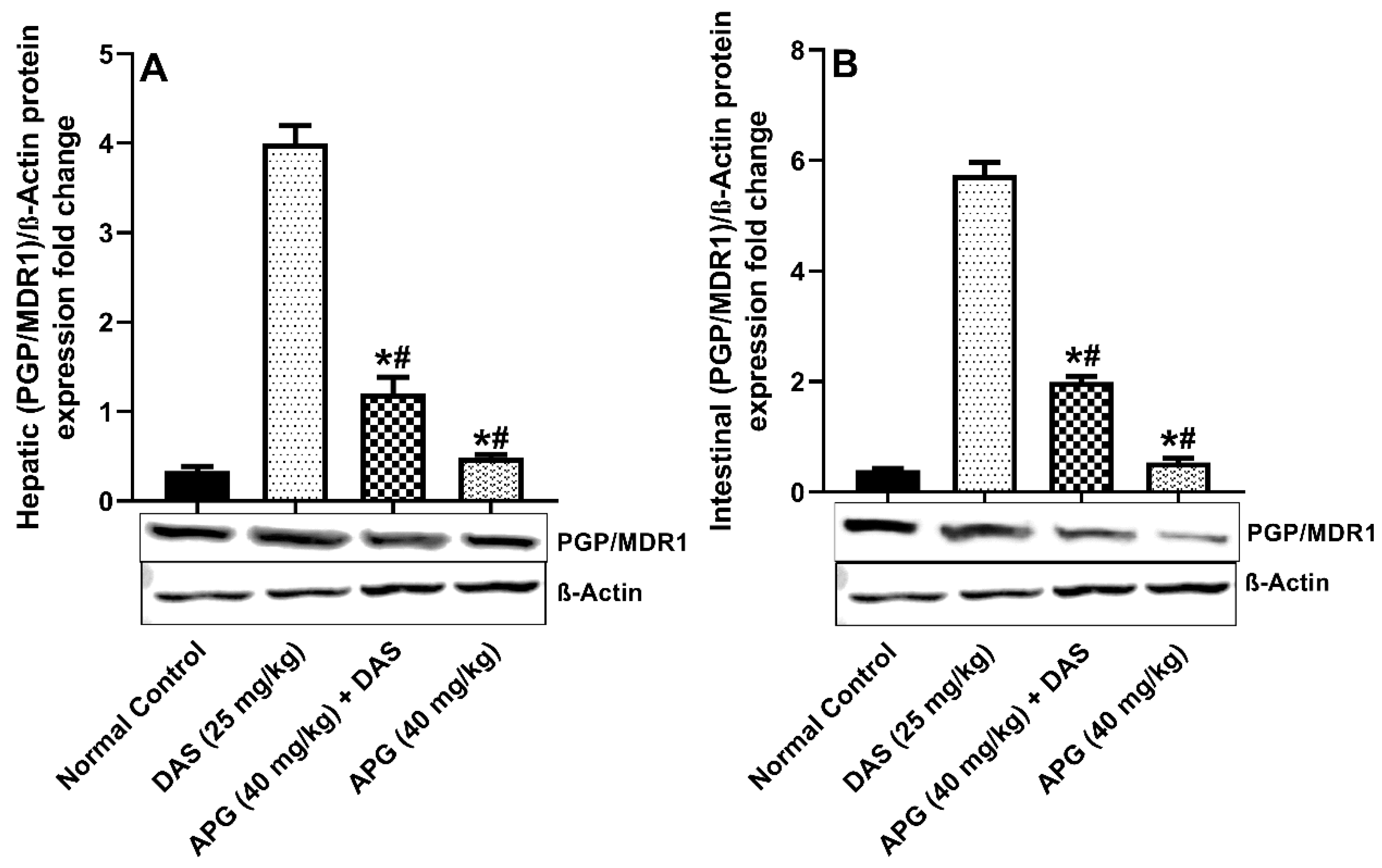
2.3. Effect of Apigenin on Hepatic and Intestinal Pgp/MDR1 Protein Expression
2.4. Effect of APG on Hepatic and Intestinal BCRP/ABCG2 Protein Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Pharmacokinetic Studies
4.3. Experimental Design
4.4. Mass Spectrometry and UPLC Chromatographic Conditions
4.5. Sample Preparation
4.6. Pharmacokinetic Analysis
4.7. Protein Expression Analysis
4.8. Statistical Analysis
5. Conclusions
6. Limitations and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.L.; Ho, H.K.; Chan, A. Metabolism-related pharmacokinetic drug-drug interactions with tyrosine kinase inhibitors: Current understanding, challenges and recommendations. Br. J. Clin. Pharmacol. 2015, 79, 241–253. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Haap, M.; Kopp, H.G.; Lipp, H.P. Tyrosine kinase inhibitors—A review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009, 10, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Bonvin, A.; Mesnil, A.; Nicolini, F.E.; Cotte, L.; Michallet, M.; Descotes, J.; Vial, T. Dasatinib-induced acute hepatitis. Leuk. Lymphoma 2008, 49, 1630–1632. [Google Scholar] [CrossRef] [PubMed]
- Herviou, P.; Thivat, E.; Richard, D.; Roche, L.; Dohou, J.; Pouget, M.; Eschalier, A.; Durando, X.; Authier, N. Therapeutic drug monitoring and tyrosine kinase inhibitors. Oncol. Lett. 2016, 12, 1223–1232. [Google Scholar] [CrossRef]
- Rochat, B.; Fayet, A.; Widmer, N.; Lahrichi, S.L.; Pesse, B.; Decosterd, L.A.; Biollaz, J. Imatinib metabolite profiling in parallel to imatinib quantification in plasma of treated patients using liquid chromatography-mass spectrometry. J. Mass Spectrom. 2008, 43, 736–752. [Google Scholar] [CrossRef]
- Van Erp, N.P.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat. Rev. 2009, 35, 692–706. [Google Scholar] [CrossRef]
- Chen, Y.; Agarwal, S.; Shaik, N.M.; Chen, C.; Yang, Z.; Elmquist, W.F. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J. Pharmacol. Exp. Ther. 2009, 330, 956–963. [Google Scholar] [CrossRef]
- Haouala, A.; Widmer, N.; Duchosal, M.A.; Montemurro, M.; Buclin, T.; Decosterd, L.A. Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 2011, 117, e75–e87. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Alam, M.A.; Raish, M.; Mohammed, I.E.; Hassan Mohammed, A.E.; Ansari, M.A.; Al Jenoobi, F.I. Dasatinib significantly reduced in vivo exposure to cyclosporine in a rat model: The possible involvement of CYP3A induction. Pharmacol. Rep. 2019, 71, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, X.; Wang, Y.; Wen, C.; Wang, C.; Zhou, Z.; Lin, G. Development of UPLC-MS/MS Method for Studying the Pharmacokinetic Interaction Between Dasatinib and Posaconazole in Rats. Drug Des. Devel. Ther. 2021, 15, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; Garcia-Gutierrez, V.; Hochhaus, A.; Kim, D.W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Lin, S.; Kuo, G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008, 17, 275–279. [Google Scholar] [PubMed]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Marjanovic Vicentic, J.; Popovic, J.; Golic Grdadolnik, S.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Lampropoulos, P.; Lambropoulou, M.; Papalois, A.; Basios, N.; Manousi, M.; Simopoulos, C.; Tsaroucha, A.K. The role of apigenin in an experimental model of acute pancreatitis. J. Surg. Res. 2013, 183, 129–137. [Google Scholar] [CrossRef]
- Ho, P.-C.; Saville, D.J.; Wanwimolruk, S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J. Pharm. Sci. 2001, 4, 217–227. [Google Scholar]
- Saeed, M.; Kadioglu, O.; Khalid, H.; Sugimoto, Y.; Efferth, T. Activity of the dietary flavonoid, apigenin, against multidrug-resistant tumor cells as determined by pharmacogenomics and molecular docking. J. Nutr. Biochem. 2015, 26, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Priyanka, L.; Gnananath, K.; Babu, P.R.; Sujatha, S. Pharmacokinetic drug interactions between apigenin, rutin and paclitaxel mediated by P-glycoprotein in rats. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Xu, T.; Li, W.S.; Luo, J.; Geng, P.W.; Wang, L.; Xia, M.M.; Chen, M.C.; Yu, L.; Hu, G.X. The effect of apigenin on pharmacokinetics of imatinib and its metabolite N-desmethyl imatinib in rats. Biomed. Res. Int. 2013, 2013, 789184. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction With Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef]
- Miller, M.F.; Bellizzi, K.M.; Sufian, M.; Ambs, A.H.; Goldstein, M.S.; Ballard-Barbash, R. Dietary supplement use in individuals living with cancer and other chronic conditions: A population-based study. J. Am. Diet. Assoc. 2008, 108, 483–494. [Google Scholar] [CrossRef]
- Gardiner, P.; Phillips, R.S.; Shaughnessy, A. Herbal and dietary supplement-drug interactions in patients with chronic illnesses. Am. Fam. Physician 2008, 77, 73–78. [Google Scholar]
- Alzoman, N.Z.; Maher, H.M.; Shehata, S.M.; Abanmy, N.O. UPLC-MS/MS study of the effect of dandelion root extract on the plasma levels of the selected irreversible tyrosine kinase inhibitors dasatinib, imatinib and nilotinib in rats: Potential risk of pharmacokinetic interactions. Biomed. Chromatogr. 2019, 33, e4674. [Google Scholar] [CrossRef]
- Fleisher, B.; Unum, J.; Shao, J.; An, G. Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: A new mechanism of beverage-drug interaction. J. Pharm. Sci. 2015, 104, 266–275. [Google Scholar] [CrossRef]
- Durmus, S.; Hendrikx, J.J.; Schinkel, A.H. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv. Cancer Res. 2015, 125, 1–41. [Google Scholar]
- Kamath, A.V.; Wang, J.; Lee, F.Y.; Marathe, P.H. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): A potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother. Pharmacol. 2008, 61, 365–376. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, R.; Bae, S.; Murry, D.J.; An, G. TKI combination therapy: Strategy to enhance dasatinib uptake by inhibiting Pgp- and BCRP-mediated efflux. Biopharm Drug Dispos. 2016, 37, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Early, Durable Responses Seen with Sprycel (dasatinib) in First- and Second-Line Treatment of Pediatric Patients with Chronic Myeloid Leukemia in Chronic Phase (CP-CML). Bristol-Myers Squibb. 2017. Available online: https://news.bms.com/news/details/2017/Early-Durable-Responses-Seen-with-Sprycel-dasatinib-in-First--and-Second-Line-Treatment-of-Pediatric-Patients-with-Chronic-Myeloid-Leukemia-in-Chronic-Phase-CP-CML/default.aspx (accessed on 1 November 2022).
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Lee, B.H.; Kim, D.S.; Song, H.J.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol. Rep. 2017, 38, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Durmus, S.; Xu, N.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (MDR1/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) restrict brain accumulation of the JAK1/2 inhibitor, CYT387. Pharmacol. Res. 2013, 76, 9–16. [Google Scholar] [CrossRef]
- Saric Mustapic, D.; Debeljak, Z.; Males, Z.; Bojic, M. The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme. Molecules 2018, 23, 2553. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, L.L.; Weemhoff, J.L.; Bedir, E.; Khan, I.A.; Harmatz, J.S.; Goldman, P.; Greenblatt, D.J. Inhibition of human cytochromes P450 by components of Ginkgo biloba. J. Pharm. Pharmacol. 2004, 56, 1039–1044. [Google Scholar] [CrossRef]
- Ezzeldin, E.; Iqbal, M.; Herqash, R.N.; ElNahhas, T. Simultaneous quantitative determination of seven novel tyrosine kinase inhibitors in plasma by a validated UPLC-MS/MS method and its application to human microsomal metabolic stability study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1136, 121851. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Iqbal, M.; Raish, M.; Ahmad, A.; Ali, E.A.; Bin Jardan, Y.A.; Ansari, M.A.; Shahid, M.; Ahad, A.; Alkharfy, K.M.; Al-Jenoobi, F.I. Cytochrome P450 3A2 and PGP-MDR1-Mediated Pharmacokinetic Interaction of Sinapic Acid with Ibrutinib in Rats: Potential Food/Herb-Drug Interaction. Processes 2022, 10, 1066. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Effects of sinapic acid on hepatic cytochrome P450 3A2, 2C11, and intestinal P-glycoprotein on the pharmacokinetics of oral carbamazepine in rats: Potential food/herb-drug interaction. Epilepsy Res. 2019, 153, 14–18. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
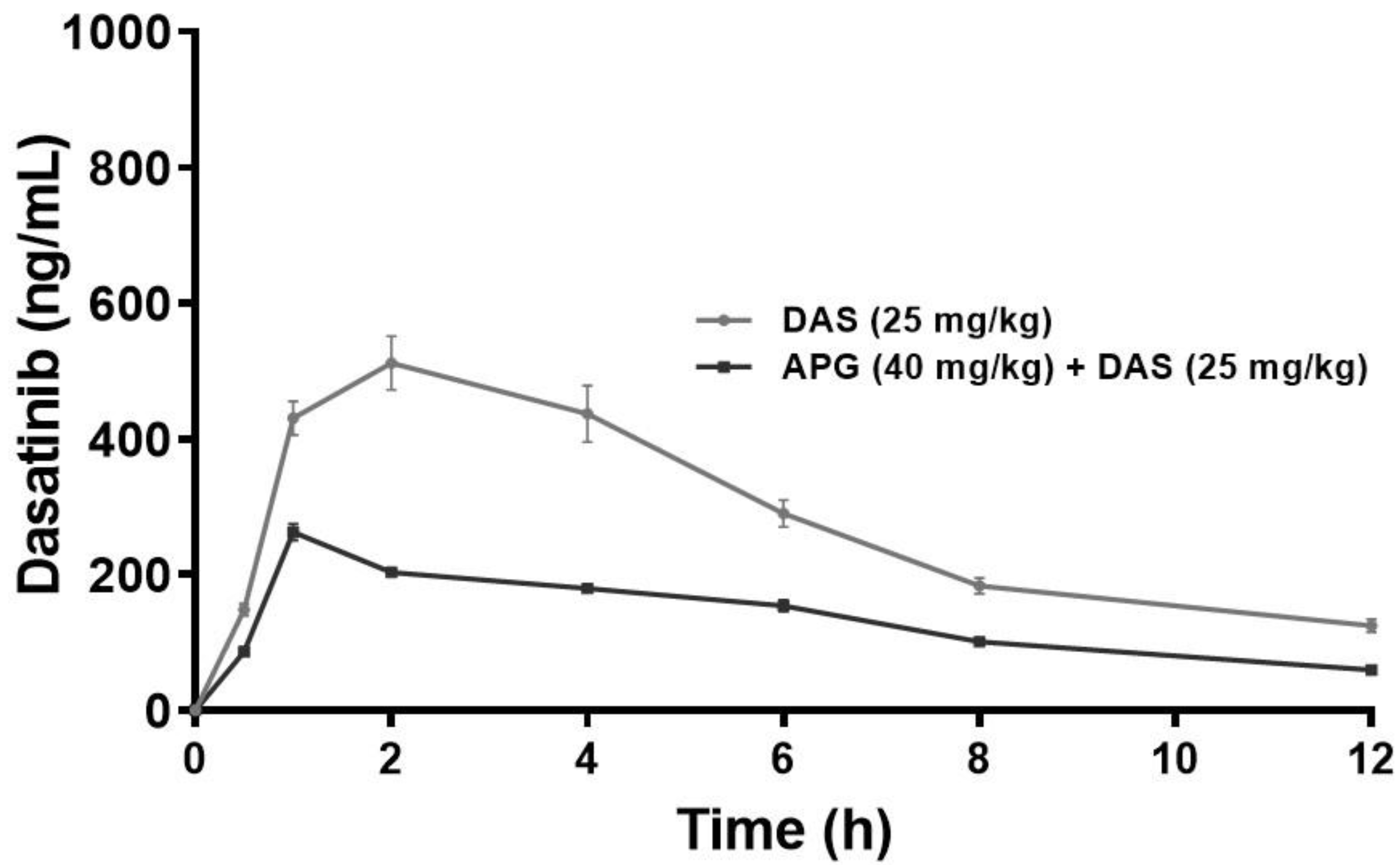


| Parameter (Unit) | DAS 25 mg/kg ± SD | DAS + APG ± SD | % Change |
|---|---|---|---|
| Kel (1/h) | 0.14 ± 0.01 | 0.11 ± 0.004 * | 24.83 |
| T1/2 (h) | 4.97 ± 0.48 | 6.56 ± 0.26 * | 32.16 |
| Tmax (h) | 1 ± 0 | 1.5 ± 0.00 * | 50 |
| Cmax (ng/mL) | 263.06 ± 12.33 | 511.81 ± 39.50 * | 94.56 |
| AUC0-t (ng/mL × h) | 1635.84 ± 45.29 | 2947.80 ± 168.32 * | 80.20 |
| AUC0-inf_obs (ng/mL × h) | 2063.49 ± 96.99 | 4129.07 ± 361.71 * | 100.10 |
| AUMC0-inf_obs (ng/mL × h2) | 16200.10 ± 1656.57 | 39472.75 ± 3665.28 * | 143.65 |
| MRT 0-inf_obs (h) | 7.84 ± 0.46 | 9.55 ± 0.43 * | 21.90 |
| Vd (mg/kg)/(ng/mL) | 0.087 ± 0.005 | 0.06 ± 0.004 * | 33.69 |
| Cl (mg/kg)/(ng/mL)/h | 0.012 ± 0.0005 | 0.006 ± 0.00 * | 49.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raish, M.; Ahmad, A.; Shahid, M.; Jardan, Y.A.B.; Ahad, A.; Kalam, M.A.; Ansari, M.A.; Iqbal, M.; Ali, N.; Alkharfy, K.M.; et al. Effects of Apigenin on Pharmacokinetics of Dasatinib and Probable Interaction Mechanism. Molecules 2023, 28, 1602. https://doi.org/10.3390/molecules28041602
Raish M, Ahmad A, Shahid M, Jardan YAB, Ahad A, Kalam MA, Ansari MA, Iqbal M, Ali N, Alkharfy KM, et al. Effects of Apigenin on Pharmacokinetics of Dasatinib and Probable Interaction Mechanism. Molecules. 2023; 28(4):1602. https://doi.org/10.3390/molecules28041602
Chicago/Turabian StyleRaish, Mohammad, Ajaz Ahmad, Mudassar Shahid, Yousef A. Bin Jardan, Abdul Ahad, Mohd Abul Kalam, Mushtaq Ahmad Ansari, Muzaffar Iqbal, Naushad Ali, Khalid M. Alkharfy, and et al. 2023. "Effects of Apigenin on Pharmacokinetics of Dasatinib and Probable Interaction Mechanism" Molecules 28, no. 4: 1602. https://doi.org/10.3390/molecules28041602







