Antimalarial Activity of Tri- and Tetra-Substituted Anilino Pyrazoles
Abstract
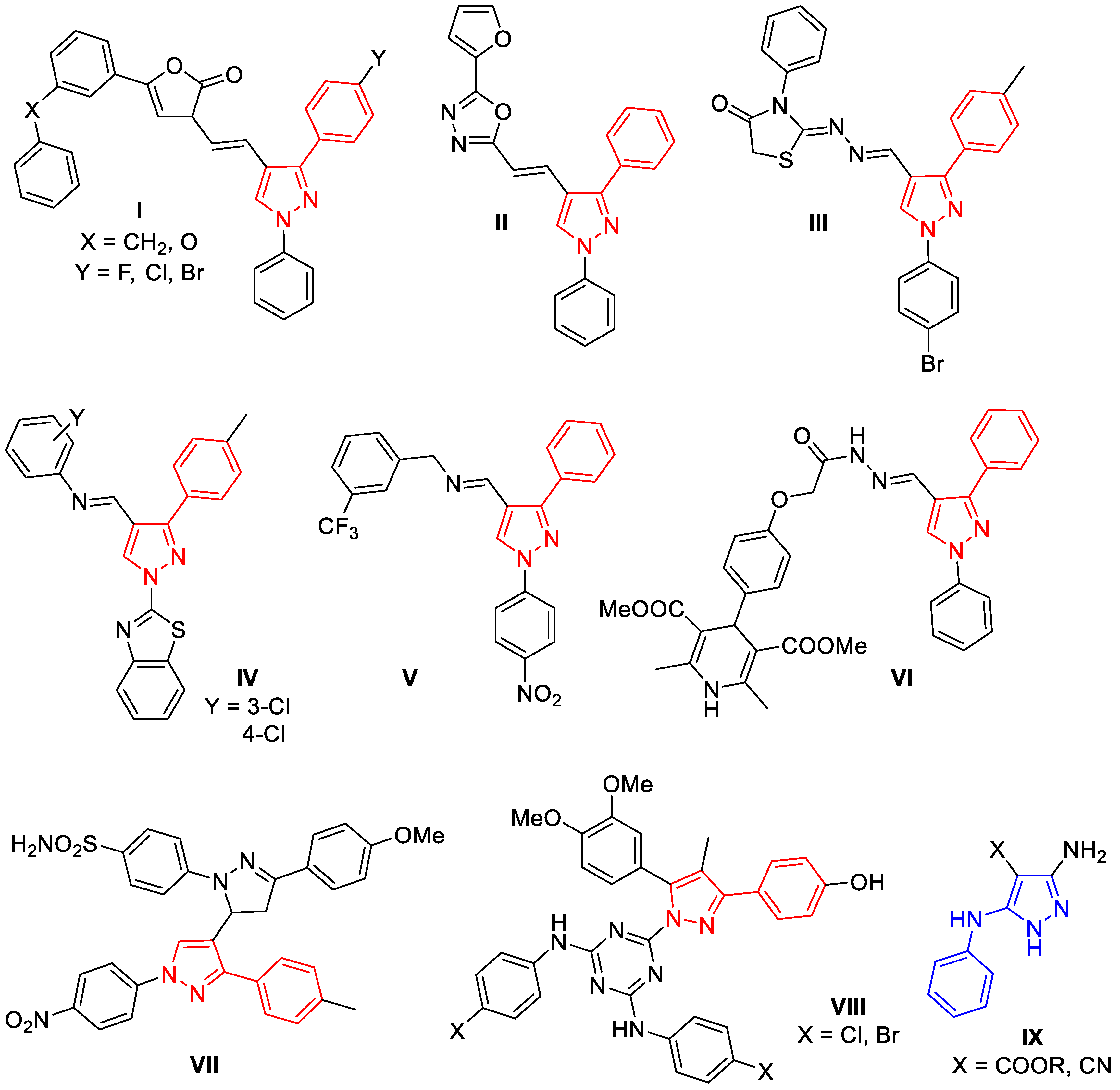
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antiplasmodial Activity
2.3. Pharmacokinetic Properties and Drug-Likeness Prediction
3. Materials and Methods
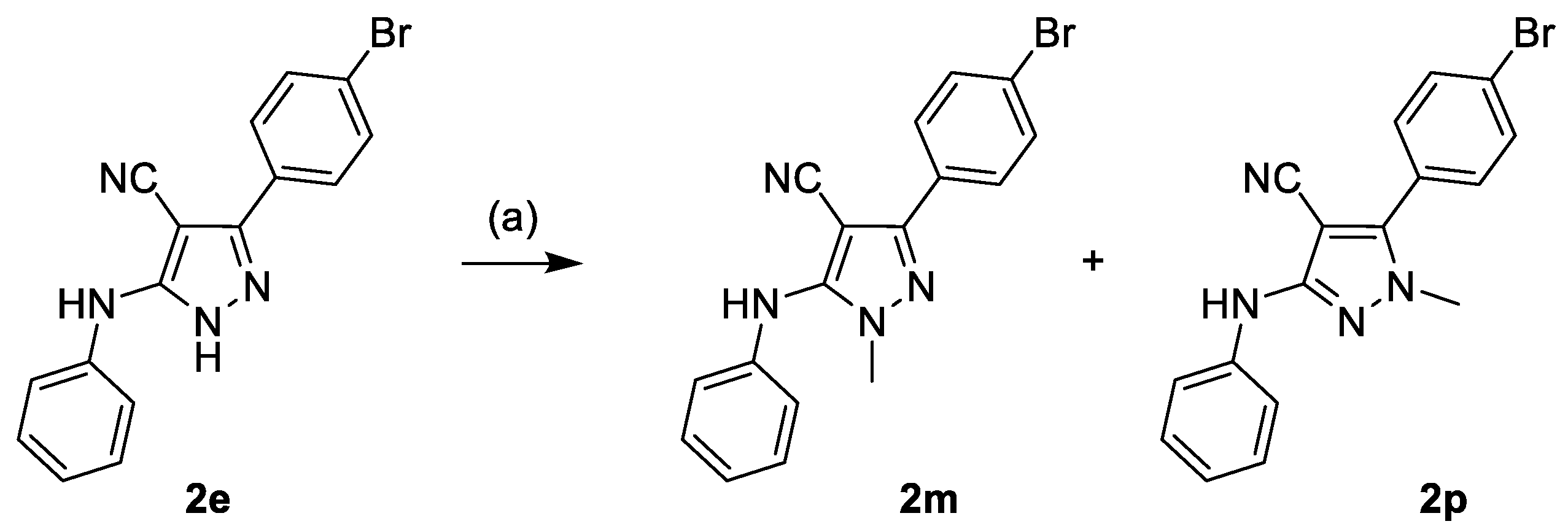
3.1. Chemistry
Synthesis of Compounds 2m and 2p
3.2. Plasmodium Cultures and Compound Susceptibility Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. World Malaria Report; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. WHO Guidelines for Malaria, 31 March 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Patel, P.; Bharti, P.K.; Bansal, D.; Ali, N.A.; Raman, R.K.; Mohapatra, P.K.; Sehgal, R.; Mahanta, J.; Sultan, A.A.; Singh, N. Prevalence of mutations linked to antimalarial resistance in Plasmodium falciparum from Chhattisgarh, Central India: A malaria elimination point of view. Sci. Rep. 2017, 7, 16690. [Google Scholar] [CrossRef] [PubMed]
- Menard, D.; Dondorp, A. Antimalarial drug resistance: A threat to malaria elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030, 2021 Update; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa, I.A., Jr.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; Castro, P.F.S.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic compounds: Pharmacology of pyrazole analogs from rational structural considerations. Front. Pharmacol. 2021, 12, 666725. [Google Scholar] [CrossRef]
- Hill, B.T.; Whelan, R.D.H. Antitumour activity and cell kinetic effects of pyrazofurin in vitro. Eur. J. Cancer 1980, 16, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, T.; Nabeshima, T.; Yoshida, N.; Yamaguchi, K. Neurochemical studies of an analgesic, 1,3-diphenyl-5-(2-dimethylaminopropionamide)-pyrazole [difenamizole]. Res. Commun. Chem. Pathol. Pharmacol. 1981, 31, 31–53. [Google Scholar]
- Clemett, D.; Goa, K.L. Celecoxib. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.; Tomlinson, B.; Taheri, S.; Thomas, G. Rimonabant for the treatment of obesity. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Straube, S. Anti-inflammatory and antipyretic analgesics and drugs used in gout. Side Effects Drugs Annu. 2012, 34, 181–193. [Google Scholar] [CrossRef]
- Dopp, J.M.; Agapitov, A.V.; Sinkey, C.A.; Haynes, W.G.; Phillips, B.G. Sildenafil increases sympathetically mediated vascular tone in humans. Am. J. Hypertens. 2013, 26, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Mitou, G.; Frentzel, J.; Desquesnes, A.; Le Gonidec, S.; AlSaati, T.; Beau, I.; Lamant, L.; Meggetto, F.; Espinos, E.; Codogno, P.; et al. Targeting autophagy enhances the anti-tumoral action of crizotinib in ALK-positive anaplastic large cell lymphoma. Oncotarget 2015, 6, 30149–30164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.; Al-Aizari, F.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Choudhary, D.; Rani, I.; Monga, J.; Goyal, R.; Husain, A.; Garg, P.; Khokra, S.L. Pyrazole based furanone hybrids as novel antimalarial: A combined experimental, pharmacological and computational study. Cent. Nerv. Syst. Agents Med. Chem. 2022, 22, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Khan, M.F.; Nainwal, L.M.; Ishaq, M.; Akhter, M.; Bakht, A.; Anwer, T.; Afrin, F.; Islamuddin, M.; Husain, I.; et al. Targeting malaria and leishmaniasis: Synthesis and pharmacological evaluation of novel pyrazole-1,3,4-oxadiazole hybrids. Part II. Bioorg. Chem. 2019, 89, 102986–102997. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Hassan, A.M.; Abd El Razik, H.A.; El-Miligy, M.M.; El-Agroudy, E.J.; Bekhit, A.D. New heterocyclic hybrids of pyrazole and its bioisosteres: Design, synthesis and biological evaluation as dual acting antimalarial-antileishmanial agents. Eur. J. Med. Chem. 2015, 94, 30–44. [Google Scholar] [CrossRef]
- Aggarwal, S.; Paliwal, D.; Kaushik, D.; Gupta, G.K.; Kumar, A. Pyrazole Schiff base hybrids as anti-malarial agents: Synthesis, in vitro screening and computational study. Comb. Chem. High Throughput Screen. 2018, 21, 194–203. [Google Scholar] [CrossRef]
- Rathelot, P.; Azas, N.; El-Kashef, H.; Delmas, F.; Di Giorgio, C.; Timon-David, P.; Maldonado, J.; Vanelle, P. 1,3-Diphenylpyrazoles: Synthesis and antiparasitic activities of azomethine derivatives. Eur. J. Med. Chem. 2002, 37, 671–679. [Google Scholar] [CrossRef]
- Kumar, P.; Kadyan, K.; Duhan, M.; Sindhu, J.; Singh, V.; Saharan, B.S. Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents. Chem. Cent. J. 2017, 11, 115–128. [Google Scholar] [CrossRef]
- Kumar, G.; Tanwar, O.; Kumar, J.; Akhter, M.; Sharma, S.; Pillai, C.R.; Alam, M.M.; Zama, M.S. Pyrazole-pyrazoline as promising novel antimalarial agents: A mechanistic study. Eur. J. Med. Chem. 2018, 149, 139–147. [Google Scholar] [CrossRef]
- Gogoi, P.; Shakya, A.; Ghosh, S.K.; Gogoi, N.; Gahtori, P.; Singh, N.; Bhattacharyya, D.R.; Singh, U.P.; Bhat, H.R. In silico study, synthesis, and evaluation of the antimalarial activity of hybrid dimethoxy pyrazole 1,3,5-triazine derivatives. J. Biochem. Mol. Toxicol. 2021, 35, e22682. [Google Scholar] [CrossRef]
- Domínguez, J.N.; Charris, J.E.; Caparelli, M.; Riggione, F. Synthesis and antimalarial activity of substituted pyrazole derivatives. Arzneimittelforschung 2002, 52, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Gupta, V.; Nema, R.K.; Misra, U. Synthesis of some substituted pyrazole derivatives and their evaluation as antiprotozoal agents. Int. J. Chem. Sci. 2008, 6, 179–184. [Google Scholar]
- Lusardi, M.; Rotolo, C.; Ponassi, M.; Iervasi, E.; Rosano, C.; Spallarossa, A. One-pot synthesis and antiproliferative activity of highly functionalized pyrazole derivatives. ChemMedChem 2022, 17, e202100670. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.; Profumo, A.; Rotolo, C.; Iervasi, E.; Rosano, C.; Spallarossa, A.; Ponassi, M. Regioselective synthesis, structural characterization, and antiproliferative activity of novel tetra-substituted phenylaminopyrazole derivatives. Molecules 2022, 27, 5814. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717–42729. [Google Scholar] [CrossRef] [PubMed]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Teague, S.; Davis, A.; Leeson, P.; Oprea, T. The design of leadlike combinatorial libraries. Angew. Chem. Int. Ed. 1999, 38, 3743–3748. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef]

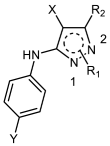 | ||||||
|---|---|---|---|---|---|---|
| IC50 (µM) a | ||||||
| Cpd | R1 | R2 | X | Y | D10 | W2 |
| 2a | H | C6H5 | CN | H | 65.60 | 52.96 |
| 2b | H | (3-Br)C6H4 | CN | H | 27.77 | 21.03 |
| 2c | H | (4-F)C6H4 | CN | H | 57.84 | 42.97 |
| 2d | H | (4-Cl)C6H4 | CN | H | 34.27 | 32.69 |
| 2e | H | (4-Br)C6H4 | CN | H | 19.75 | 19.87 |
| 2f | H | 2-thienyl | CN | H | NA | NA |
| 2g | H | (4-Cl)C6H4 | CN | NO2 | NA | NA |
| 2h | H | (4-Cl)C6H4 | CN | OMe | NA | NA |
| 2i | H | C6H5 | COPh | H | NA | NA |
| 2j | H | C6H5 | COOEt | H | 54.48 | 38.25 |
| 2k | H | (4-NO2)C6H5 | COOEt | H | 25.67 | 18.74 |
| 2l | (1)-Me | C6H5 | CN | H | 18.08 | 12.14 |
| 2m | (1)-Me | (4-Br)C6H4 | CN | H | NA | 46.64 |
| 2n | (1)-Me | C6H5 | COPh | H | 36.29 | 30.43 |
| 2o | (1)-CH2C6H5 | C6H5 | CN | H | 51.13 | 51.85 |
| 2p | (2)-Me | (4-Br)C6H4 | CN | H | NA | 44.67 |
| CQ | 0.03 | 0.47 | ||||
| 2b | 2e | 2k | 2l | |
|---|---|---|---|---|
| Physicochemical prop. | ||||
| MW (g/mol) | 339.19 | 339.19 | 352.34 | 274.32 |
| Fraction Csp3 | 0.00 | 0.00 | 0.11 | 0.06 |
| Rotatable bonds | 3 | 3 | 7 | 3 |
| H-bond acceptors | 2 | 2 | 5 | 2 |
| H-bond donors | 2 | 2 | 2 | 1 |
| TPSA a (Å2) | 64.50 | 64.0 | 112.83 | 53.64 |
| Lipophilicity | ||||
| LogP b | 4.71 | 4.71 | 4.35 | 4.04 |
| Water solubility | ||||
| Solubility (mg/mL) c | 0.0017 | 0.0017 | 0.0058 | 0.0037 |
| Pharmacokinetics | ||||
| GI absorption | High | High | High | High |
| BBB permeant | Yes | Yes | No | Yes |
| P-gp substrate | No | No | No | No |
| CYP1A2 inhibitor | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes |
| CYP2C9 inhibitor | Yes | Yes | Yes | Yes |
| CYP2D6 inhibitor | Yes | Yes | No | Yes |
| CYP3A4 inhibitor | Yes | Yes | No | Yes |
| Druglikeness | ||||
| Lipinski violations | 0 | 0 | 0 | 0 |
| Medicinal chemistry | ||||
| PAINS alerts | 0 | 0 | 0 | 0 |
| Brenk alerts | 0 | 0 | 1 | 0 |
| Leadlikeness violations | 1 | 1 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lusardi, M.; Basilico, N.; Rotolo, C.; Parapini, S.; Spallarossa, A. Antimalarial Activity of Tri- and Tetra-Substituted Anilino Pyrazoles. Molecules 2023, 28, 1712. https://doi.org/10.3390/molecules28041712
Lusardi M, Basilico N, Rotolo C, Parapini S, Spallarossa A. Antimalarial Activity of Tri- and Tetra-Substituted Anilino Pyrazoles. Molecules. 2023; 28(4):1712. https://doi.org/10.3390/molecules28041712
Chicago/Turabian StyleLusardi, Matteo, Nicoletta Basilico, Chiara Rotolo, Silvia Parapini, and Andrea Spallarossa. 2023. "Antimalarial Activity of Tri- and Tetra-Substituted Anilino Pyrazoles" Molecules 28, no. 4: 1712. https://doi.org/10.3390/molecules28041712
APA StyleLusardi, M., Basilico, N., Rotolo, C., Parapini, S., & Spallarossa, A. (2023). Antimalarial Activity of Tri- and Tetra-Substituted Anilino Pyrazoles. Molecules, 28(4), 1712. https://doi.org/10.3390/molecules28041712







