Juice Powders from Rosehip (Rosa canina L.): Physical, Chemical, and Antiglycation Properties
Abstract
:1. Introduction
2. Results and Discussion
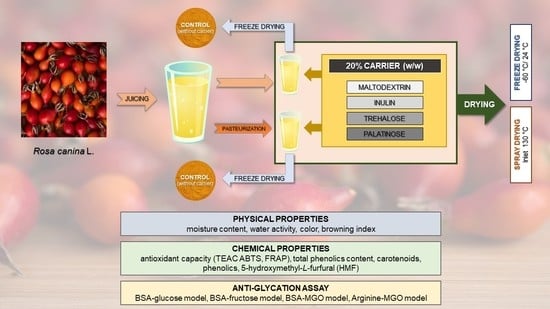
2.1. Rosehip Juice Powder Production by Freeze- and Spray Drying
2.2. Physical Properties
2.2.1. Moisture Content (MC)
2.2.2. Water Activity (aw)
2.2.3. Color and Browning Index (BI)
2.3. Phenolics and HMF
2.4. Carotenoids
2.5. Total Phenolic Content (TPC)
2.6. Antioxidant Capacity In Vitro
2.7. Antiglycation Properties
2.8. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Material
3.2. Chemicals and Reagents
3.3. Juicing
3.4. Freeze- and Spray-Drying
3.5. Physical Properties
3.5.1. Moisture Content (MC) and Dry Matter (DM)
3.5.2. Water Activity (aw)
3.5.3. Color and Browning Index (BI)
3.6. Antioxidant Capacity In Vitro
3.7. Total Phenolic Content (TPC)
3.8. Sample Preparation for Antiglycation Assays
3.9. Antiglycation Assay
3.9.1. BSA-Glucose/Fructose Model
3.9.2. BSA-MGO Model
3.9.3. L-arginine–MGO Model
3.10. Carotenoid Chromatographic Analysis
3.11. Phenolics and 5-Hydroxymethyl-L-furfural (HMF) Chromatographic Analysis
3.12. Statistical Analysis and Chemometrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nađpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anačkov, G.T.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Comparative Study of Biological Activities and Phytochemical Composition of Two Rose Hips and Their Preserves: Rosa Canina L. and Rosa Arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic Fruits as a Source of Important Phytochemicals: Improving the Traditional Use of Rosa Canina Fruits in Portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Fan, C.; Pacier, C.; Martirosyan, D.M. Rose Hip (Rosa Canina L): A Functional Food Perspective. Funct. Foods Health Dis. 2014, 4, 493–509. [Google Scholar] [CrossRef]
- Selahvarzian, A.; Alizadeh, A.; Baharvand, P.A.; Eldahshan, O.A.; Rasoulian, B. Medicinal Properties of Rosa Canina L. Herb. Med. J. 2018, 3, 77–84. [Google Scholar] [CrossRef]
- Patel, S. Rose Hip as an Underutilized Functional Food: Evidence-Based Review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Ozkan, G.; Esatbeyoglu, T.; Capanoglu, E. Bioavailability of Rosehip (Rosa canina L.) Infusion Phenolics Prepared by Thermal, Pulsed Electric Field and High Pressure Processing. Foods 2022, 11, 1955. [Google Scholar] [CrossRef]
- Igual, M.; García-Herrera, P.; Cámara, R.M.; Martínez-Monzó, J.; García-Segovia, P.; Cámara, M. Bioactive Compounds in Rosehip (Rosa Canina) Powder with Encapsulating Agents. Molecules 2022, 27, 4737. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Atalar, I.; Yilmaz, V.A.; Odabas, H.I.; Gul, O. Application of Multi Pass High Pressure Homogenization to Improve Stability, Physical and Bioactive Properties of Rosehip (Rosa canina L.) Nectar. Food Chem. 2019, 282, 67–75. [Google Scholar] [CrossRef]
- Sagdic, O.; Toker, O.S.; Polat, B.; Arici, M.; Yilmaz, M.T. Bioactive and Rheological Properties of Rose Hip Marmalade. J. Food Sci. Technol. 2015, 52, 6465–6474. [Google Scholar] [CrossRef]
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of Pasteurization Process and Storage on Color and Shelf-Life of Pomegranate Juices. LWT Food Sci. Technol. 2013, 54, 592–596. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of Wall Material Physicochemical Characteristics on the Stability of Encapsulated Phytochemicals: A Review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A.; Ciska, E.; Majerska, J. Chemometric Contribution for Deeper Understanding of Thermally-Induced Changes of Polyphenolics and the Formation of Hydroxymethyl-L-Furfural in Chokeberry Powders. Food Chem. 2021, 342, 128335. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.B. Chapter 6—Flavor Enhancement Ingredients. In Aging, Nutrition and Taste; Marcus, J.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 173–206. ISBN 978-0-12-813527-3. [Google Scholar]
- Del Castillo, M.D.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Gonzalez, I.; Medrano, A.; Filip, R.; Uribarri, J. Healthy Eating Recommendations: Good for Reducing Dietary Contribution to the Body’s Advanced Glycation/Lipoxidation End Products Pool? Nutr. Res. Rev. 2021, 34, 48–63. [Google Scholar] [CrossRef]
- Tripodo, G.; Mandracchia, D. Inulin as a Multifaceted (Active) Substance and Its Chemical Functionalization: From Plant Extraction to Applications in Pharmacy, Cosmetics and Food. Eur. J. Pharm. Biopharm. 2019, 141, 21–36. [Google Scholar] [CrossRef]
- Illippangama, A.U.; Jayasena, D.D.; Jo, C.; Mudannayake, D.C. Inulin as a Functional Ingredient and Their Applications in Meat Products. Carbohydr. Polym. 2022, 275, 118706. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, E.; Sadowska, A.; Sawicka, D.; Kotulska-Bąblińska, I.; Car, H. A Head-to-Head Comparison Review of Biological and Toxicological Studies of Isomaltulose, d-Tagatose, and Trehalose on Glycemic Control. Crit. Rev. Food Sci. Nutr. 2022, 62, 5679–5704. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Ciechanowska, A.; Hendrysiak, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A. How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts? Foods 2021, 10, 1864. [Google Scholar] [CrossRef]
- Nagai, R.; Shirakawa, J.-I.; Ohno, R.-I.; Moroishi, N.; Nagai, M. Inhibition of AGEs Formation by Natural Products. Amino Acids 2014, 46, 261–266. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of Extracts of Bioactive Compounds Obtained from Acerola (Malpighia Emarginata DC) Pulp and Residue by Spray and Freeze Drying: Chemical, Morphological and Chemometric Characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Michalska-Ciechanowska, A.; Turkiewicz, I.P.; Lech, K.; Nowicka, P. Influence Carrier Agents, Drying Methods, Storage Time on Physico-Chemical Properties and Bioactive Potential of Encapsulated Sea Buckthorn Juice Powders. Molecules 2020, 25, 3801. [Google Scholar] [CrossRef]
- Sawale, P.D.; Shendurse, A.M.; Mohan, M.S.; Patil, G.R. Isomaltulose (Palatinose)—An Emerging Carbohydrate. Food Biosci. 2017, 18, 46–52. [Google Scholar] [CrossRef]
- Michalska, A.; Lech, K. The Effect of Carrier Quantity and Drying Method on the Physical Properties of Apple Juice Powders. Beverages 2018, 4, 2. [Google Scholar] [CrossRef]
- Caliskan, G.; Dirim, S.N. The Effect of Different Drying Processes and the Amounts of Maltodextrin Addition on the Powder Properties of Sumac Extract Powders. Powder Technol. 2016, 287, 308–314. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of Spray Drying Conditions on the Physical Properties of Cagaita (Eugenia Dysenterica DC.) Fruit Extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Majerska, J.; Brzezowska, J.; Wojdyło, A.; Figiel, A. The Influence of Maltodextrin and Inulin on the Physico-Chemical Properties of Cranberry Juice Powders. ChemEngineering 2020, 4, 12. [Google Scholar] [CrossRef]
- Handbook of Food Preservation, 2nd ed.; Rahman, M.S. (Ed.) CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-19108-4. [Google Scholar]
- Da Silva Carvalho, A.G.; da Costa Machado, M.T.; da Silva, V.M.; Sartoratto, A.; Rodrigues, R.A.F.; Hubinger, M.D. Physical Properties and Morphology of Spray Dried Microparticles Containing Anthocyanins of Jussara (Euterpe Edulis Martius) Extract. Powder Technol. 2016, 294, 421–428. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Lech, K.; Figiel, A. Functional Relationships between Phytochemicals and Drying Conditions during the Processing of Blackcurrant Pomace into Powders. Adv. Powder Technol. 2017, 28, 1340–1348. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Jó, J.; de Oliveira, G.A.; Chaimsohn, F.P.; Sierakowski, M.R.; de Freitas, R.A.; Ribani, R.H. Microencapsulation of Juçara (Euterpe edulis M.) Pulp by Spray Drying Using Different Carriers and Drying Temperatures. Dry. Technol. 2015, 33, 153–161. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Honke, J.; Ciska, E.; Andlauer, W. Drying-Induced Physico-Chemical Changes in Cranberry Products. Food Chem. 2018, 240, 448–455. [Google Scholar] [CrossRef]
- Chikpah, S.K.; Korese, J.K.; Sturm, B.; Hensel, O. Colour Change Kinetics of Pumpkin (Cucurbita Moschata) Slices during Convective Air Drying and Bioactive Compounds of the Dried Products. J. Agric. Food Res. 2022, 10, 100409. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Barros, R.M.; Almeida, D.P.F.; Pintado, M. Peach Polyphenol and Carotenoid Content as Affected by Frozen Storage and Pasteurization. LWT Food Sci. Technol. 2016, 66, 361–368. [Google Scholar] [CrossRef]
- Ménabréaz, T.; Dorsaz, M.; Bocquel, D.; Udrisard, I.; Kosinska-Cagnazzo, A.; Andlauer, W. Goji Berry and Whey Protein Concentrate Enriched Rice Extrudates—Physical Properties and Accessibility of Bioactives. Pol. J. Food Nutr. Sci. 2021, 71, 29–37. [Google Scholar] [CrossRef]
- Margean, A.; Lupu, M.I.; Alexa, E.; Padureanu, V.; Canja, C.M.; Cocan, I.; Negrea, M.; Calefariu, G.; Poiana, M.-A. An Overview of Effects Induced by Pasteurization and High-Power Ultrasound Treatment on the Quality of Red Grape Juice. Molecules 2020, 25, 1669. [Google Scholar] [CrossRef] [PubMed]
- Astiti, M.A.; Jittmittraphap, A.; Leaungwutiwong, P.; Chutiwitoonchai, N.; Pripdeevech, P.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. LC-QTOF-MS/MS Based Molecular Networking Approach for the Isolation of α-Glucosidase Inhibitors and Virucidal Agents from Coccinia Grandis (L.) Voigt. Foods 2021, 10, 3041. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Lech, K.; Michalska-Ciechanowska, A.; Nowicka, P. The Influence of Different Carrier Agents and Drying Techniques on Physical and Chemical Characterization of Japanese Quince (Chaenomeles Japonica) Microencapsulation Powder. Food Chem. 2020, 323, 126830. [Google Scholar] [CrossRef] [PubMed]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Evaluation of Different Methods for the Production of Juice Concentrates and Fruit Powders from Cactus Pear. Innov. Food Sci. Emerg. Technol. 2006, 7, 275–287. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Figiel, A. Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa Spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The Use of Food By-Products as a Novel for Functional Foods: Their Use as Ingredients and for the Encapsulation Process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Manickavasagan, A.; Al-Ismaili, A.M.; Al-Mulla, Y.A. Effect of Carrier Agents on Physicochemical Properties of Foam-Mat Freeze-Dried Date Powder. Dry. Technol. 2018, 36, 1292–1303. [Google Scholar] [CrossRef]
- Romero-González, J.; Shun Ah-Hen, K.; Lemus-Mondaca, R.; Muñoz-Fariña, O. Total Phenolics, Anthocyanin Profile and Antioxidant Activity of Maqui, Aristotelia Chilensis (Mol.) Stuntz, Berries Extract in Freeze-Dried Polysaccharides Microcapsules. Food Chem. 2020, 313, 126115. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Lin, X.; Bu, C.; Zhang, X. Role of Advanced Glycation End Products in Mobility and Considerations in Possible Dietary and Nutritional Intervention Strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Wang, Y.; Zhang, X.; Jia, H.; Guo, L.; Huang, L.; Gao, W. Comparative Studies on Characterization, Saccharide Mapping and Antiglycation Activity of Polysaccharides from Different Polygonatum Ssp. J. Pharm. Biomed. Anal. 2020, 186, 113243. [Google Scholar] [CrossRef]
- Wang, W.; Yagiz, Y.; Buran, T.J.; do Nascimento Nunes, C.; Gu, L. Phytochemicals from Berries and Grapes Inhibited the Formation of Advanced Glycation End-products by Scavenging Reactive Carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory Effect of Phenolic Compounds and Plant Extracts on the Formation of Advance Glycation End Products: A Comprehensive Review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative Effect of Fruit and Vegetable Seed Extracts: Inhibition of AGE Formation and Carbonyl-Trapping Abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef]
- Ho, S.-C.; Chang, P.-W.; Tong, H.-T.; Yu, P.-Y. Inhibition of Fluorescent Advanced Glycation End-Products and N-Carboxymethyllysine Formation by Several Floral Herbal Infusions. Int. J. Food Prop. 2014, 17, 617–628. [Google Scholar] [CrossRef]
- Figiel, A. Drying Kinetics and Quality of Beetroots Dehydrated by Combination of Convective and Vacuum-Microwave Methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of Colour Change of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Andlauer, W. Characterisation of Bioactive Compounds in Berry Juices by Traditional Photometric and Modern Microplate Methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Jolliffe, I.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; New York, NY, USA, 2022. Available online: https://www.xlstat.com/en (accessed on 15 December 2022).

| Type of Juice | Carrier | Inlet Air Temperature | Outlet Air Temperature | Volume Flow | Feed Flow |
|---|---|---|---|---|---|
| Non-pasteurized | Maltodextrin | 130 °C | 82 °C | 26 m3/h | 4 mL/m3 |
| Inulin | 82 °C | 35 m3/h | |||
| Trehalose | 74 °C | 26 m3/h | |||
| Palatinose | 77 °C | 26 m3/h | |||
| Pasteurized | Maltodextrin | 130 °C | 82 °C | 35 m3/h | 4 mL/m3 |
| Inulin | 84 °C | 35 m3/h | |||
| Trehalose | 83 °C | 35 m3/h | |||
| Palatinose | 77 °C | 26 m3/h |
| Type of Juice | Drying Technique | Carrier | MC | aw | Color | BI | ||
|---|---|---|---|---|---|---|---|---|
| (%) | (-) | L* | a* | b* | (AU) | |||
| Non-Pasteurized | FD | (-) | 4.82 ± 0.46 d | 0.24 ± 0.01 e | 79.57 ± 0.18 cde | 6.39 ± 0.04 efg | 31.52 ± 0.02 g | 0.40 ± 0.00 ef |
| M | 7.10 ± 0.34 f | 0.38 ± 0.00 h | 81.50 ± 0.59 def | 7.41 ± 0.45 gh | 30.30 ± 1.16 g | 0.40 ± 0.01 de | ||
| I | 2.44 ± 0.35 abc | 0.11 ± 0.01 b | 89.26 ± 0.13 hi | 1.86 ± 0.02 a–d | 19.43 ± 0.43 cd | 0.35 ± 0.00 abc | ||
| T | 6.96 ± 0.01 ef | 0.34 ± 0.00 g | 63.41 ± 5.35 a | 5.32 ± 0.90 e | 26.10 ± 2.74 f | 0.41 ± 0.01 ef | ||
| P | 6.51 ± 0.24 ef | 0.30 ± 0.00 f | 70.84 ± 0.64 b | 7.11 ± 0.42 fgh | 30.75 ± 1.32 g | 0.42 ± 0.01 f | ||
| SD | M | 2.33 ± 0.18 abc | 0.18 ± 0.00 cd | 90.61 ± 1.11 hi | 0.90 ± 0.16 a | 13.76 ± 0.96 a | 0.34 ± 0.00 a | |
| I | 2.12 ± 0.37 ab | 0.11 ± 0.01 b | 91.81 ± 0.22 i | 1.63 ± 0.02 abc | 16.15 ± 0.09 a–d | 0.34 ± 0.00 ab | ||
| T | 2.71 ± 0.08 abc | 0.16 ± 0.00 c | 90.75 ± 2.40 hi | 2.25 ± 0.86 a-d | 16.11 ± 2.04 abc | 0.35 ± 0.01 ab | ||
| P | 2.37 ± 0.08 abc | 0.18 ± 0.00 cd | 81.81 ± 0.96 def | 1.81 ± 0.02 a-d | 15.39 ± 0.11 ab | 0.35 ± 0.00 ab | ||
| Pasteurized | FD | (-) | 4.80 ± 0.35 d | 0.24 ± 0.00 e | 75.94 ± 1.61 c | 7.46 ± 0.27 gh | 30.88 ± 0.27 g | 0.41 ± 0.00 ef |
| M | 6.41 ± 0.19 ef | 0.37 ± 0.00 h | 79.12 ± 1.10 cd | 5.75 ± 0.63 ef | 25.29 ± 1.99 f | 0.38 ± 0.01 d | ||
| I | 2.94 ± 0.19 bc | 0.07 ± 0.01 a | 84.02 ± 0.07 efg | 2.83 ± 0.06 cd | 23.22 ± 0.18 ef | 0.37 ± 0.00 c | ||
| T | 6.73 ± 0.06 ef | 0.30 ± 0.00 f | 75.86 ± 0.26 c | 7.89 ± 0.01 h | 30.73 ± 0.08 g | 0.41 ± 0.00 ef | ||
| P | 6.12 ± 0.16 e | 0.24 ± 0.00 e | 74.82 ± 0.41 bc | 8.11 ± 0.27 h | 30.98 ± 0.72 g | 0.41 ± 0.00 ef | ||
| SD | M | 1.81 ± 0.06 a | 0.12 ± 0.01 b | 86.93 ± 1.48 fgh | 3.04 ± 0.36 d | 18.40 ± 1.24 bcd | 0.35 ± 0.00 abc | |
| I | 2.29 ± 0.13 abc | 0.10 ± 0.00 b | 83.31 ± 4.84 d–g | 1.25 ± 0.07 ab | 16.49 ± 0.51 a-d | 0.35 ± 0.00 ab | ||
| T | 1.79 ± 0.18 a | 0.11 ± 0.00 b | 89.06 ± 1.29 ghi | 2.24 ± 0.34 bcd | 17.44 ± 0.67 bcd | 0.35 ± 0.00 ab | ||
| P | 3.18 ± 0.09 c | 0.18 ± 0.00 d | 87.77 ± 1.00 ghi | 3.04 ± 0.14 d | 19.76 ± 0.41 de | 0.36 ± 0.00 bc | ||
| Type of Juice | Drying Technique | Carrier | Catechin | Gallic Acid | Hydroxybenzoic Acid | Rutin | Sum of Phenolics | HMF | Carotenoids |
|---|---|---|---|---|---|---|---|---|---|
| (mg/g DM) | (µg/kg DM) | ||||||||
| Non-Pasteurized | FD | (-) | 2.28 ± 0.19 bc | 0.32 ± 0.03 d | 0.14 ± 0.01 e | 0.07 ± 0.01 fg | 2.81 ± 0.24 bc | nd | 140 |
| M | 1.10 ± 0.04 a | 0.13 ± 0.01 a | 0.05 ± 0.00 a | 0.04 ± 0.00 ab | 1.31 ± 0.05 | nd | nd | ||
| I | 2.26 ± 0.09 bc | 0.21 ± 0.01 c | 0.09 ± 0.00 c | 0.04 ± 0.01 ab | 2.60 ± 0.07 bc | nd | nd | ||
| T | 0.95 ± 0.02 a | 0.13 ± 0.01 a | 0.04 ± 0.00 a | 0.03 ± 0.00 ab | 1.15 ± 0.03 a | nd | nd | ||
| P | 0.98 ± 0.07 a | 0.14 ± 0.01 a | 0.04 ± 0.00 a | 0.05 ± 0.01 bc | 1.20 ± 0.08 a | nd | nd | ||
| SD | M | 1.33 ± 0.00 a | 0.14 ± 0.02 ab | 0.05 ± 0.00 ab | 0.03 ± 0.00 ab | 1.56 ± 0.01 a | nd | nd | |
| I | 2.51 ± 0.06 cd | 0.21 ± 0.00 bc | 0.09 ± 0.00 c | 0.04 ± 0.00 bc | 2.84 ± 0.06 cd | nd | nd | ||
| T | 1.32 ± 0.00 a | 0.13 ± 0.02 a | 0.05 ± 0.00 ab | 0.02 ± 0.00 a | 1.53 ± 0.01 a | nd | nd | ||
| P | 1.22 ± 0.04 a | 0.14 ± 0.01 a | 0.05 ± 0.00 a | 0.04 ± 0.00 ab | 1.44 ± 0.04 a | nd | nd | ||
| Pasteurized | FD | (-) | 2.76 ± 0.22 d | 0.33 ± 0.05 d | 0.11 ± 0.01 d | 0.09 ± 0.01 ab | 3.29 ± 0.29 d | nd | nd |
| M | 1.09 ± 0.04 a | 0.10 ± 0.01 a | 0.04 ± 0.00 a | 0.03 ± 0.00 g | 1.26 ± 0.05 a | nd | nd | ||
| I | 2.10 ± 0.14 b | 0.15 ± 0.01 abc | 0.07 ± 0.00 bc | 0.06 ± 0.00 cde | 2.37 ± 0.15 b | nd | nd | ||
| T | 1.06 ± 0.08 a | 0.10 ± 0.01 a | 0.04 ± 0.00 a | 0.06 ± 0.00 def | 1.26 ± 0.09 a | nd | nd | ||
| P | 1.05 ± 0.07 a | 0.09 ± 0.01 a | 0.04 ± 0.00 a | 0.07 ± 0.01 efg | 1.26 ± 0.08 a | nd | nd | ||
| SD | M | 1.27 ± 0.05 a | 0.13 ± 0.00 a | 0.05 ± 0.00 ab | 0.03 ± 0.00 ab | 1.49 ± 0.05 a | nd | nd | |
| I | 2.11 ± 0.11 bc | 0.15 ± 0.02 abc | 0.09 ± 0.01 c | 0.05 ± 0.01 bcd | 2.39 ± 0.13 bc | nd | nd | ||
| T | 1.29 ± 0.12 a | 0.14 ± 0.00 ab | 0.05 ± 0.00 ab | 0.02 ± 0.00 a | 1.51 ± 0.11 a | nd | nd | ||
| P | 1.25 ± 0.10 a | 0.05 ± 0.00 a | 1.25 ± 0.10 a | 0.02 ± 0.00 a | 1.44 ± 0.10 a | nd | nd | ||
| Type of Juice | Drying Technique | Carrier | TPC | TEAC ABTS | FRAP |
|---|---|---|---|---|---|
| (g Gallic Acid/ 100 g DM) | (mmol Trolox/ 100 g DM) | ||||
| Non-Pasteurized | FD | (-) | 6.98 ± 0.71 h | 48.58 ± 1.50 h | 35.90 ± 0.57 d |
| Maltodextrin | 2.13 ± 0.14 a–e | 15.48 ± 0.13 ab | 12.14 ± 1.39 ab | ||
| Inulin | 1.84 ± 0.54 ab | 22.88 ± 0.90 def | 19.00 ± 0.35 c | ||
| Trehalose | 1.34 ± 0.21 a | 24.45 ± 0.64 ef | 21.49 ± 0.35 c | ||
| Palatinose | 2.37 ± 0.30 a–f | 24.38 ± 0.94 ef | 19.50 ± 1.05 c | ||
| SD | Maltodextrin | 2.03 ± 0.54 abc | 18.29 ± 0.59 c | 14.51 ± 0.81 b | |
| Inulin | 2.21 ± 0.32 a-e | 22.93 ± 0.49 def | 20.18 ± 0.25 c | ||
| Trehalose | 2.35 ± 0.21 a-g | 24.75 ± 0.02 ef | 19.97 ± 0.01 c | ||
| Palatinose | 3.74 ± 0.34 fg | 24.28 ± 0.14 ef | 20.61 ± 0.24 c | ||
| Pasteurized | FD | (-) | 6.52 ± 0.26 h | 44.50 ± 0.35 g | 34.46 ± 1.10 d |
| Maltodextrin | 3.36 ± 0.20 efg | 17.10 ± 1.16 bc | 13.85 ± 0.51 ab | ||
| Inulin | 3.77 ± 0.12 g | 21.72 ± 0.05 d | 19.30 ± 0.17 c | ||
| Trehalose | 3.17 ± 0.07 c–g | 22.70 ± 0.16 def | 19.21 ± 0.16 c | ||
| Palatinose | 3.53 ± 0.27 efg | 23.40 ± 0.51 def | 20.67 ± 1.19 c | ||
| SD | Maltodextrin | 1.90 ± 0.28 a–d | 14.30 ± 0.69 a | 11.60 ± 0.05 a | |
| Inulin | 2.89 ± 0.16 b–g | 22.11 ± 0.23 de | 18.83 ± 0.16 c | ||
| Trehalose | 2.70 ± 0.48 b–g | 23.78 ± 0.34 def | 20.31 ± 0.06 c | ||
| Palatinose | 3.24 ± 0.26 d–g | 22.88 ± 0.24 def | 19.24 ± 1.17 c | ||
| Type of Juice | Drying Technique | Carrier | BSA-glucose | BSA-fructose | BSA-MGO | L-arginine–MGO |
|---|---|---|---|---|---|---|
| Fluorescent AGE Inhibition [%] | ||||||
| Non-Pasteurized | FD | (-) | 60.24 ± 0.99 b | 88.00 ± 0.03 bcd | 38.03 ± 0.85 g | 44.61 ± 0.22 c |
| M | 62.54 ± 0.65 bc | 87.10 ± 0.05 be | 30.02 ± 0.60 cde | 14.61 ± 0.55 ab | ||
| I | 65.54 ± 0.94 c | 87.47 ± 0.08 bcd | 29.87 ± 1.22 cde | 15.64 ± 1.32 ab | ||
| T | 63.73 ± 0.50 bc | 87.36 ± 0.17 bcd | 28.06 ± 0.99 c | 18.87 ± 1.54 b | ||
| P | 64.40 ± 0.79 c | 87.37 ± 0.23 bcd | 30.51 ± 0.59 c–f | 20.50 ± 0.81 b | ||
| SD | M | 62.39 ± 0.12 bc | 86.77 ± 0.59 b | 31.20 ± 0.29 c–f | 18.22 ± 1.29 ab | |
| I | 64.99 ± 0.76 c | 88.43 ± 0.66 cd | 35.13 ± 1.01 fg | 24.01 ± 1.11 b | ||
| T | 63.42 ± 1.23 bc | 87.52 ± 0.16 bcd | 27.85 ± 1.59 c | 19.36 ± 0.31 b | ||
| P | 64.78 ± 0.33 c | 87.60 ± 0.17 bcd | 28.57 ± 0.08 cd | 22.39 ± 0.17 b | ||
| Pasteurized | FD | (-) | 62.42 ± 1.07 bc | 88.72 ± 0.23 d | 43.33 ± 1.06 h | 46.75 ± 0.44 c |
| M | 62.33 ± 0.89 bc | 87.07 ± 0.09 be | 28.77 ± 0.38 cd | 14.78 ± 1.03 ab | ||
| I | 64.65 ± 1.42 c | 86.87 ± 0.84 b | 29.82 ± 4.05 cde | 18.04 ± 0.44 ab | ||
| T | 64.80 ± 0.15 c | 87.64 ± 0.05 bcd | 33.38 ± 1.12 d–g | 21.74 ± 2.36 b | ||
| P | 65.43 ± 0.14 c | 87.90 ± 0.00 bcd | 31.14 ± 0.08 c–f | 20.74 ± 0.65 b | ||
| SD | M | 64.51 ± 0.11 c | 87.83 ± 0.06 bcd | 33.8 ± 1.49 efg | 17.42 ± 2.65 ab | |
| I | 64.37 ± 0.89 c | 86.97 ± 0.88 be | 20.43 ± 0.31 b | 23.62 ± 0.97 ab | ||
| T | 63.43 ± 0.55 bc | 87.82 ± 0.26 bcd | 32.61 ± 0.06 c–f | 21.40 ± 1.78 b | ||
| P | 65.81 ± 0.35 c | 88.14 ± 0.09 bcd | 33.43 ± 0.62 d–g | 22.34 ± 2.13 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrysiak, A.; Brzezowska, J.; Nicolet, N.; Bocquel, D.; Andlauer, W.; Michalska-Ciechanowska, A. Juice Powders from Rosehip (Rosa canina L.): Physical, Chemical, and Antiglycation Properties. Molecules 2023, 28, 1674. https://doi.org/10.3390/molecules28041674
Hendrysiak A, Brzezowska J, Nicolet N, Bocquel D, Andlauer W, Michalska-Ciechanowska A. Juice Powders from Rosehip (Rosa canina L.): Physical, Chemical, and Antiglycation Properties. Molecules. 2023; 28(4):1674. https://doi.org/10.3390/molecules28041674
Chicago/Turabian StyleHendrysiak, Aleksandra, Jessica Brzezowska, Nancy Nicolet, Dimitri Bocquel, Wilfried Andlauer, and Anna Michalska-Ciechanowska. 2023. "Juice Powders from Rosehip (Rosa canina L.): Physical, Chemical, and Antiglycation Properties" Molecules 28, no. 4: 1674. https://doi.org/10.3390/molecules28041674
APA StyleHendrysiak, A., Brzezowska, J., Nicolet, N., Bocquel, D., Andlauer, W., & Michalska-Ciechanowska, A. (2023). Juice Powders from Rosehip (Rosa canina L.): Physical, Chemical, and Antiglycation Properties. Molecules, 28(4), 1674. https://doi.org/10.3390/molecules28041674