Juçara Fruit (Euterpe Edulis Martius) Valorization Combining Emergent Extraction Technologies and Aqueous Solutions of Alkanediols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of PLE Extraction Conditions
2.2. Optimization of UAE Extraction Conditions
2.3. Solvent Effect on UAE Recovery of Anthocyanin-Rich Extract
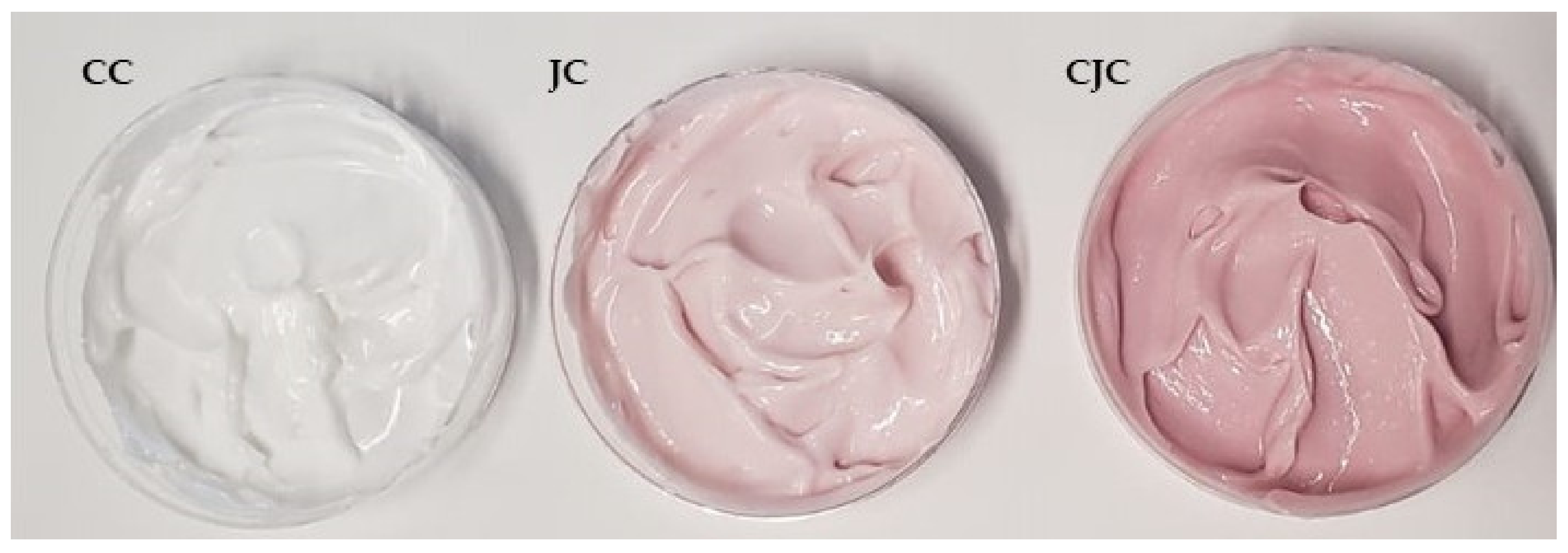
2.4. Characterization of Formulations Containing the Juçara Extract
3. Material and Methods
3.1. Raw Material
3.2. Chemicals
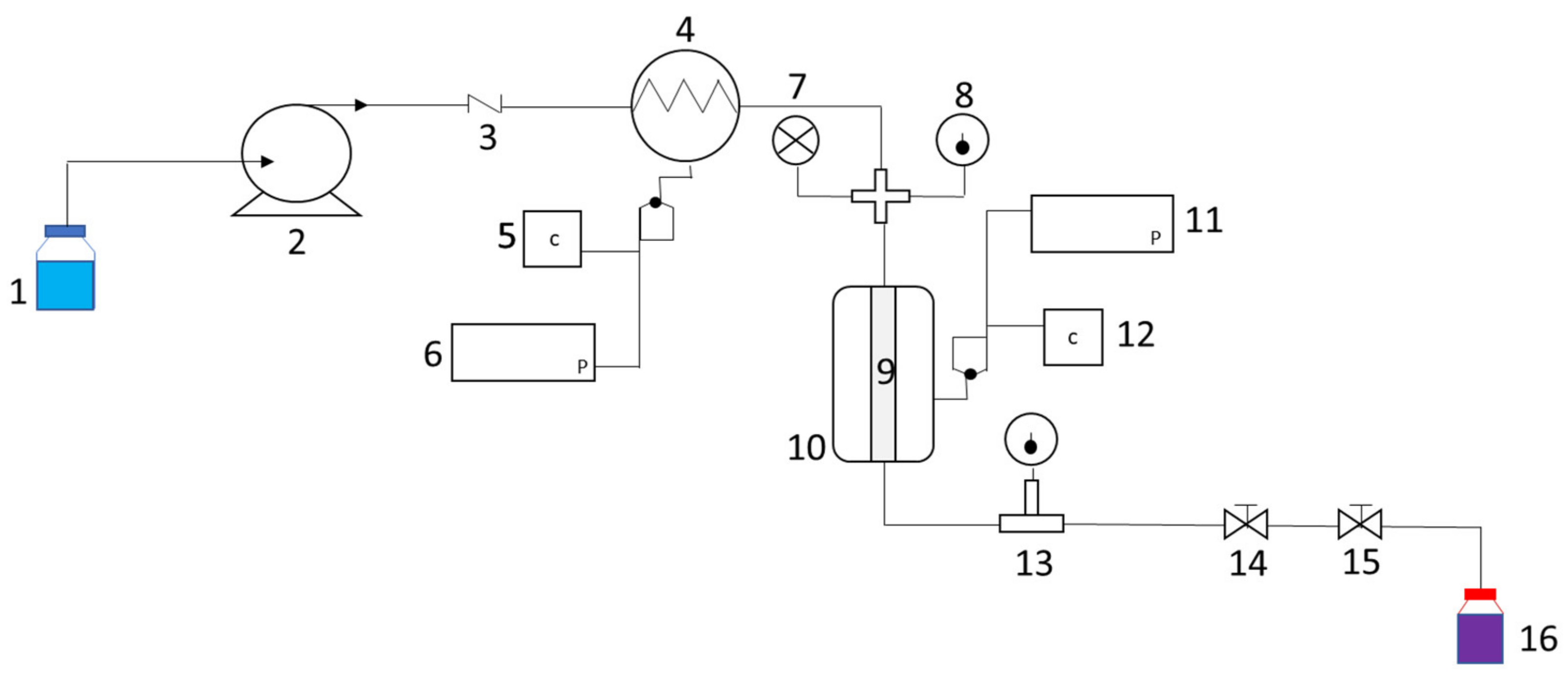
3.3. Extraction Methodologies
3.3.1. Pressurized Liquid Extraction
3.3.2. Ultrasound-Assisted Extraction (UAE)
3.3.3. Response Surface Methodology
3.4. Anthocyanin-Rich Extracts Characterization
3.5. Comparing Alkanediol Aqueous Solutions with Other Solvents
3.5.1. Total Phenolic Content (TPC)
3.5.2. ABTS Radical Scavenging Assay
3.5.3. Statistical Analysis
3.6. Product Formulations Using Juçara Anthocyanin-Rich Extract as Additive
3.6.1. Natural Soap Production by Hot Process Saponification
3.6.2. Incorporation of the Extract in a Cosmetic Cream
3.6.3. Antioxidant Activity Analysis and pH of Product Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Trevisan, A.C.D.; Fantini, A.C.; Schmitt-Filho, A.L.; Farley, J. Market for Amazonian Açaí (Euterpe Oleraceae) Stimulates Pulp Production from Atlantic Forest Juçara Berries (Euterpe Edulis). Agroecol. Sustain. Food Syst. 2015, 39, 762–781. [Google Scholar] [CrossRef]
- Schulz, M.; Borges, G.d.S.C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Juçara Fruit (Euterpe Edulis Mart.): Sustainable Exploitation of a Source of Bioactive Compounds. Food Res. Int. 2016, 89, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Gaiotto, F.A.; Grattapaglia, D.; Vencovsky, R. Genetic Structure, Mating System, and Long-Distance Gene Flow in Heart of Palm (Euterpe Edulis Mart.). J. Hered. 2003, 94, 399–406. [Google Scholar] [CrossRef]
- Carvalho, C.d.S.; Ballesteros-Mejia, L.; Ribeiro, M.C.; Côrtes, M.C.; Santos, A.S.; Collevatti, R.G. Climatic Stability and Contemporary Human Impacts Affect the Genetic Diversity and Conservation Status of a Tropical Palm in the Atlantic Forest of Brazil. Conserv. Genet. 2017, 18, 467–478. [Google Scholar] [CrossRef]
- Garcia, J.A.A.; Corrêa, R.C.G.; Barros, L.; Pereira, C.; Abreu, R.M.V.; Alves, M.J.; Calhelha, R.C.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Chemical Composition and Biological Activities of Juçara (Euterpe Edulis Martius) Fruit by-Products, a Promising Underexploited Source of High-Added Value Compounds. J. Funct. Foods 2019, 55, 325–332. [Google Scholar] [CrossRef]
- Cardoso, A.L.; De Liz, S.; Rieger, D.K.; Farah, A.C.A.; Kunradi Vieira, F.G.; Altenburg De Assis, M.A.; Di Pietro, P.F. An Update on the Biological Activities of Euterpe Edulis (Juçara). Planta Med. 2018, 84, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.S.; Marques, A.S.F.; Machado, M.T.C.; Silva, V.M.; Hubinger, M.D. Determination of Anthocyanins and Non-Anthocyanin Polyphenols by Ultra Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry (UPLC/ESI–MS) in Jussara (Euterpe Edulis) Extracts. J. Food Sci. Technol. 2017, 54, 2135–2144. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Ribani, R.H.; Beta, T. Anthocyanins, Phenolic Acids and Antioxidant Properties of Juçara Fruits (Euterpe Edulis M.) along the On-Tree Ripening Process. Plant Foods Hum. Nutr. 2014, 69, 142–147. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of Anthocyanins: Gaps in Knowledge, Challenges and Future Research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Mesquita, L.M.d.S.; Martins, P.L.G.; Habu, S.; Rosso, V.V. de Lactobacillus Fermentation of Jussara Pulp Leads to the Enzymatic Conversion of Anthocyanins Increasing Antioxidant Activity. J. Food Compos. Anal. 2018, 69, 162–170. [Google Scholar] [CrossRef]
- Peron, D.V.; Fraga, S.; Antelo, F. Thermal Degradation Kinetics of Anthocyanins Extracted from Juçara (Euterpe Edulis Martius) and “Italia” Grapes (Vitis Vinifera L.), and the Effect of Heating on the Antioxidant Capacity. Food Chem. 2017, 232, 836–840. [Google Scholar] [CrossRef]
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles from Herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of Emerging Technologies for the Extraction of Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. Overview of Modern Extraction Techniques for Food and Agricultural Samples. In Modern Extraction Techniques; ACS Publications: Washington, DC, USA, 2006; pp. 3–19. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of Extraction of Natural Products Using Ultrasonic Irradiations-a Review of Current Status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric Constants of Water, Methanol, Ethanol, Butanol and Acetone: Measurement and Computational Study. J. Solution Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Herrero, M.; Sánchez-Camargo, A.d.P.; Cifuentes, A.; Ibáñez, E. Plants, Seaweeds, Microalgae and Food by-Products as Natural Sources of Functional Ingredients Obtained Using Pressurized Liquid Extraction and Supercritical Fluid Extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Henry, M.C.; Yonker, C.R. Supercritical Fluid Chromatography, Pressurized Liquid Extraction, and Supercritical Fluid Extraction. Anal. Chem. 2006, 78, 3909–3915. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef]
- Machmudah, S.; Lestari, S.D.; Widiyastuti; Wahyudiono; Kanda, H.; Winardi, S.; Goto, M. Subcritical Water Extraction Enhancement by Adding Deep Eutectic Solvent for Extracting Xanthone from Mangosteen Pericarps. J. Supercrit. Fluids 2018, 133, 615–624. [Google Scholar] [CrossRef]
- Tommasi, E.; Cravotto, G.; Galletti, P.; Grillo, G.; Mazzotti, M.; Sacchetti, G.; Samorì, C.; Tabasso, S.; Tacchini, M.; Tagliavini, E. Enhanced and Selective Lipid Extraction from the Microalga P. Tricornutum by Dimethyl Carbonate and Dupercritical CO2 Dsing Deep Eutectic Solvents and Microwaves as Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 8316–8322. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized Aqueous Solutions of Deep Eutectic Solvent (DES): A Green Emergent Extraction of Anthocyanins from a Brazilian Berry Processing by-Product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Wan Mahmood, W.M.A.; Lorwirachsutee, A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Polyol-Based Deep Eutectic Solvents for Extraction of Natural Polyphenolic Antioxidants from Chlorella Vulgaris. ACS Sustain. Chem. Eng. 2019, 7, 5018–5026. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Composed of Choline Chloride and Polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.P.; Mafra, M.R. Extraction of Phenolic Compounds from Rosemary Using Choline Chloride–Based Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 258, 117975. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Pressurized Limonene as an Alternative Bio-Solvent for the Extraction of Lipids from Marine Microorganisms. J. Supercrit. Fluids 2014, 92, 1–7. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef]
- Bermejo, D.V.; Luna, P.; Manic, M.S.; Najdanovic-Visak, V.; Reglero, G.; Fornari, T. Extraction of Caffeine from Natural Matter Using a Bio-Renewable Agrochemical Solvent. Food Bioprod. Process. 2013, 91, 303–309. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Z.; Glasius, M.; Kristensen, K.; Xiao, L.; Xu, X. Pressurized Liquid Extraction of Ginger (Zingiber Officinale Roscoe) with Bioethanol: An Efficient and Sustainable Approach. J. Chromatogr. A 2011, 1218, 5765–5773. [Google Scholar] [CrossRef] [PubMed]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol Based Solvents: Synthesis, Properties and Applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved Utilization of Renewable Resources: New Important Derivatives of Glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J.; Agarwal, L. Microbial Production and Applications of 1,2-Propanediol. Indian J. Microbiol. 2010, 50, 2–11. [Google Scholar] [CrossRef]
- FDA GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 21 October 2022).
- Günter Verheugen Cosmetic Ingredients Other than Perfume and Aromatic Raw Materials. Off. J. Eur. Union. 2006, 1–13.
- Vieira, V.; Calhelha, R.C.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans Regia L. Leaves as a Source of Bioactive Compounds. Molecules 2020, 25, 2497. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Ultrasonic-Assisted Virgin Coconut Oil Based Extraction for Maximizing Polyphenol Recovery and Bioactivities of Mangosteen Peels. J. Food Sci. Technol. 2020, 57, 4032–4043. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Jakštas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Drakšiene, G.; Masteikova, R.; Bernatoniene, J. Optimization of Carvacrol, Rosmarinic, Oleanolic and Ursolic Acid Extraction from Oregano Herbs (Origanum Onites L., Origanum Vulgare spp. Hirtum and Origanum Vulgare L.). Nat. Prod. Res. 2016, 30, 672–674. [Google Scholar] [CrossRef]
- Hubbermann, E.M.; Heins, A.; Stöckmann, H.; Schwarz, K. Influence of Acids, Salt, Sugars and Hydrocolloids on the Colour Stability of Anthocyanin Rich Black Currant and Elderberry Concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of Phenolic Compounds and Anthocyanins from Juçara (Euterpe Edulis Mart.) Residues Using Pressurized Liquids and Supercritical Fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De La Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Torskangerpoll, K.; Andersen, Ø.M. Colour Stability of Anthocyanins in Aqueous Solutions at Various PH Values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]
- Vieira, G.S.; Cavalcanti, R.N.; Meireles, M.A.A.; Hubinger, M.D. Chemical and Economic Evaluation of Natural Antioxidant Extracts Obtained by Ultrasound-Assisted and Agitated Bed Extraction from Jussara Pulp (Euterpe Edulis). J. Food Eng. 2013, 119, 196–204. [Google Scholar] [CrossRef]
- Madalão, M.C.M.; Lima, E.M.F.; Benincá, D.B.; Saraiva, S.H.; de Carvalho, R.V.; Silva, P.I. Extraction of Bioactive Compounds from Juçara Pulp (Euterpe Edulis M.) Is Affected by Ultrasonic Power and Temperature. Cienc. Agrotecnologia 2021, 45, 1–11. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Abranches, D.O.; Soares, B.P.; Ferreira, A.M.; Shimizu, S.; Pinho, S.P.; Coutinho, J.A.P. The Impact of Size and Shape in the Performance of Hydrotropes: A Case-Study of Alkanediols. Phys. Chem. Chem. Phys. 2022, 24, 7624–7634. [Google Scholar] [CrossRef]
- Soares, B.P.; Abranches, D.O.; Sintra, T.E.; Leal-Duaso, A.; García, J.I.; Pires, E.; Shimizu, S.; Pinho, S.P.; Coutinho, J.A.P. Glycerol Ethers as Hydrotropes and Their Use to Enhance the Solubility of Phenolic Acids in Water. ACS Sustain. Chem. Eng. 2020, 8, 5742–5749. [Google Scholar] [CrossRef]
- Atolani, O.; Olabiyi, E.T.; Issa, A.A.; Azeez, H.T.; Onoja, E.G.; Ibrahim, S.O.; Zubair, M.F.; Oguntoye, O.S.; Olatunji, G.A. Green Synthesis and Characterisation of Natural Antiseptic Soaps from the Oils of Underutilised Tropical Seed. Sustain. Chem. Pharm. 2016, 4, 32–39. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics-a Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Rambabu, K.; Edathil, A.A.; Nirmala, G.S.; Hasan, S.W.; Yousef, A.F.; Show, P.L.; Banat, F. Date-Fruit Syrup Waste Extract as a Natural Additive for Soap Production with Enhanced Antioxidant and Antibacterial Activity. Environ. Technol. Innov. 2020, 20, 101153. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal Ph of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físicos-Quimicos Para Análise de Alimentos; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC-Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Talib, R.A.; Law, C.L. Optimization of Total Monomeric Anthocyanin (TMA) and Total Phenolic Content (TPC) Extractions from Mangosteen (Garcinia Mangostana Linn.) Hull Using Ultrasonic Treatments. Ind. Crop. Prod. 2013, 50, 1–7. [Google Scholar] [CrossRef]
- Koşar, M.; Dorman, H.J.; Hiltunen, R. Effect of an Acid Treatment on the Phytochemical and Antioxidant Characteristics of Extracts from Selected Lamiaceae Species. Food Chem. 2005, 91, 525–533. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying in Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
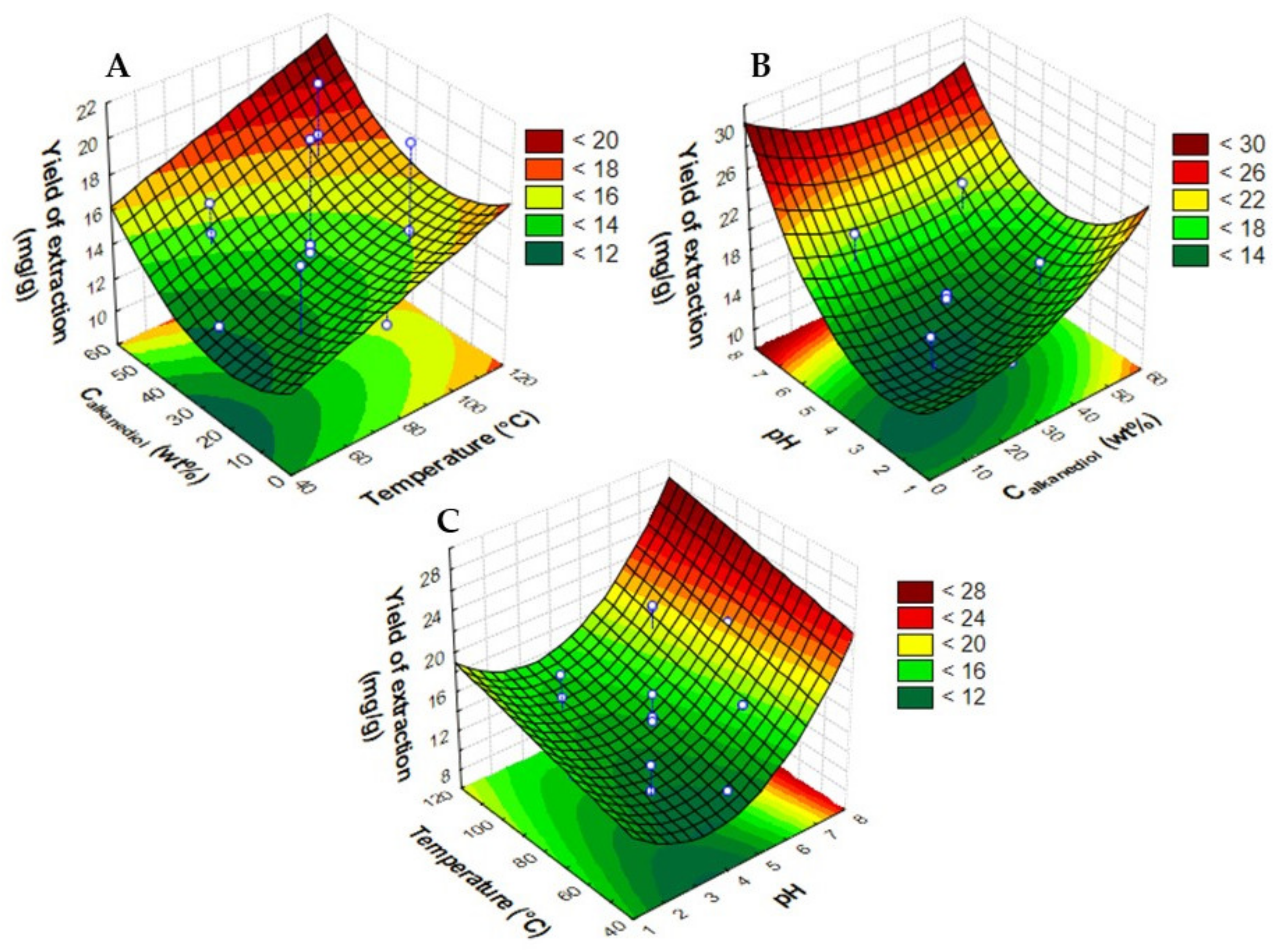
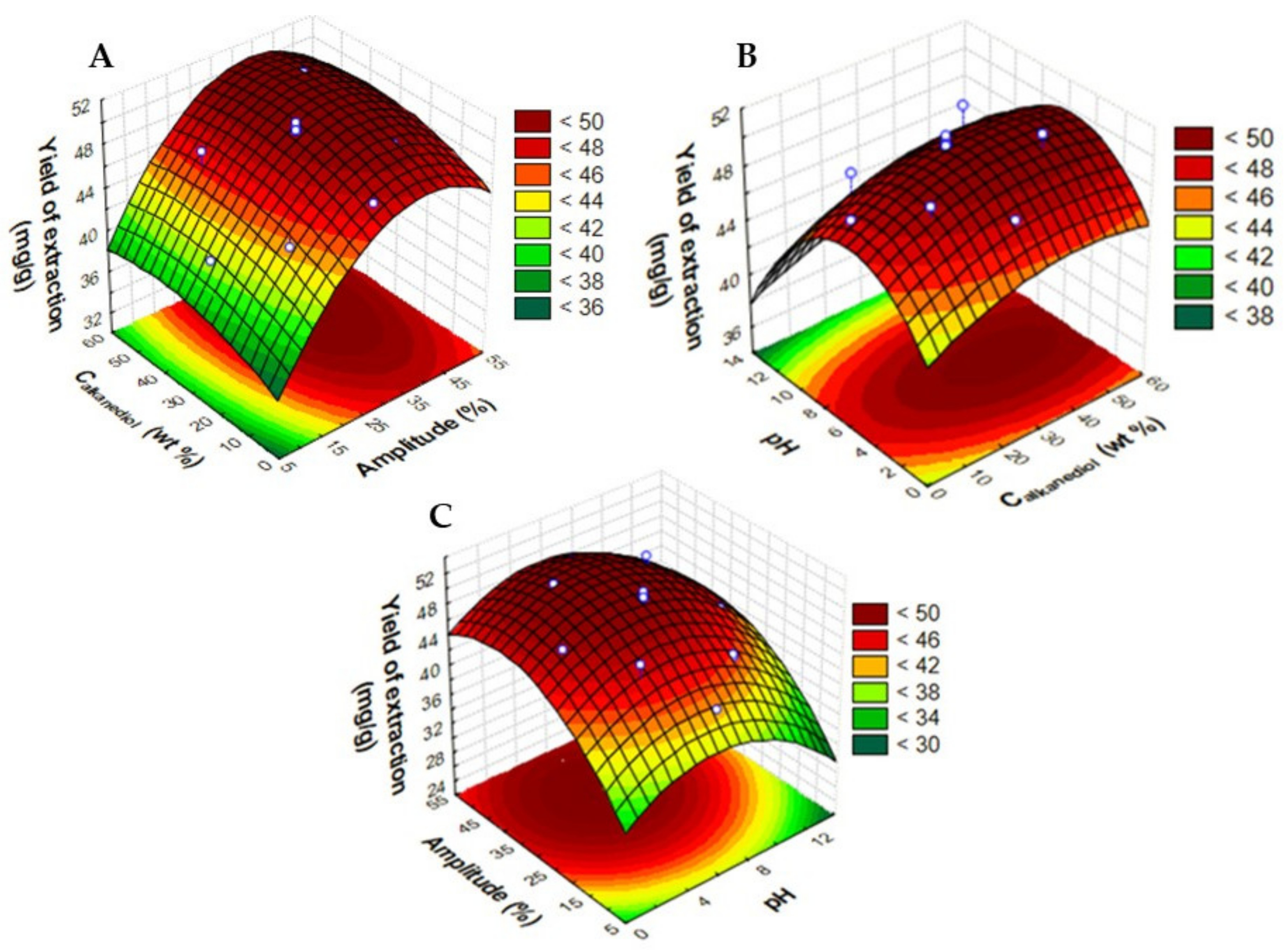


| Assay | Temperature (°C) | Calkanediol (wt%) | pH | Ccyanidin-3-O-glucoside (mg·gdry biomass−1) | Ccyanidin-3-O-rutinoside (mg·gdry biomass−1) | Yield of Extraction (mganthocyanins·gdry biomass−1) |
|---|---|---|---|---|---|---|
| 1 | 60 (−1) | 15 (−1) | 3.0 (−1) | 2.73 | 9.62 | 12.35 |
| 2 | 100 (+1) | 15 (−1) | 3.0 (−1) | 3.52 | 12.08 | 15.60 |
| 3 | 60 (−1) | 45 (+1) | 3.0 (−1) | 3.40 | 11.51 | 14.91 |
| 4 | 100 (+1) | 45 (+1) | 3.0 (−1) | 4.06 | 13.82 | 17.88 |
| 5 | 60 (−1) | 15 (−1) | 6.0 (+1) | 3.62 | 12.94 | 16.56 |
| 6 | 100 (+1) | 15 (−1) | 6.0 (+1) | 4.72 | 15.81 | 20.53 |
| 7 | 60 (−1) | 45 (+1) | 6.0 (+1) | 3.86 | 12.72 | 16.58 |
| 8 | 100 (+1) | 45 (+1) | 6.0 (+1) | 4.81 | 15.91 | 20.72 |
| 9 | 47 (−1.67) | 30 (0) | 4.5 (0) | 2.70 | 9.52 | 12.22 |
| 10 | 114 (+1.67) | 30 (0) | 4.5 (0) | 2.91 | 10.35 | 13.26 |
| 11 | 80 (0) | 4.8 (−1.67) | 4.5 (0) | 2.79 | 10.12 | 12.91 |
| 12 | 80 (0) | 55.2 (+1.67) | 4.5 (0) | 3.89 | 12.88 | 16.77 |
| 13 | 80 (0) | 30 (0) | 2.0 (−1.67) | 2.70 | 9.52 | 12.22 |
| 14 | 80 (0) | 30 (0) | 7.0 (+1.67) | 4.62 | 15.87 | 20.49 |
| 15 | 80 (0) | 30 (0) | 4.5 (0) | 2.97 | 10.51 | 13.48 |
| 16 | 80 (0) | 30 (0) | 4.5 (0) | 3.32 | 11.17 | 14.48 |
| 17 | 80 (0) | 30 (0) | 4.5 (0) | 3.27 | 11.23 | 14.50 |
| 18 | 80 (0) | 30 (0) | 4.5 (0) | 2.92 | 10.19 | 13.11 |
| 19 | 80 (0) | 30 (0) | 4.5 (0) | 3.13 | 10.89 | 14.01 |
| Assay | Amplitude (%) | Calkanediol (wt%) | pH | Ccyanidin-3-O-glucoside (mg·gdry biomass−1) | Ccyanidin-3-O-rutinoside (mg·gdry biomass−1) | Yield of Extraction (mganthocyanins·gdry biomass−1) |
|---|---|---|---|---|---|---|
| 1 | 18 (−1) | 15 (−1) | 4.0 (−1) | 7.64 | 36.64 | 44.29 |
| 2 | 42 (+1) | 15 (−1) | 4.0 (−1) | 8.72 | 40.67 | 49.39 |
| 3 | 18 (−1) | 45 (+1) | 4.0 (−1) | 8.72 | 38.94 | 47.66 |
| 4 | 42 (+1) | 45 (+1) | 4.0 (−1) | 9.45 | 41.50 | 50.95 |
| 5 | 18 (−1) | 15 (−1) | 10.0 (+1) | 7.64 | 35.66 | 43.30 |
| 6 | 42 (+1) | 15 (−1) | 10.0 (+1) | 8.54 | 39.55 | 48.10 |
| 7 | 18 (−1) | 45 (+1) | 10.0 (+1) | 7.63 | 35.89 | 43.52 |
| 8 | 42 (+1) | 45 (+1) | 10.0 (+1) | 9.12 | 40.51 | 49.63 |
| 9 | 10 (−1.67) | 30 (0) | 7.0 (0) | 7.21 | 34.47 | 41.68 |
| 10 | 50 (+1.67) | 30 (0) | 7.0 (0) | 8.98 | 40.20 | 49.18 |
| 11 | 30 (0) | 4.8 (−1.67) | 7.0 (0) | 8.13 | 39.69 | 47.82 |
| 12 | 30 (0) | 55.2 (+1.67) | 7.0 (0) | 8.83 | 40.18 | 49.00 |
| 13 | 30 (0) | 30 (0) | 1.9 (−1.67) | 8.79 | 39.14 | 47.93 |
| 14 | 30 (0) | 30 (0) | 12 (+1.67) | 7.69 | 36.62 | 44.30 |
| 15 | 30 (0) | 30 (0) | 7.0 (0) | 8.98 | 40.19 | 49.16 |
| 16 | 30 (0) | 30 (0) | 7.0 (0) | 9.29 | 40.87 | 50.16 |
| 17 | 30 (0) | 30 (0) | 7.0 (0) | 9.35 | 40.72 | 50.08 |
| 18 | 30 (0) | 30 (0) | 7.0 (0) | 9.48 | 41.32 | 50.80 |
| 19 | 30 (0) | 30 (0) | 7.0 (0) | 9.30 | 40.84 | 50.14 |
| Solvent | Yield of Extraction ± s (mganthocyanins·gdry biomass−1) | TPC ± s (mgGAE·gdry biomass−1) | ABTS ± s (μmolTE·gdry biomass−1) |
|---|---|---|---|
| Water | 41.8 ± 0.7 e | 104.1 ± 0.8 d | 1163 ± 21 d |
| 1,2-ethanediol | 49.60 ± 0.09 d | 107 ± 8 d | 1241 ± 8 bc |
| 1,2-propanediol | 50 ± 1 cd | 117 ± 5 c | 1302 ± 32 a |
| 1,2-butanediol | 51.0 ± 0.2 bcd | 130 ± 6 a | 1192 ± 16 cd |
| 1,2-pentanediol | 52.63 ± 0.05 bc | 127 ± 4 ab | 1267 ± 35 ab |
| 1,2-hexanediol | 55.5 ± 0.8 a | 126 ± 5 abc | 1302 ± 48 a |
| Ethanol | 49.0 ± 0.7 d | 120 ± 5 abc | 1229 ± 26 bc |
| (1.0.1) | 50 ± 1 d | 125 ± 11 abc | 1222 ± 13 bc |
| (2.0.2) | 49.8 ± 0.5 cd | 126 ± 1 abc | 1215 ± 25 bcd |
| (2.0.0) | 53.7 ± 0.4 ab | 119 ± 2 bc | 1255 ± 35 ab |
| Formulation | ABTS (μmolTE·gformulation−1) |
|---|---|
| Base bar (BB) | 6.6 ± 0.6 |
| Juçara bar (JB) | 16.0 ± 0.5 |
| Control Cream (CC) | 0.10 ± 0.01 |
| Juçara Cream (JC) | 1.5 ± 0.1 |
| Concentrated Juçara Cream (CJC) | 3.3 ± 0.2 |
| Compound | CAS Number | Source | Purity (wt%) |
|---|---|---|---|
| 1,2-ethanediol | 107-21-1 | Fisher Scientific (Geel, Belgium) | >99.0 |
| 1,2-propanediol | 57-55-6 | Sigma-Aldrich (Steinheim, Germany) | 99.5 |
| 1,2-butanediol | 584-03-2 | Sigma-Aldrich (Steinheim, Germany) | >98.0 |
| 1,2-pentanediol | 5343-92-0 | TCI (Zwijndrecht, Belgium) | 97.0 |
| 1,2-hexanediol | 6920-22-5 | Alfa Aesar (Kandel, Germany) | 97.0 |
| 1,3-dimethoxypropan-2-ol (1.0.1) | 623-69-8 | Synthesized (Aveiro, Portugal) | >99.0 |
| 1,3-diethoxypropan-2-ol (2.0.2) | 4043-59-8 | Synthesized (Aveiro, Portugal) | >99.0 |
| 3-Ethoxypropane-1,2-diol (2.0.0) | 1874-62-0 | Synthesized (Aveiro, Portugal) | >99.0 |
| 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) | 30931-67-0 | Sigma-Aldrich (Steinheim, Germany) | 98.0 |
| Acetone | 67-64-1 | Fisher Scientific (Geel, Belgium) | >99.0 |
| Acetonitrile | 75-05-8 | Fisher Scientific (Geel, Belgium) | 99.9 |
| Cyanidin 3-O-glucoside chloride | 7084-24-4 | Sigma-Aldrich (Steinheim, Germany) | >95.0 |
| Cyanidin-3-O-rutinoside chloride | 18719-76-1 | Sigma-Aldrich (Steinheim, Germany) | >98.0 |
| Ethanol | 64-17-5 | Fisher Scientific (Steinheim, Germany) | 99.8 |
| Folin–Ciocalteu reagent 2M | - | Panreac (Barcelona, Spain) | - |
| Gallic acid | 149-91-7 | Sigma-Aldrich (Steinheim, Germany) | 99.5 |
| Hydrochloric acid (HCl) | 7647-01-0 | Fisher Scientific (Steinheim, Germany) | 37.0 |
| Methanol | 67-56-1 | Fisher Scientific (Steinheim, Germany) | 99.8 |
| Potassium persulfate (K2S2O8) | 7727-21-1 | Scharlau (Barcelona, Spain) | 99.0 |
| Sodium carbonate (Na2CO3) | 497-19-8 | Prolabo (Geel, Belgium) | 99.0 |
| Sodium hydroxide (NaOH) | 1310-73-2 | Fisher Scientific (Steinheim, Germany) | 98.0 |
| Sulfuric acid (H2SO4) | 7664-93-9 | Sigma-Aldrich (Steinheim, Germany) | 95.0 |
| Trolox | 53188-07-1 | Acros Organics (Geel, Belgium) | 97.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, B.P.; Ferreira, A.M.; Justi, M.; Rodrigues, L.G.G.; Oliveira, J.V.; Pinho, S.P.; Coutinho, J.A.P. Juçara Fruit (Euterpe Edulis Martius) Valorization Combining Emergent Extraction Technologies and Aqueous Solutions of Alkanediols. Molecules 2023, 28, 1607. https://doi.org/10.3390/molecules28041607
Soares BP, Ferreira AM, Justi M, Rodrigues LGG, Oliveira JV, Pinho SP, Coutinho JAP. Juçara Fruit (Euterpe Edulis Martius) Valorization Combining Emergent Extraction Technologies and Aqueous Solutions of Alkanediols. Molecules. 2023; 28(4):1607. https://doi.org/10.3390/molecules28041607
Chicago/Turabian StyleSoares, Bruna P., Ana M. Ferreira, Marina Justi, Luiz Gustavo Gonçalves Rodrigues, J. Vladimir Oliveira, Simão P. Pinho, and João A. P. Coutinho. 2023. "Juçara Fruit (Euterpe Edulis Martius) Valorization Combining Emergent Extraction Technologies and Aqueous Solutions of Alkanediols" Molecules 28, no. 4: 1607. https://doi.org/10.3390/molecules28041607
APA StyleSoares, B. P., Ferreira, A. M., Justi, M., Rodrigues, L. G. G., Oliveira, J. V., Pinho, S. P., & Coutinho, J. A. P. (2023). Juçara Fruit (Euterpe Edulis Martius) Valorization Combining Emergent Extraction Technologies and Aqueous Solutions of Alkanediols. Molecules, 28(4), 1607. https://doi.org/10.3390/molecules28041607