Alkyl Gallates as Potential Antibiofilm Agents: A Review
Abstract
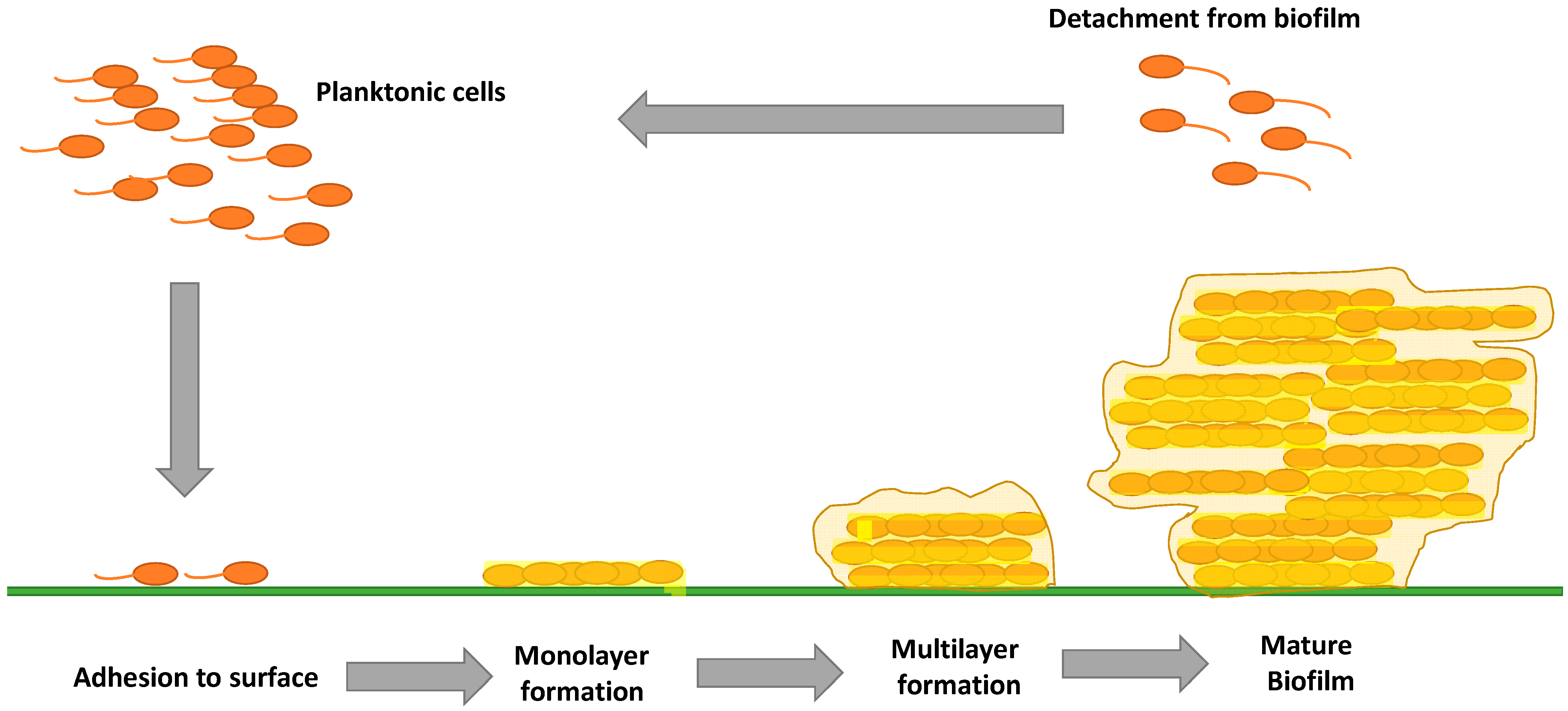
:1. Introduction
2. Gallic Acid
3. Methyl Gallate
4. Ethyl Gallate
5. Propyl Gallate
6. Butyl Gallate
7. Hexyl Gallate
8. Octyl Gallate
9. Dodecyl Gallate
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vestby, L.K.; Gronseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121 (Suppl. 136)), 1–51. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Kalesinskas, P.; Masalha, M.; Falah, M.; Abu-Farich, B.; Melninkaitis, A.; Zeidan, M.; Rayan, A. Inhibitory Effects of Ethyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry and Gene Expression Analysis. Molecules 2019, 24, 529. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; Zeidan, M.; et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Kouidhi, B.; Al Qurashi, Y.M.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Rayan, M.; Masalha, M.; Abu-Farich, B.; Al-Jaas, H.; Abu-Lafi, M.; Rayan, A. Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masalha, M.; Rayan, M.; Adawi, A.; Abdallah, Z.; Rayan, A. Capturing antibacterial natural products with in silico techniques. Mol. Med. Rep. 2018, 18, 763–770. [Google Scholar] [CrossRef]
- Rayan, M.; Abdallah, Z.; Abu-Lafi, S.; Masalha, M.; Rayan, A. Indexing Natural Products for their Antifungal Activity by Filters-based Approach: Disclosure of Discriminative Properties. Curr. Comput. Aided Drug Des. 2019, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rayan, M.; Abu-Farich, B.; Basha, W.; Rayan, A.; Abu-Lafi, S. Correlation between Antibacterial Activity and Free-Radical Scavenging: In-Vitro Evaluation of Polar/Non-Polar Extracts from 25 Plants. Processes 2020, 8, 117. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, S.M.; Dykes, G.A. Potential mechanisms for the effects of tea extracts on the attachment, biofilm formation and cell size of Streptococcus mutans. Biofouling 2013, 29, 307–318. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.; Walsh, L. Potential Role for Natural Products in Dental Caries Control. Oral Health Prev. Dent. 2019, 17, 479–485. [Google Scholar] [PubMed]
- Hattarki, S.A.; Bogar, C.; Bhat, K.G. Green tea catechins showed antibacterial activity on streptococcus mutans—An In Vitro study. Indian J. Dent. Res. 2021, 32, 226–229. [Google Scholar] [CrossRef]
- Schneider-Rayman, M.; Steinberg, D.; Sionov, R.V.; Friedman, M.; Shalish, M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: An In Vitro study. BMC Oral Health 2021, 21, 447. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, A.T.W. Epigallocatechin gallate and gallic acid affect colonization of abiotic surfaces by oral bacteria. Arch. Oral Biol. 2020, 120, 104922. [Google Scholar] [CrossRef] [PubMed]
- Albutti, A.; Gul, M.S.; Siddiqui, M.F.; Maqbool, F.; Adnan, F.; Ullah, I.; Rahman, Z.; Qayyum, S.; Shah, M.A.; Salman, M. Combating Biofilm by Targeting Its Formation and Dispersal Using Gallic Acid against Single and Multispecies Bacteria Causing Dental Plaque. Pathogens 2021, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef]
- Passos, M.R.; Almeida, R.S.; Lima, B.O.; Rodrigues, J.Z.S.; Macedo Neres, N.S.; Pita, L.S.; Marinho, P.D.F.; Santos, I.A.; da Silva, J.P.; Oliveira, M.C.; et al. Anticariogenic activities of Libidibia ferrea, gallic acid and ethyl gallate against Streptococcus mutans in biofilm model. J. Ethnopharmacol. 2021, 274, 114059. [Google Scholar] [CrossRef]
- Sendamangalam, V.; Choi, O.K.; Kim, D.; Seo, Y. The anti-biofouling effect of polyphenols against Streptococcus mutans. Biofouling 2011, 27, 13–19. [Google Scholar] [CrossRef]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. The need for new antibiotics. Clin. Microbiol. Infect. 2004, 10 (Suppl. 4), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, R.Y.; Roy, A.; Bera, S. Antagonistic Roles of Gallates and Ascorbic Acid in Pyomelanin Biosynthesis of Pseudomonas aeruginosa Biofilms. Curr. Microbiol. 2021, 78, 3843–3852. [Google Scholar] [CrossRef]
- Kosuru, R.Y.; Aashique, M.; Fathima, A.; Roy, A.; Bera, S. Revealing the dual role of gallic acid in modulating ampicillin sensitivity of Pseudomonas aeruginosa biofilms. Future Microbiol. 2018, 13, 297–312. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Kanchanadumkerng, P. Influence of Gallic Acid and Thai Culinary Essential Oils on Antibacterial Activity of Nisin against Streptococcus mutans. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 5539459. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Santos, C.A.; Lima, E.M.F.; Franco, B.; Pinto, U.M. Exploring Phenolic Compounds as Quorum Sensing Inhibitors in Foodborne Bacteria. Front. Microbiol. 2021, 12, 735931. [Google Scholar] [CrossRef]
- Bali, E.B.; Turkmen, K.E.; Erdonmez, D.; Saglam, N. Comparative Study of Inhibitory Potential of Dietary Phytochemicals Against Quorum Sensing Activity of and Biofilm Formation by Chromobacterium violaceum 12472, and Swimming and Swarming Behaviour of Pseudomonas aeruginosa PAO1. Food Technol. Biotechnol. 2019, 57, 212–221. [Google Scholar] [CrossRef]
- Sherif, M.M.; Elkhatib, W.F.; Khalaf, W.S.; Elleboudy, N.S.; Abdelaziz, N.A. Multidrug Resistant Acinetobacter baumannii Biofilms: Evaluation of Phenotypic-Genotypic Association and Susceptibility to Cinnamic and Gallic Acids. Front. Microbiol. 2021, 12, 716627. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, J.; Rubini, D.; Sinsinwar, S.; Senthilkumar, M.; Nithyanand, P.; Vadivel, V. Gallic Acid an Agricultural Byproduct Modulates the Biofilm Matrix Exopolysaccharides of the Phytopathogen Ralstonia solanacearum. Curr. Microbiol. 2020, 77, 3339–3354. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Novovic, K.; Malesevic, M.; Dinic, M.; Stojkovic, D.; Jovcic, B.; Sokovic, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria-Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Sette, D.E.S.P.H.; Santana, C.P.; Sousa, I.M.O.; Foglio, M.A.; Medeiros, F.D.; Medeiros, A.C.D. Schinopsis brasiliensis Engl. to combat the biofilm-dependents diseases In Vitro. An. Acad. Bras. Ciênc. 2020, 92, e20200408. [Google Scholar] [CrossRef] [PubMed]
- Davila-Avina, J.; Gil-Solis, C.; Merino-Mascorro, J.; Garcia, S.; Heredia, N. Phenolics with Bactericidal Activity Alter Motility and Biofilm Formation in Enterotoxigenic, Enteropathogenic, and Enterohemorrhagic Escherichia coli. Foodborne Pathog. Dis. 2020, 17, 568–575. [Google Scholar] [CrossRef]
- Kang, J.; Li, Q.; Liu, L.; Jin, W.; Wang, J.; Sun, Y. The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Appl. Microbiol. Biotechnol. 2018, 102, 1837–1846. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, A.C.B.; Koga-Ito, C.Y. Effects of Acetone Fraction from Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors. Front. Microbiol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Hossain, M.A.; Park, H.C.; Park, S.W.; Park, S.C.; Seo, M.G.; Her, M.; Kang, J. Synergism of the Combination of Traditional Antibiotics and Novel Phenolic Compounds against Escherichia coli. Pathogens 2020, 9, 811. [Google Scholar] [CrossRef]
- Gobin, M.; Proust, R.; Lack, S.; Duciel, L.; Des Courtils, C.; Pauthe, E.; Gand, A.; Seyer, D. A Combination of the Natural Molecules Gallic Acid and Carvacrol Eradicates P. aeruginosa and S. aureus Mature Biofilms. Int. J. Mol. Sci. 2022, 23, 7118. [Google Scholar] [CrossRef]
- Hossain, M.A.; Lee, S.J.; Park, N.H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.W.; Park, S.C. Impact of phenolic compounds in the acyl homoserine lactone-mediated quorum sensing regulatory pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.H.; Zhang, L.; Yu, F.; Li, F.; Liu, Z.Y.; Chen, J.H. Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin. Materials 2017, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Mechesso, A.F.; Yixian, Q.; Park, S.C. Methyl gallate and tylosin synergistically reduce the membrane integrity and intracellular survival of Salmonella Typhimurium. PLoS ONE 2019, 14, e0221386. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.K.; Roy, N.; Acharyya, S.; Saha, D.R.; Koley, H.; Sarkar, P.; Bhowmik, P. In vivo fluid accumulation-inhibitory, anticolonization and anti-inflammatory and In Vitro biofilm-inhibitory activities of methyl gallate isolated from Terminalia chebula against fluoroquinolones resistant Vibrio cholerae. Microb. Pathog. 2019, 128, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Cho, C.Y.; Ho, A.; Huang, J.Y.; Martin, B.; Gilbert, E.S. 4-Ethoxybenzoic acid inhibits Staphylococcus aureus biofilm formation and potentiates biofilm sensitivity to vancomycin. Int. J. Antimicrob. Agents 2020, 56, 106086. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Par, K.J.; Choi, H.Y.; Kwak, J.H.; Kim, W.G. Differential effects of alkyl gallates on quorum sensing in Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 7741. [Google Scholar] [CrossRef]
- Aracri, F.M.; Cavalcanti, R.M.F.; Guimaraes, L.H.S. Extracellular Tannase from Aspergillus ochraceus: Influence of the Culture Conditions on Biofilm Formation, Enzyme Production, and Application. J. Microbiol. Biotechnol. 2019, 29, 1749–1759. [Google Scholar] [CrossRef]
- Ding, T.; Li, T.; Li, J. Virtual screening for quorum-sensing inhibitors of Pseudomonas fluorescens P07 from a food-derived compound database. J. Appl. Microbiol. 2019, 127, 763–777. [Google Scholar] [CrossRef]
- Oh, E.; Bae, J.; Kumar, A.; Choi, H.J.; Jeon, B. Antioxidant-based synergistic eradication of methicillin-resistant Staphylococcus aureus (MRSA) biofilms with bacitracin. Int. J. Antimicrob. Agents 2018, 52, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Gabe, V.; Kacergius, T.; Abu-Lafi, S.; Zeidan, M.; Abu-Farich, B.; Austys, D.; Masalha, M.; Rayan, A. Suppressive Effects of Octyl Gallate on Streptococcus mutans Biofilm Formation, Acidogenicity, and Gene Expression. Molecules 2019, 24, 3170. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Ahmad, K.; Khan, L.A.; Hameed, S. Octyl gallate triggers dysfunctional mitochondria leading to ROS driven membrane damage and metabolic inflexibility along with attenuated virulence in Candida albicans. Med. Mycol. 2020, 58, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Jiang, L.; Lin, S.; Jin, W.G.; Gu, Q.; Chen, Y.W.; Zhang, K.; Ettelaie, R. Ultra-efficient antimicrobial photodynamic inactivation system based on blue light and octyl gallate for ablation of planktonic bacteria and biofilms of Pseudomonas fluorescens. Food Chem. 2022, 374, 131585. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Lin, S.; Chen, W.X.; Jiang, L.; Gu, Q.; Li, D.H.; Chen, Y.W. Dual-Stage Blue-Light-Guided Membrane and DNA-Targeted Photodynamic Inactivation Using Octyl Gallate for Ultraefficient Eradication of Planktonic Bacteria and Sessile Biofilms. J. Agric. Food Chem. 2022, 70, 7547–7565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Xu, W.; Liu, Y.; Wang, W.; Wu, K.; Wang, Z.; Zhang, X. Antibacterial effects of Traditional Chinese Medicine monomers against Streptococcus pneumoniae via inhibiting pneumococcal histidine kinase (VicK). Front. Microbiol. 2015, 6, 479. [Google Scholar] [CrossRef] [PubMed]
- Gabe, V.; Zeidan, M.; Kacergius, T.; Bratchikov, M.; Falah, M.; Rayan, A. Lauryl Gallate Activity and Streptococcus mutans: Its Effects on Biofilm Formation, Acidogenicity and Gene Expression. Molecules 2020, 25, 3685. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Species | Effect of Biofilm Inhibition | Mechanism of Action | Dosage | Ref. | Note |
|---|---|---|---|---|---|
| Proteus spp. , E. coli, Pseudomonas spp. , Salmonella spp. , Streptococcus mutans, and Staphylococcus aureus and multispecies bacteria | Effective against planktonic bacterial growth and biofilm formation | Significant changes in extracellular polysaccharide | 20–200 mg/L | [19] | No significant change on pre-formed biofilm |
| Clinical isolates of Pseudomonas aeruginosa | Effective in inhibiting the pre-existing biofilms | Possibly through the inhibition of pyomelanin synthesis | 3–5 mg/mL | [25] | Synergistic effect of gallic acid and ascorbic acid seen in the inhibition of biofilm formation and associated pyomelanin synthesis |
| Streptococcus mutans | Effective against mature biofilm | Reduces the following: viable cells, production of alkali-soluble glucans, acidogenicity and aciduricity capacity, and expression of glycosyltransferase genes | at 250 µg/mL | [21] | |
| Streptococcus mutans | Effective against biofilm formation | Targeting the cell membrane | [27] | ||
| Chromobacterium violaceum | Effective against biofilm formation | - | 8.8 mM, 9.4 mM | [29,30] | Gallic acid does not affect QS |
| Clinical isolates of Acinetobacter baumannii | Effective against bacterial growth and biofilm formation | Cleavage of peptidoglycan, molecules-mediated quorum sensing, and the antioxidant activity of gallic acid probably implicated in regulating genes of biofilm formation | 1.32–2.11 mg/mL | [31] | Significant association between MDR and the biofilm-forming ability of these isolates |
| Ralstonia solanacearum | Effective against young and mature biofilm formation | - | 3 mg/mL | [32] | Gallic acid is an ecofriendly compound and could be used as a green pesticide |
| Streptococcus mutans; Streptococcus oralis; Streptococcus mitis; Streptococcus salivarius | Effective against bacterial growth | - | Less than 1.0 mg/mL | [34] | Gallic acid shows no cytotoxicity |
| Clinical isolates of E. coli | Gallic acid in combination with ampicillin is synergistically effective against bacterial growth and biofilm viability | Changes in membrane integrity and permeability of bacterial cell | The MIC value of gallic acid against E. coli (1024 µg/mL) | [38] | Gallic acid-ampicillin synergism |
| Pseudomonas aeruginosa and Staphylococcus aureus | Gallic alone and in combination with carvacrol inhibits mature biofilm | Changes in plasma membrane properties | MIC value 2.5 mg/mL | [39] | The effect observed with gallic acid and carvacrol combination seen also on dual-species mature biofilms of S. aureus and P. aeruginosa |
| Enteropathogenic E. coli [EPEC], enterohemorrhagic E. coli [EHEC], and enterotoxigenic E. coli [ETEC] | Effective against biofilm formation and its related genes | - | MBC value 2.1–2.2 mg/mL | [35] | |
| E. coli | Effective against planktonic bacterial growth and biofilm formation | Suppression of the pgaABCD genes | 2 mg/mL | [36] | Inhibition of biofilm formation and biofilm eradication were at 2 and 8 mg/mL, respectively |
| Bacterial Species | Effect of Biofilm Inhibition | Mechanism of Action | Dosage | Ref. | Note |
|---|---|---|---|---|---|
| Streptococcus mu-tans | Effective in preventing biofilm formation | By reducing the biofilm biomass, roughness and thickness | 0.55–1 mg/L | [7] | Suppression of the biofilm acidogenicity |
| Pseudomonas aeruginosa (strain PAO1) | Effective in preventing biofilm formation | By inhibiting quorum-sensing gene expression and exopolysaccharide production | 16–256 µg/mL | [40] | Decrease of the biofilm viability |
| Streptococcus mutans | Effective against biofilm formation | By inhibiting the bacterial adhesion to the dentin–resin interface | 200–600 µg/mL | [41] | Epigallocatechin-3-O-(3-O-methyl)-gallate incorporated in the adhesive system |
| Salmonella enterica Serovar Typhimurium | Effective against bacterial growth and biofilm formation | By damaging bacterial cell membrane, reducing membrane potential and causing the leakage of bacterial cell contents | 32–4096 µg/mL | [42] | The biofilm inhibitory effect of Methyl Gallate occurs in combination with antibiotic Tylosin, and the effect is synergistic |
| Vibrio cholerae (strains SG24 and PC4) | Effective against biofilm formation | By disintegrating the bacterial inner and outer membranes and leakage of cytoplasmic material | 64 µg/mL | [43] | Methyl Gallate does not cause ≥90% reduction of biofilm at the 2 × MIC (128 µg/mL) |
| Staphylococcus aureus | Effective against biofilm formation | Possibly through the attenuation of bacterial growth | 0.2 mg/mL, 0.4 mg/mL | [44] | Methyl Gallate does not potentiate the activity of antibiotic vancomycin against the biofilm-dwelling cells |
| Enteropathogenic E. coli [EPEC], enterohemorrhagic E. coli [EHEC], and enterotoxigenic E. coli [ETEC] | Effective against biofilm formation | By inhibiting expression of the biofilm-associated genes (flhC, fliA, fliC, csgA) | 0.07–2.1 mg/mL | [35] | Low concentrations of Methyl Gallate inhibit the biofilm formation without significantly reducing cell populations |
| Bacterial Species | Effect of Biofilm Inhibition | Mechanism of Action | Dosage | Ref. | Note |
|---|---|---|---|---|---|
| Streptococcus mutans | Effective against planktonic bacteria and in preventing biofilm formation | Significant changes in the gene expression of gtfC and gtfB and less on gbpB | 2.78–3.53 mM | [6] | No significant effect on gtfD, atpD, and atpF |
| Pseudomonas aeruginosa (strains PAO1 and PA14) | Inhibited biofilm formation significantly | Inhibit virulence factor production and biofilm formation while preserving cell viability | 3–30 μM | [45] | Among the key virulence reported factors: elastase, pyocyanin, and rhamnolipid |
| Streptococcus mutans | Inhibit biofilm formation | Significant reduction in the gene expression of gtfB, gtfC, and gtfD | 50 mg/mL (252 mM) [EG was tested only against ATCC25175 biofilms] | [21] | Reduced the number of viable cells, acidogenicity, and aciduricity |
| Bacterial Species | Effect of Biofilm Inhibition | Mechanism of Action | Dosage | Ref. | Note |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa (strains PAO1, PA14, and drug-resistant clinical isolates) | Effective against biofilm formation | By suppressing the production of extracellular polymeric substances, quorum-sensing signaling molecules, and quorum sensing gene expression | 30–300 µM | [45] | Propyl Gallate inhibits the biofilm formation without affecting planktonic cell viability |
| Pseudomonas fluorescens (strain P07) | Possibly effective against biofilm formation | Possibly through inhibiting the production of quorum sensing signal molecules (acyl-homoserine lactones) | <2.25 mg/mL (anti-QS action in Chromobacterium violaceum CV026), <2.50 mg/mL (anti-QS action in Agrobacterium tumefaciens A136), not determined for P. fluorescens biofilm inhibition | [47] | Inhibition of quorum-sensing signal molecules occurs under sub-MIC |
| Pseudomonas aeruginosa clinical isolates (strains PA9027 and PA27853) | Effective in inhibiting the pre-existing biofilms | Possibly through the inhibition of pyomelanin synthesis | 3–5 mg/mL | [25] | Synergistic effect of propyl gallate and ascorbic acid seen in the inhibition of biofilm formation and associated pyomelanin synthesis |
| Bacterial and Fungal Species | Effect of Biofilm Inhibition | Mechanism of Action | Dosage | Ref. | Note |
|---|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus (MRSA) | Effective against biofilm formation | By suppressing the production of extracellular polysaccharides | 2 µg/mL | [48] | Synergistic effect of Octyl Gallate and bacitracin (10−3 U/mL) occurs in the inhibition of biofilm formation |
| Streptococcus mu-tans | Effective in preventing biofilm formation | By inhibiting the expression of biofilm-associated genes (gbpB, gtfB, gtfC, gtfD) | 97.4–100.24 µM | [49] | Suppression of the biofilm acidogenicity through the inhibition of atpD gene expression |
| Pseudomonas aeruginosa (strains PAO1, PA14, and drug-resistant clinical isolates) | Enhancement of the biofilm formation | By increasing the production of extracellular polymeric substances | 100–300 µM | [45] | Octyl Gallate reduces pyocyanin and rhamnolipid synthesis by inhibiting the PqsR system |
| Candida albicans | Effective in preventing biofilm formation and eliminating the preformed biofilm | By suppressing the transition of fungal cells from yeast to hyphae and damaging fungal cell membrane | 20 µg/mL | [50] | Octyl Gallate causes mitochondrial dysfunction and induces oxidative stress in C. albicans cells |
| Pseudomonas fluorescens | Effective in preventing biofilm formation and eliminating the preformed biofilm | By inducing the production of reactive oxygen species and damaging the bacterial cell membrane | 0.05 mM, 0.1 mM, 0.4 mM | [51] | Synergistic effect of Octyl Gallate and blue light (photodynamic inactivation system) |
| Vibrio parahaemolyticus | Effective in preventing biofilm formation and eliminating the preformed biofilm | By inducing the production of reactive oxygen species and damaging the bacterial cell membrane | 0.1 mM, 0.2 mM | [52] | Synergistic effect of Octyl Gallate and blue light (photodynamic inactivation system) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayan, M.; Abu Lafi, S.; Falah, M.; Kacergius, T.; Kirkliauskiene, A.; Gabe, V.; Rayan, A. Alkyl Gallates as Potential Antibiofilm Agents: A Review. Molecules 2023, 28, 1751. https://doi.org/10.3390/molecules28041751
Rayan M, Abu Lafi S, Falah M, Kacergius T, Kirkliauskiene A, Gabe V, Rayan A. Alkyl Gallates as Potential Antibiofilm Agents: A Review. Molecules. 2023; 28(4):1751. https://doi.org/10.3390/molecules28041751
Chicago/Turabian StyleRayan, Mahmoud, Saleh Abu Lafi, Mizied Falah, Tomas Kacergius, Agne Kirkliauskiene, Vika Gabe, and Anwar Rayan. 2023. "Alkyl Gallates as Potential Antibiofilm Agents: A Review" Molecules 28, no. 4: 1751. https://doi.org/10.3390/molecules28041751
APA StyleRayan, M., Abu Lafi, S., Falah, M., Kacergius, T., Kirkliauskiene, A., Gabe, V., & Rayan, A. (2023). Alkyl Gallates as Potential Antibiofilm Agents: A Review. Molecules, 28(4), 1751. https://doi.org/10.3390/molecules28041751






