Analyses of Transcriptomics upon IL-1β-Stimulated Mouse Chondrocytes and the Protective Effect of Catalpol through the NOD2/NF-κB/MAPK Signaling Pathway
Abstract
:1. Introduction
2. Results
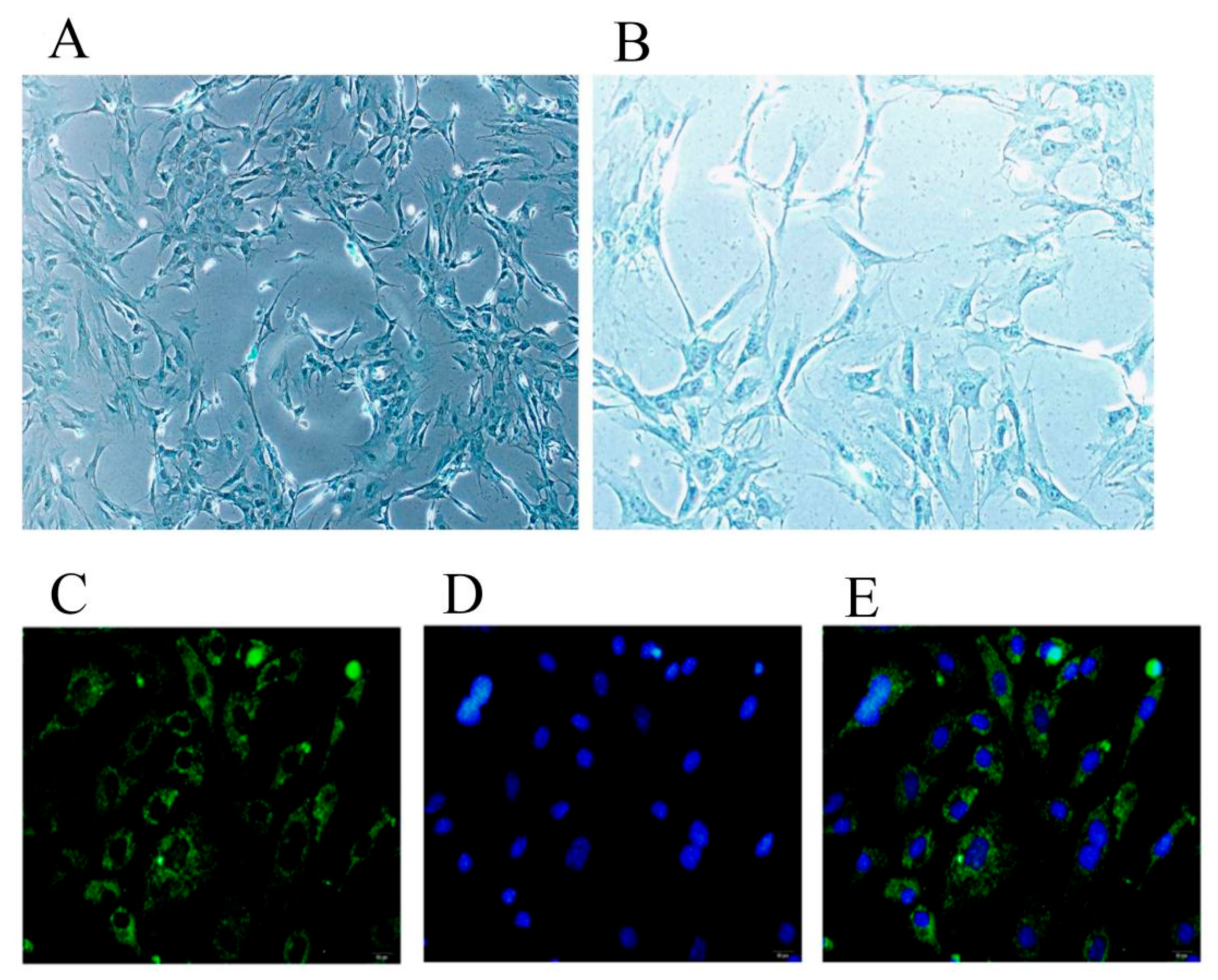
2.1. Identification of Chondrocytes and Inflammatory Model Conditions
2.2. Signaling Pathway Prediction Based on GO and KEGG Analyses
2.3. Effect of Catalpol on Cell Viability of Chondrocytes
2.4. Catalpol Inhibits IL-1β-Induced Inflammatory Cytokine Release
2.5. The Effects of Catalpol on the Levels of Inflammasome Activation-Associated Proteins and mRNA
2.6. Catalpol Inhibits Activation of the NOD2-NF-κB/MAPK Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Culturing and Identification of Chondrocytes
4.3. Establishment of an Inflammation Model
4.4. Transcriptome Analysis
4.5. Effect of Catalpol on Chondrocyte Viability
4.6. Q-PCR
4.7. ELISA and the Griess Reaction
4.8. Western Blotting
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. A report on the use of a single intra-articular administration of autologous platelet therapy in a naturally occurring canine osteoarthritis model—A preliminary study. BMC Musculoskelet. Disord. 2020, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Liu, S.; Tong, C.; Tan, H.; Yang, Y.; He, C. LIM mineralization protein-1 inhibits IL-1β-induced human chondrocytes injury by altering the NF-κB and MAPK/JNK pathways. Exp. Ther. Med. 2022, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Mucientes, A.; Herranz, E.; Moro, E.; Lajas, C.; Candelas, G.; Fernández-Gutiérrez, B.; Lamas, J.R. Differential Expression of HOX Genes in Mesenchymal Stem Cells from Osteoarthritic Patients is Independent of Their Promoter Methylation. Cells 2018, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Natsuka, M.; Uehara, A.; Yang, S.; Echigo, S.; Takada, H. A polymer-type water-soluble peptidoglycan exhibited both Toll-like receptor 2- and NOD2-agonistic activities, resulting in synergistic activation of human monocytic cells. Innate Immun. 2008, 14, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, C.; Kang, H.S.; Shin, S.W.; Kim, S.Y.; Park, H.C.; Hong, S.N. NOD2 Supports Crypt Survival and Epithelial Regeneration after Radiation-Induced Injury. Int. J. Mol. Sci. 2019, 20, 4297. [Google Scholar] [CrossRef]
- Ren, Y.; Wan, T.; Zuo, Z.; Cui, H.; Peng, X.; Fang, J.; Deng, J.; Hu, Y.; Yu, S.; Shen, L.; et al. Resistin increases the expression of NOD2 in mouse monocytes. Exp. Ther. Med. 2017, 13, 2523–2528. [Google Scholar] [CrossRef]
- Ma, C.; Wu, L.; Song, L.; He, Y.; Adel Abdo Moqbel, S.; Yan, S.; Sheng, K.; Wu, H.; Ran, J.; Wu, L. The pro-inflammatory effect of NR4A3 in osteoarthritis. J. Cell. Mol. Med. 2020, 24, 930–940. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, N.; Cheng, G.; Zhang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Zhu, B.; Zhang, Q.; Qin, L. Rehmannia glutinosa Libosch Extracts Prevent Bone Loss and Architectural Deterioration and Enhance Osteoblastic Bone Formation by Regulating the IGF-1/PI3K/mTOR Pathway in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 3964. [Google Scholar] [CrossRef]
- Lim, D.W.; Kim, Y.T. Dried root of Rehmannia glutinosa prevents bone loss in ovariectomized rats. Molecules 2013, 18, 5804–5813. [Google Scholar] [CrossRef]
- Liu, C.; Ma, R.; Wang, L.; Zhu, R.; Liu, H.; Guo, Y.; Zhao, B.; Zhao, S.; Tang, J.; Li, Y.; et al. Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J. Ethnopharmacol. 2017, 198, 351–362. [Google Scholar] [CrossRef]
- Jhun, J.Y.; Na, H.S.; Shin, J.W.; Jung, K.A.; Seo, H.B.; Ryu, J.Y.; Choi, J.W.; Moon, S.J.; Park, H.J.; Oh, S.W.; et al. Notoginseng Radix and Rehmanniae Radix Preparata Extract Combination (YH23537) Reduces Pain and Cartilage Degeneration in Rats with Monosodium Iodoacetate-Induced Osteoarthritis. J. Med. Food 2018, 21, 745–754. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, R.; Bian, Y.; Chen, W.; Peng, L. Catalpol Attenuates IL-1β Induced Matrix Catabolism, Apoptosis and Inflammation in Rat Chondrocytes and Inhibits Cartilage Degeneration. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6649–6659. [Google Scholar] [CrossRef]
- Ji, C.; Qiu, M.; Ruan, H.; Li, C.; Cheng, L.; Wang, J.; Li, C.; Qi, J.; Cui, W.; Deng, L. Transcriptome Analysis Revealed the Symbiosis Niche of 3D Scaffolds to Accelerate Bone Defect Healing. Adv. Sci. 2022, 9, e2105194. [Google Scholar] [CrossRef]
- Stanton, L.A.; Underhill, T.M.; Beier, F. MAP kinases in chondrocyte differentiation. Dev. Biol. 2003, 263, 165–175. [Google Scholar] [CrossRef]
- Priya, G.B.; Nagaleekar, V.K.; Milton, A.A.P.; Saminathan, M.; Kumar, A.; Sahoo, A.R.; Wani, S.A.; Kumar, A.; Gupta, S.K.; Sahoo, A.P.; et al. Genome wide host gene expression analysis in mice experimentally infected with Pasteurella multocida. PLoS ONE 2017, 12, e0179420. [Google Scholar] [CrossRef]
- Franca, R.; Vieira, S.M.; Talbot, J.; Peres, R.S.; Pinto, L.G.; Zamboni, D.S.; Louzada-Junior, P.; Cunha, F.Q.; Cunha, T.M. Expression and activity of NOD1 and NOD2/RIPK2 signalling in mononuclear cells from patients with rheumatoid arthritis. Scand. J. Rheumatol. 2016, 45, 8–12. [Google Scholar] [CrossRef]
- Ma, C.H.; Wu, C.H.; Jou, I.M.; Tu, Y.K.; Hung, C.H.; Hsieh, P.L.; Tsai, K.L. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biol. 2018, 14, 72–81. [Google Scholar] [CrossRef]
- Xu, R.; Luo, C.; Ge, Q.; Ying, J.; Zhang, P.; Xia, C.; Fang, L.; Xu, H.; Yuan, W.; Xu, T.; et al. Radix Rehmanniae Praeparata promotes bone fracture healing through activation of TGF-β signaling in mesenchymal progenitors. Biomed. Pharmacother. 2020, 130, 110581. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Koh, H.M.; Lim, S.Y.; Choudhury, H.; Pandey, M. Molecular and Biochemical Pathways of Catalpol in Alleviating Diabetes Mellitus and Its Complications. Biomolecules 2021, 11, 323. [Google Scholar] [CrossRef]
- He, L.; Zhao, R.; Wang, Y.; Liu, H.; Wang, X. Research Progress on Catalpol as Treatment for Atherosclerosis. Front. Pharmacol. 2021, 12, 716125. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, X.; Zhang, W.; Zhang, Y.; Bi, Y.; Meng, Q.; Bian, H.; Li, Y. Catalpol Inhibits Macrophage Polarization and Prevents Postmenopausal Atherosclerosis Through Regulating Estrogen Receptor Alpha. Front. Pharmacol. 2021, 12, 655081. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Peng, H.; Liu, J.; Cai, M.; Wu, H.; Zhang, Z.; Bai, J.; Yao, Y.; Dong, X.; Yin, X.; et al. Catalpol Protects ARPE-19 Cells against Oxidative Stress via Activation of the Keap1/Nrf2/ARE Pathway. Cells 2021, 10, 2635. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, H.; Chen, S.; Wu, X.; Chen, X.; Wang, F. Catalpol inhibits the proliferation, migration and metastasis of HCC cells by regulating miR-140-5p expression. Mol. Med. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Zhao, L.; Du, W.; Zhao, D.; Ji, X.; Huang, Y.; Pang, Y.; Guo, K.; Yin, X. Catalpol Protects Against High Glucose-Induced Bone Loss by Regulating Osteoblast Function. Front. Pharmacol. 2021, 12, 626621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Jia, Y.; Xu, J.; Chai, Y. Catalpol promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.; Shao, X.; Wang, Z.; Du, Y.; Zhu, C.; Du, W.; Tang, D.; Ji, S. Prenylated phenolic compounds from licorice (Glycyrrhiza uralensis) and their anti-inflammatory activity against osteoarthritis. Food Funct. 2022, 13, 795–805. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Liang, W.; Li, H.; Liu, F.; Weng, X.; Lin, P.; Chen, W.; Zheng, C.; Xu, H.; et al. Bushen Zhuangjin Decoction promotes chondrocyte proliferation by stimulating cell cycle progression. Exp. Ther. Med. 2015, 9, 839–844. [Google Scholar] [CrossRef]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhang, B.; Sheng, Y.; Xu, W.; Luo, Y.; He, X.; Huang, K. Comprehensive Analysis of the Characteristics and Differences in Adult and Newborn Brown Adipose Tissue (BAT): Newborn BAT is a More Active/Dynamic BAT. Cells 2020, 9, 201. [Google Scholar] [CrossRef]
- Ren, C.; Jin, J.; Hu, W.; Chen, Q.; Yang, J.; Wu, Y.; Zhou, Y.; Sun, L.; Gao, W.; Zhang, X.; et al. Betulin Alleviates the Inflammatory Response in Mouse Chondrocytes and Ameliorates Osteoarthritis via AKT/Nrf2/HO-1/NF-κB Axis. Front. Pharmacol. 2021, 12, 754038. [Google Scholar] [CrossRef]
- Song, X.; Ma, T.; Hu, H.; Zhao, M.; Bai, H.; Wang, X.; Liu, L.; Li, T.; Sheng, X.; Xu, X.; et al. Chronic Circadian Rhythm Disturbance Accelerates Knee Cartilage Degeneration in Rats Accompanied by the Activation of the Canonical Wnt/β-Catenin Signaling Pathway. Front. Pharmacol. 2021, 12, 760988. [Google Scholar] [CrossRef]







| Oligo Name | Sequence (5′–3′) |
|---|---|
| IL-1 | F: CTG CAC TAC AGG CTC CGA |
| R: GCC ACA GGT ATT TTG TCG TT | |
| IL-6 | F: TTA GCC ACT CCT TCT GTG ACT CC |
| R: ACC CCA ATT TCC AAT GCT CT | |
| IL-12a | F: GACCTGTTTACCACTGGAACTA |
| R: GATCTGCTGATGGTTGTGATTC | |
| TNF-α | F: TCG TAT GAA ATG GCA AAT CG |
| R: GGT CCC AAC AAG GAG GAG | |
| INOS | F: ATC CCG AAA CGA TAC ACT T |
| R: TCT GGC GAA GAA CAA TCC | |
| COX2 | F: TCT ACA AGA CGC CAC ATC CC |
| R: ACG GGG TTG TTG ATT TCG TCT | |
| MMP3 | F: GGA GGC AGC AGA GAA CCT AC |
| R: TCC AAC CCG AGG AAC TTC TG | |
| MMP13 | F: CAG TGC TGC GGT TCA CTT TG |
| R: TCA TCA TAA CTC CAC ACG TGG TT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Zhao, L.; Ji, X.; Guo, K.; Yin, X. Analyses of Transcriptomics upon IL-1β-Stimulated Mouse Chondrocytes and the Protective Effect of Catalpol through the NOD2/NF-κB/MAPK Signaling Pathway. Molecules 2023, 28, 1606. https://doi.org/10.3390/molecules28041606
Pang Y, Zhao L, Ji X, Guo K, Yin X. Analyses of Transcriptomics upon IL-1β-Stimulated Mouse Chondrocytes and the Protective Effect of Catalpol through the NOD2/NF-κB/MAPK Signaling Pathway. Molecules. 2023; 28(4):1606. https://doi.org/10.3390/molecules28041606
Chicago/Turabian StylePang, Yong, Lu Zhao, Xueyan Ji, Kaijin Guo, and Xiaoxing Yin. 2023. "Analyses of Transcriptomics upon IL-1β-Stimulated Mouse Chondrocytes and the Protective Effect of Catalpol through the NOD2/NF-κB/MAPK Signaling Pathway" Molecules 28, no. 4: 1606. https://doi.org/10.3390/molecules28041606
APA StylePang, Y., Zhao, L., Ji, X., Guo, K., & Yin, X. (2023). Analyses of Transcriptomics upon IL-1β-Stimulated Mouse Chondrocytes and the Protective Effect of Catalpol through the NOD2/NF-κB/MAPK Signaling Pathway. Molecules, 28(4), 1606. https://doi.org/10.3390/molecules28041606






