Synthesis of Disubstituted Carboxonium Derivatives of Closo-Decaborate Anion [2,6-B10H8O2CC6H5]−: Theoretical and Experimental Study
Abstract
1. Introduction
2. Results and Discussion
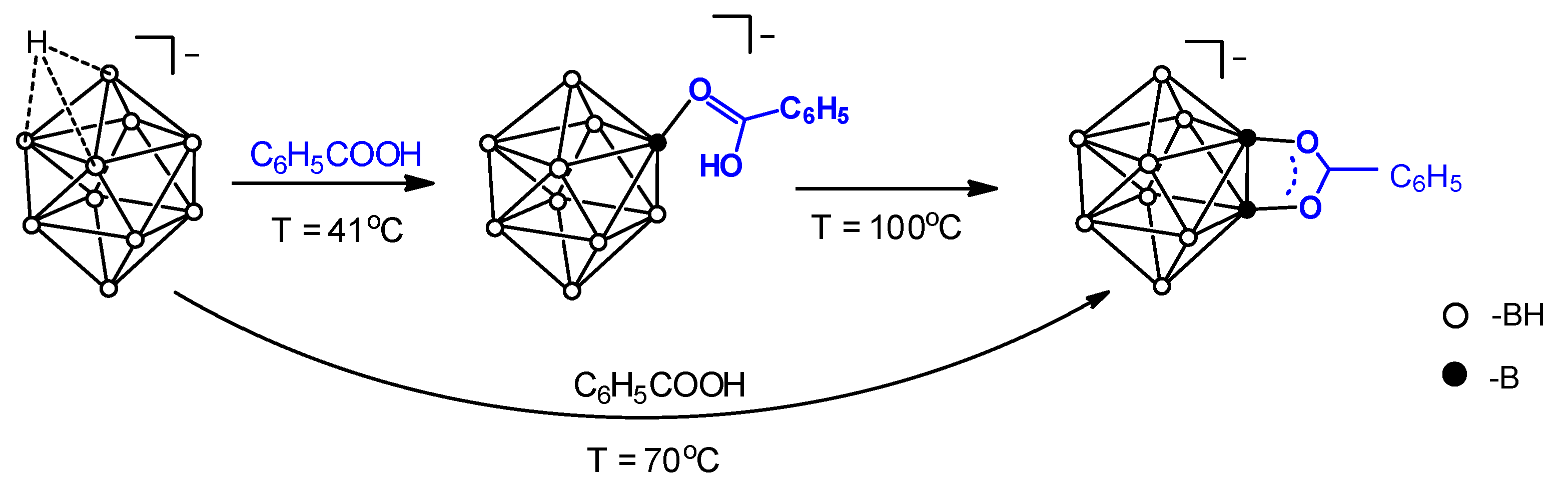
2.1. Synthesis of [B10H8O2CC6H5]−
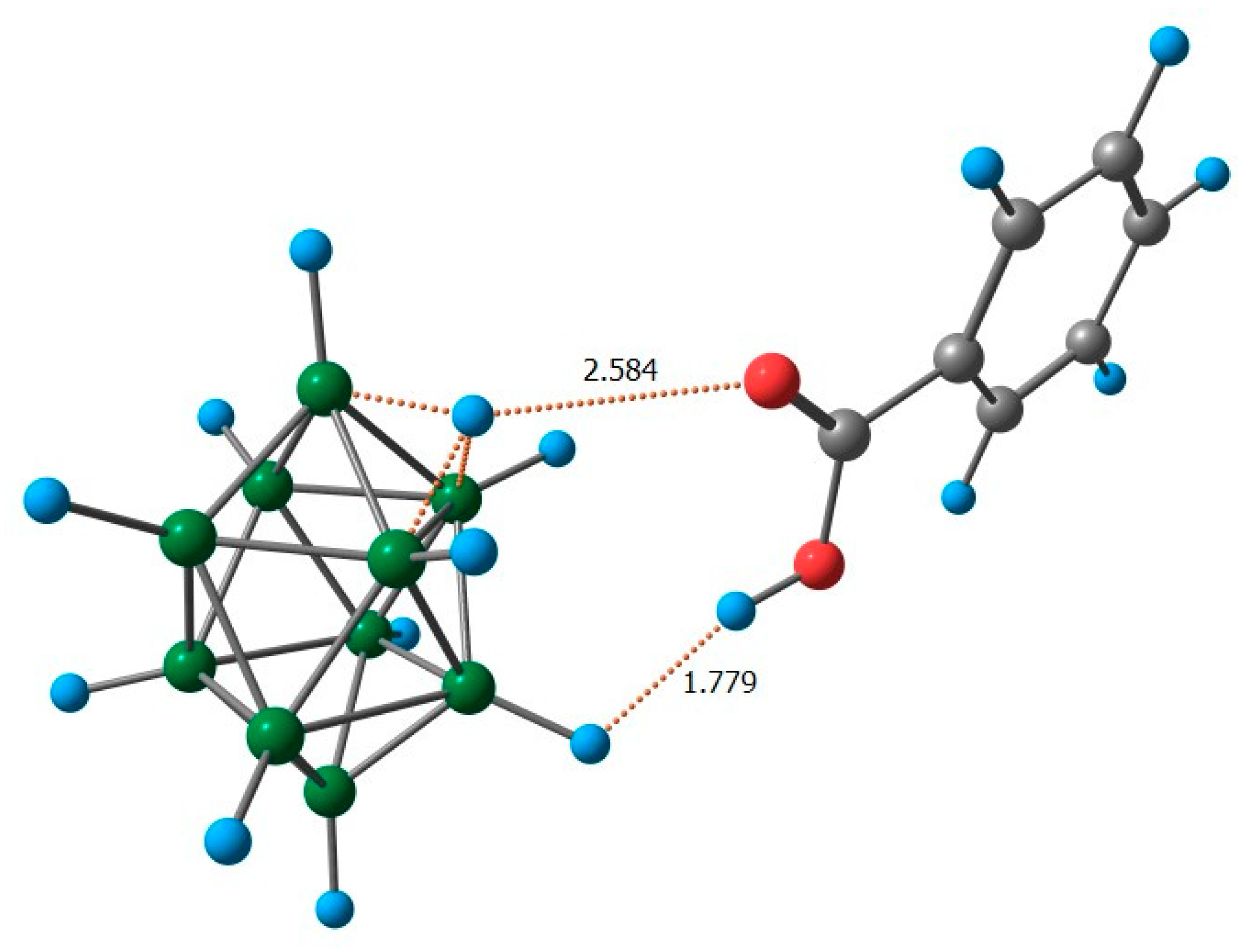
2.2. Reaction Mechanism Investigation Based on DFT Calculations
3. Materials and Methods
3.1. IR Spectra
3.2. NMR Spectra
3.3. Electrospray Ionisation Mass Spectrometry (ESI-MS)
3.4. X-ray Crystal Structure Determination
3.5. Computational Details
3.6. Materials
3.7. Synthesis of ((C4H9)4N)[2,6-B10H8O2CC6H5]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adelusi, T.I.; Oyedele, A.-Q.K.; Boyenle, I.D.; Ogunlana, A.T.; Adeyemi, R.O.; Ukachi, C.D.; Idris, M.O.; Olaoba, O.T.; Adedotun, I.O.; Kolawole, O.E.; et al. Molecular modeling in drug discovery. Inform. Med. Unlocked 2022, 29, 100880. [Google Scholar] [CrossRef]
- Gao, K.; Wang, R.; Chen, J.; Cheng, L.; Frishcosy, J.; Huzumi, Y.; Qiu, Y.; Schluckbier, T.; Wei, X.; Wei, G.-W. Methodology-Centered Review of Molecular Modeling, Simulation, and Prediction of SARS-CoV-2. Chem. Rev. 2022, 122, 11287–11368. [Google Scholar] [CrossRef] [PubMed]
- Maurer, R.J.; Freysoldt, C.; Reilly, A.M.; Brandenburg, J.G.; Hofmann, O.T.; Björkman, T.; Lebègue, S.; Tkatchenko, A. Advances in Density-Functional Calculations for Materials Modeling. Annu. Rev. Mater. Res. 2019, 49, 1–30. [Google Scholar] [CrossRef]
- Daniel, C. Density Functional Theories and Coordination Chemistry. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Jones, R.O. Density functional theory: Its origins, rise to prominence, and future. Rev. Mod. Phys. 2015, 87, 897–923. [Google Scholar] [CrossRef]
- Datta, D.; Gordon, M.S. A Massively Parallel Implementation of the CCSD(T) Method Using the Resolution-of-the-Identity Approximation and a Hybrid Distributed/Shared Memory Parallelization Model. J. Chem. Theory Comput. 2021, 17, 4799–4822. [Google Scholar] [CrossRef]
- Rojas, S.; Parravicini, O.; Vettorazzi, M.; Tosso, R.; Garro, A.; Gutiérrez, L.; Andújar, S.; Enriz, R. Combined MD/QTAIM techniques to evaluate ligand-receptor interactions. Scope and limitations. Eur. J. Med. Chem. 2020, 208, 112792. [Google Scholar] [CrossRef]
- Morgante, P.; Peverati, R. The devil in the details: A tutorial review on some undervalued aspects of density functional theory calculations. Int. J. Quantum Chem. 2020, 120, e26332. [Google Scholar] [CrossRef]
- Luo, J.; Dai, H.; Zeng, C.; Wu, D.; Cao, M. A Theoretical Study of the Halogen Bond between Heteronuclear Halogen and Benzene. Molecules 2022, 27, 8078. [Google Scholar] [CrossRef]
- Zhao, L.; Pan, S.; Holzmann, N.; Schwerdtfeger, P.; Frenking, G. Chemical Bonding and Bonding Models of Main-Group Compounds. Chem. Rev. 2019, 119, 8781–8845. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.-L.; Liu, Z.-P. Reaction prediction via atomistic simulation: From quantum mechanics to machine learning. Iscience 2020, 24, 102013. [Google Scholar] [CrossRef]
- Koistinen, O.-P.; Ásgeirsson, V.; Vehtari, A.; Jónsson, H. Minimum Mode Saddle Point Searches Using Gaussian Process Regression with Inverse-Distance Covariance Function. J. Chem. Theory Comput. 2019, 16, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, M.; Neese, F. Effect of the Solute Cavity on the Solvation Energy and its Derivatives within the Framework of the Gaussian Charge Scheme. J. Comput. Chem. 2020, 41, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.; Ghafoor, S.; de Visser, S.P. Density Functional Theory Study into the Reaction Mechanism of Isonitrile Biosynthesis by the Nonheme Iron Enzyme ScoE. Top. Catal. 2022, 65, 528–543. [Google Scholar] [CrossRef]
- Xie, C.; Sun, Y.; Zhu, B.; Xu, M.; Yu, H.; Liu, E. Density functional theory study on the reaction mechanism of selective catalytic reduction of NO by NH3 over the γ-Fe2O3 (0 0 1) surface. Comput. Theor. Chem. 2020, 1192, 113052. [Google Scholar] [CrossRef]
- Du, L.; Jin, S.; Nie, P.; She, C.; Wang, J. Initial Decomposition Mechanism of 3-Nitro-1,2,4-triazol-5-one (NTO) under Shock Loading: ReaxFF Parameterization and Molecular Dynamic Study. Molecules 2021, 26, 4808. [Google Scholar] [CrossRef]
- Cortes-Guzman, F.; Bader, R. Complementarity of QTAIM and MO theory in the study of bonding in donor-acceptor complexes. Coord. Chem. Rev. 2005, 249, 633–662. [Google Scholar] [CrossRef]
- Firme, C.L. Local potential energy: A novel QTAIM tool to quantify the binding energy of classical hydrogen bonds. Chem. Phys. Lett. 2020, 754, 137593. [Google Scholar] [CrossRef]
- Alikhani, M.E. On the chemical bonding features in boron containing compounds: A combined QTAIM/ELF topological analysis. Phys. Chem. Chem. Phys. 2013, 15, 12602–12609. [Google Scholar] [CrossRef]
- Lepetit, C.; Fau, P.; Fajerwerg, K.; Kahn, M.L.; Silvi, B. Topological analysis of the metal-metal bond: A tutorial review. Coord. Chem. Rev. 2017, 345, 150–181. [Google Scholar] [CrossRef]
- Jacobsen, H. Chemical bonding in view of electron charge density and kinetic energy density descriptors. J. Comput. Chem. 2009, 30, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, P.; Chamorro, E.; Chattaraj, P.K.; De Proft, F.; Gázquez, J.L.; Liu, S.; Morell, C.; Toro-Labbé, A.; Vela, A.; Ayers, P. Conceptual density functional theory: Status, prospects, issues. Theor. Chem. Accounts 2020, 139, 36. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Huang, Y.; Rong, C.; Zhang, R.; Liu, S. Evaluating frontier orbital energy and HOMO/LUMO gap with descriptors from density functional reactivity theory. J. Mol. Model. 2017, 23, 3. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Giri, S. Electrophilicity index within a conceptual DFT framework. Annu. Rep. Prog. Chem. Sect. C 2009, 105, 13–39. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual density functional theory based electronic structure principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-F.; Wu, H.-S.; Jiao, H. Structures and aromaticity of closo-BnHn−1CO− (n=5 − 12). J. Mol. Struct. THEOCHEM 2007, 822, 111–115. [Google Scholar] [CrossRef]
- Sethio, D.; Daku, L.M.L.; Hagemann, H.; Kraka, E. Quantitative Assessment of B−B−B, B−Hb−B, and B−Ht Bonds: From BH3 to B12H12 2−. ChemPhysChem 2019, 20, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.; Yan, H.; Poater, J.; Solà, M. The nido-Cage···π Bond: A Non-covalent Interaction between Boron Clusters and Aromatic Rings and Its Applications. Angew. Chem. Int. Ed. 2020, 59, 9018–9025. [Google Scholar] [CrossRef]
- Piña, M.D.L.N.; Bauzá, A.; Frontera, A. Halogen···halogen interactions in decahalo-closo-carboranes: CSD analysis and theoretical study. Phys. Chem. Chem. Phys. 2020, 22, 6122–6130. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.; Malinina, E.; Kuznetsov, N. Boron cluster anions and their derivatives in complexation reactions. Coord. Chem. Rev. 2022, 469, 214636. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in three-dimensional aromatic-like closo-dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium derivatives of polyhedral boron compounds (boranes, carboranes, metallocarboranes): Synthesis and reactivity. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Bogdanova, E.V.; Stogniy, M.Y.; Chekulaeva, L.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Grin, M.A.; Mironov, A.F.; Bregadze, V.I. Synthesis and reactivity of propionitrilium derivatives of cobalt and iron bis(dicarbollides). New J. Chem. 2020, 44, 15836–15848. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008, 11, 977–992. [Google Scholar] [CrossRef] [PubMed]
- Semioshkin, A.; Bregadze, V.; Godovikov, I.; Ilinova, A.; Laskova, J.; Starikova, Z. Synthesis and structure of 1-iodo-7-dioxonium-decahydro-closo-dodecaborate. J. Organomet. Chem. 2011, 696, 2760–2762. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Bogdanova, E.V.; Anufriev, S.A.; Sivaev, I.B. Synthesis of New Rhodacarborane [3,3-(1′,5′-COD)-8-PrNH=C(Et)NH-3,1,2-RhC2B9H10]. Russ. J. Inorg. Chem. 2022, 67, 1537–1544. [Google Scholar] [CrossRef]
- Laskova, J.; Ananiev, I.; Kosenko, I.; Serdyukov, A.; Stogniy, M.; Sivaev, I.; Grin, M.; Semioshkin, A.; Bregadze, V.I. Nucleophilic addition reactions to nitrilium derivatives [B12H11NCCH3]− and [B12H11NCCH2CH3]−. Synthesis and structures of closo-dodecaborate-based iminols, amides and amidines. Dalton Trans. 2022, 51, 3051–3059. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically Active Compounds Based on Membranotropic Cage Carriers–Derivatives of Adamantane and Polyhedral Boron Clusters (Review). Russ. J. Inorg. Chem. 2022, 67, 28–47. [Google Scholar] [CrossRef]
- Zhu, T.-C.; Xing, Y.-Y.; Sun, Y.; Duttwyler, S.; Hong, X. Directed B–H functionalization of the closo-dodecaborate cluster via concerted iodination–deprotonation: Reaction mechanism and origins of regioselectivity. Org. Chem. Front. 2020, 7, 3648–3655. [Google Scholar] [CrossRef]
- Zhdanov, A.; Zhdanova, K.; Bykov, A.; Kochnev, V.; Grigoriev, M.; Zhizhin, K.; Kuznetsov, N. Selective synthesis of the [2-B10H9I]2− anion and some theoretical aspects of its iodination process. Polyhedron 2018, 139, 125–130. [Google Scholar] [CrossRef]
- Kochnev, V.K.; Kuznetsov, N.T. Theoretical study of protonation of the B10H102− anion and subsequent hydrogen removal due to substitution reaction in acidic medium. Comput. Theor. Chem. 2016, 1075, 77–81. [Google Scholar] [CrossRef]
- Klyukin, I.; Vlasova, Y.; Novikov, A.; Zhdanov, A.; Zhizhin, K.; Kuznetsov, N. Theoretical Study of closo-Borate Anions [BnHn]2− (n = 5–12): Bonding, Atomic Charges, and Reactivity Analysis. Symmetry 2021, 13, 464. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Vlasova, Y.S.; Novikov, A.S.; Zhdanov, A.P.; Hagemann, H.R.; Zhizhin, K.Y.; Kuznetsov, N.T. B-F bonding and reactivity analysis of mono- and perfluoro-substituted derivatives of closo-borate anions (6, 10, 12): A computational study. Polyhedron 2022, 211, 115559. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Burianova, V.K.; Novikov, A.S.; Demakova, M.Y.; Pretorius, C.; Mokolokolo, P.P.; Roodt, A.; Bokach, N.A.; Suslonov, V.V.; Zhdanov, A.P.; et al. Nucleophilicity of Oximes Based upon Addition to a Nitrilium closo-Decaborate Cluster. Organometallics 2016, 35, 3612–3623. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Karpechenko, N.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Primary Amine Nucleophilic Addition to Nitrilium Closo-Dodecaborate [B12H11NCCH3]−: A Simple and Effective Route to the New BNCT Drug Design. Int. J. Mol. Sci. 2021, 22, 13391. [Google Scholar] [CrossRef]
- Jelinek, T.; Štibr, B.; Mareš, F.; Plešek, J.; Heřmánek, S. Halogenation of 4,5-dicarba-arachno- nonaborane(13),4,5-C2B7H13. Polyhedron 1987, 6, 1737–1740. [Google Scholar] [CrossRef]
- Frank, R.; Adhikari, A.K.; Auer, H.; Hey-Hawkins, E. Electrophile-Induced Nucleophilic Substitution of the nido-Dicarbaundecaborate Anionnido-7,8-C2B9H12−by Conjugated Heterodienes. Chem. A Eur. J. 2013, 20, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Laila, Z.; Yazbeck, O.; Ghaida, F.A.; Diab, M.; El Anwar, S.; Srour, M.; Mehdi, A.; Naoufal, D. Clean-activation of the B–H bond in closo-decahydrodecaborate [B10H10]2− anion via soft-route. J. Organomet. Chem. 2020, 910, 121132. [Google Scholar] [CrossRef]
- Mahfouz, N.; Ghaida, F.A.; El Hajj, Z.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent Achievements on Functionalization within closo-Decahydrodecaborate [B10H10]2− Clusters. Chemistryselect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- Peymann, T.; Lork, E.; Gabel, D. Hydroxoundecahydro-closo-dodecaborate (2−) as a Nucleophile. Preparation and Structural Characterization of O-Alkyl and O-Acyl Derivatives of Hydroxoundecahydro-closo-dodecaborate (2−). Inorg. Chem. 1996, 35, 1355–1360. [Google Scholar] [CrossRef]
- Kubasov, A.; Turishev, E.; Kopytin, A.; Shpigun, L.; Zhizhin, K.; Kuznetsov, N. Sulfonium closo-hydridodecaborate anions as active components of a potentiometric membrane sensor for lidocaine hydrochloride. Inorg. Chim. Acta 2020, 514, 119992. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Turyshev, E.S.; Novikov, I.V.; Gurova, O.M.; Starodubets, P.A.; Golubev, A.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Theoretical and experimental comparison of the reactivity of the sulfanyl-closo-decaborate and sulfanyl-closo-dodecaborate anions and their mono-S-substituted derivatives. Polyhedron 2021, 206, 115347. [Google Scholar] [CrossRef]
- Golub, I.; Filippov, O.; Belkova, N.; Epstein, L.; Shubina, E. The Reaction of Hydrogen Halides with Tetrahydroborate Anion and Hexahydro-closo-hexaborate Dianion. Molecules 2021, 26, 3754. [Google Scholar] [CrossRef] [PubMed]
- Golub, I.E.; Filippov, O.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. The Mechanism of Halogenation of Decahydro-closo-Decaborate Dianion by Hydrogen Chloride. Russ. J. Inorg. Chem. 2021, 66, 1639–1648. [Google Scholar] [CrossRef]
- Kochnev, V.K.; Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Theoretical study of molecular hydrogen elimination from the undecahydrodecaborate monoanion [B10H11]−. Exopolyhedral substitution intermediates: [B10H9]− monoanion and neutral [B10H10] cluster. Russ. J. Inorg. Chem. 2014, 59, 706–712. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, C.-Y.; Xu, T.-T.; Wu, J.; Wen, X.-Y.; Jiang, W.-J.; Chen, M.; Yang, J. Synthesis of Polyhedral Borane Cluster Fused Heterocycles via Transition Metal Catalyzed B-H Activation. Molecules 2020, 25, 391. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Zhdanov, A.P.; Bykov, A.Y.; Razgonyaeva, G.A.; Grigor’ev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. A new method for the synthesis of carboxonium derivatives of the closo-decaborate anion [2,6-B10H8(O2CR)]–, where R = CH3, C2H5. Russ. J. Inorg. Chem. 2017, 62, 1479–1482. [Google Scholar] [CrossRef]
- Safronova, E.F.; Avdeeva, V.V.; Polyakova, I.N.; Vologzhanina, A.V.; Goeva, L.V.; Malinina, E.A.; Kuznetsov, N.T. Synthesis and Structure of Disubstituted Closo-Decaborate Anion Derivatives Ph4P(2,6-B10H8O2CCH3) and 1,2-B10H8Phen with Bifunctional O,O′- and N,N′-Substituents. In Doklady Chemistry; Springer: New York, NY, USA, 2013; Volume 452, pp. 240–244. [Google Scholar] [CrossRef]
- Klyukin, I.; Zhdanov, A.; Matveev, E.; Razgonyaeva, G.; Grigoriev, M.; Zhizhin, K.; Kuznetsov, N. Synthesis and reactivity of closo-decaborate anion derivatives with multiple carbon–oxygen bonds. Inorg. Chem. Commun. 2014, 50, 28–30. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Kolbunova, A.V.; Novikov, A.S.; Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukina, A.A.; Zhdanov, A.P.; Zhizhin, K.Y.; Kuznetsov, N.T. Protonation of Borylated Carboxonium Derivative [2,6-B10H8O2CCH3]–: Theoretical and Experimental Investigation. Int. J. Mol. Sci. 2022, 23, 4190. [Google Scholar] [CrossRef]
- Nikolova, V.; Cheshmedzhieva, D.; Ilieva, S.; Galabov, B. Atomic Charges in Describing Properties of Aromatic Molecules. J. Org. Chem. 2019, 84, 1908–1915. [Google Scholar] [CrossRef]
- Oller, J.; Pérez, P.; Ayers, P.W.; Vöhringer-Martinez, E. Global and local reactivity descriptors based on quadratic and linear energy models for α,β-unsaturated organic compounds. Int. J. Quantum Chem. 2018, 118, e25706. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Geng, D.; Lu, J.; Wu, J. Thermal stability mechanism via energy absorption by chemical bonds bending and stretching in free space and the interlayer reaction of layered molecular structure explosives. Phys. Chem. Chem. Phys. 2020, 22, 13248–13260. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Selivanov, N.A.; Bykov, A.Y.; Kubasov, A.S.; Zhizhin, K.Y.; Kuznetsov, N.T. New Aspects of the Synthesis of closo-Dodecaborate Nitrilium Derivatives [B12H11NCR]–; (R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7): Experimental and Theoretical Studies. Inorganics 2022, 10, 196. [Google Scholar] [CrossRef]
- Bruker, SAINT; Bruker AXS Inc.: Madison, WI, USA, 2018.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 7.0: New vistas in localized and delocalized chemical bonding theory. J. Comput. Chem. 2019, 40, 2234–2241. [Google Scholar] [CrossRef]
- Glendening, J.K.; Badenhoop, A.E.R.; Carpenter, J.A.; Bohmann, C.M.; Morales, P.K.; Landis, C.R.; Weinhold, F. NBO 7.0; University of Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W.; Legare, D.A. Properties of atoms in molecules: Structures and reactivities of boranes and carboranes. Can. J. Chem. 1992, 70, 657–677. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 23 January 2023).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyukin, I.N.; Kolbunova, A.V.; Novikov, A.S.; Nelyubin, A.V.; Zhdanov, A.P.; Kubasov, A.S.; Selivanov, N.A.; Bykov, A.Y.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis of Disubstituted Carboxonium Derivatives of Closo-Decaborate Anion [2,6-B10H8O2CC6H5]−: Theoretical and Experimental Study. Molecules 2023, 28, 1757. https://doi.org/10.3390/molecules28041757
Klyukin IN, Kolbunova AV, Novikov AS, Nelyubin AV, Zhdanov AP, Kubasov AS, Selivanov NA, Bykov AY, Zhizhin KY, Kuznetsov NT. Synthesis of Disubstituted Carboxonium Derivatives of Closo-Decaborate Anion [2,6-B10H8O2CC6H5]−: Theoretical and Experimental Study. Molecules. 2023; 28(4):1757. https://doi.org/10.3390/molecules28041757
Chicago/Turabian StyleKlyukin, Ilya N., Anastasia V. Kolbunova, Alexander S. Novikov, Alexey V. Nelyubin, Andrey P. Zhdanov, Alexey S. Kubasov, Nikita A. Selivanov, Alexander Yu. Bykov, Konstantin Yu. Zhizhin, and Nikolay T. Kuznetsov. 2023. "Synthesis of Disubstituted Carboxonium Derivatives of Closo-Decaborate Anion [2,6-B10H8O2CC6H5]−: Theoretical and Experimental Study" Molecules 28, no. 4: 1757. https://doi.org/10.3390/molecules28041757
APA StyleKlyukin, I. N., Kolbunova, A. V., Novikov, A. S., Nelyubin, A. V., Zhdanov, A. P., Kubasov, A. S., Selivanov, N. A., Bykov, A. Y., Zhizhin, K. Y., & Kuznetsov, N. T. (2023). Synthesis of Disubstituted Carboxonium Derivatives of Closo-Decaborate Anion [2,6-B10H8O2CC6H5]−: Theoretical and Experimental Study. Molecules, 28(4), 1757. https://doi.org/10.3390/molecules28041757










