In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites
Abstract
:1. Introduction
2. Results
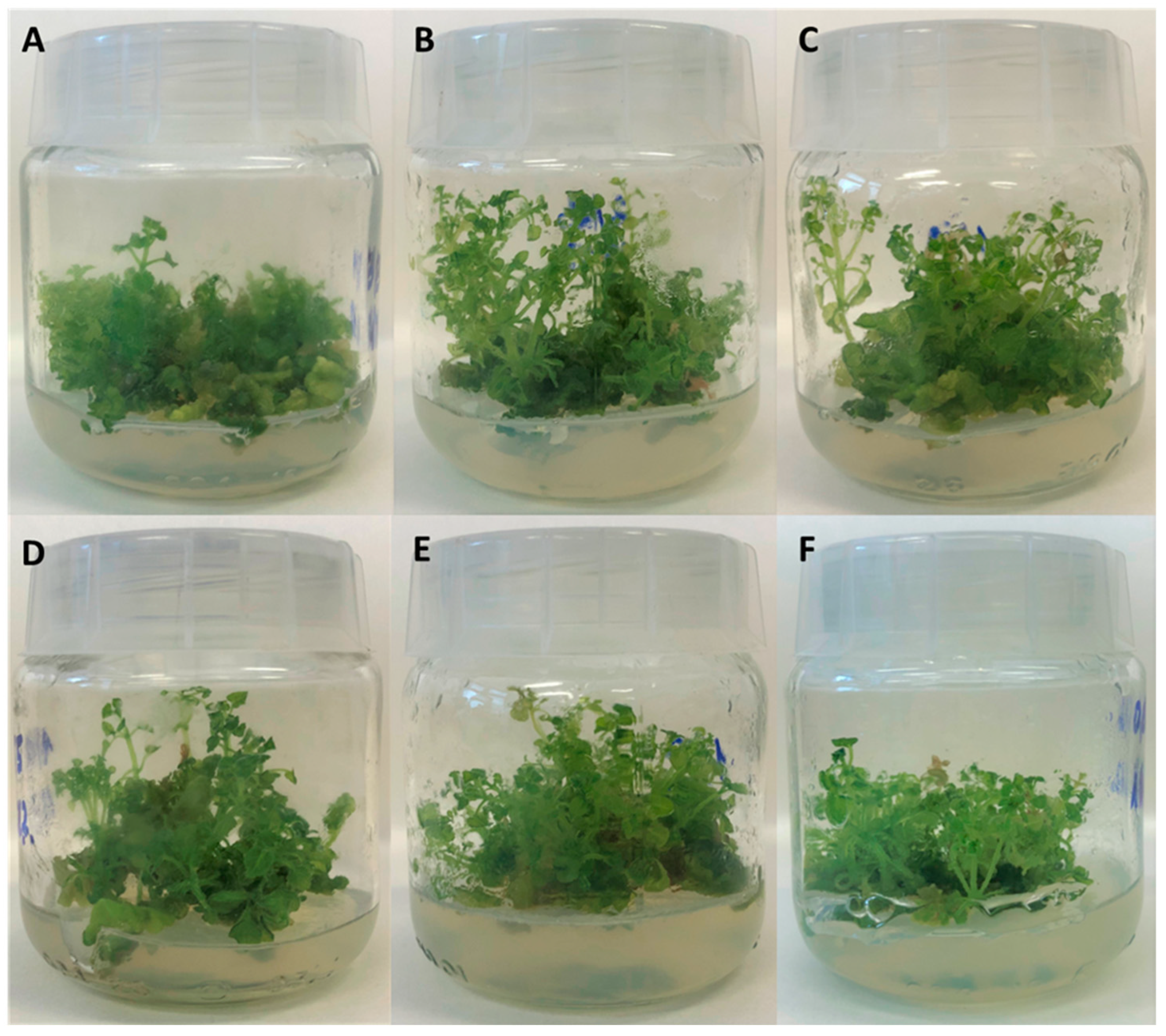
2.1. Appearance and Growth of Biomass Cultured In Vitro
2.2. Endogenous Accumulation of Metabolites
2.3. Activity of the Selected Extract
2.3.1. Antioxidant Activity and Total Phenolic Content
2.3.2. Antibacterial Activity
2.3.3. Antifungal Activity
2.3.4. Artemia Salina Lethality Bioassay
3. Discussion
3.1. In Vitro Cultures
3.2. Biological Activity of the Extract
4. Materials and Methods
4.1. Establishing of In Vitro Cultures
4.2. Experimental In Vitro Cultures
4.3. Sample Preparation
4.4. RP-HPLC Analysis of Secondary Metabolites
4.5. Determination of Total Phenolic Content
4.6. Free Radical Scavenging Activity
4.7. Reducing Power Assay
4.8. Ferrous Ions (Fe2+) Chelating Activity
4.9. Antibacterial Activity
4.10. Antifungal Activity
4.11. Artemia Salina Leach Lethality Test
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASE | ascorbic acid equivalent |
| BAP | 6-benzylaminopurine |
| BHT | butylated hydroxytoluene |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DW | dry weight |
| GAE | gallic acid equivalents |
| MIC | minimum inhibitory concentration |
| MS | Murashige and Skoog |
| NAA | 1-naphthaleneacetic acid |
| PGR | plant growth regulator |
References
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.; Çiçek, M.; Demirci, B.; Başer, K.H.C. Essential oil compositions of subspecies of Scutellaria brevibracteata Stapf from Turkey. J. Essent. Oil Res. 2019, 31, 255–262. [Google Scholar] [CrossRef]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 23 January 2023).
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 11th ed.; European Directorate for the Quality of Medicines: Strasburg, France, 2023.
- Pharmacopoeia of the People’s Republic of China; Ministry of Health of the People’s Republic of China, Chinese Pharmacopoeia Commission: Beijing, China, 2015; Volume 1.
- The Japanese Pharmacopoeia, 13th ed.; Ministry of Health and Welfare of Japan: Tokyo, Japan, 1996.
- Pharmacopoeia of the Republic of Korea, 7th ed.; Ministry of Health and Welfare of Korea: Seoul, Republic of Korea, 1998.
- Radix Scutellariae. WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 2007; Volume 3. [Google Scholar]
- Upton, R. Scutellaria lateriflora. In American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines; Upton, R., Graff, A., Jolliffe, G., Länger, R., Williamson, E., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 599–603. [Google Scholar]
- Millspaugh, C.F. Scutellaria. In American Medicinal Plants; Dover Publications: New York, NY, USA, 1974; pp. 469–472. [Google Scholar]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef]
- WFO. Scutellaria brevibracteata subsp subvelutina (Rech.f.) Greuter & Burdet. 2023. Available online: http://www.worldfloraonline.org/taxon/wfo-0000307669 (accessed on 17 January 2023).
- International Plant Name Index (IPNI) Scutellaria subvelutina Rech.f. Available online: https://www.ipni.org/n/458687-1 (accessed on 22 January 2023).
- Minareci, E.; Pekönür, S. An Important Euroasian Genus: Scutellaria L. Int. J. Second. Metab. 2017, 4, 35–46. [Google Scholar]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Santagostini, L.; Papini, A.; Flamini, G.; Fico, G. Scutellaria brevibracteata subsp. subvelutina (Rech.f.) Greuter & Burdet: Morphological and phytochemical characterization. Nat. Prod. Res. 2022, 36, 54–62. [Google Scholar] [CrossRef]
- Von Bothmer, R. Differentiation patterns in the E. Mediterranean Scutellaria rubicunda group (Lamiaceae). Plant Syst. Evol. 1987, 155, 219–249. [Google Scholar] [CrossRef]
- Özçelik, H.; Ay, G.; Öztürk, M. Some traditional plants of East and Southern Anatolia. In Proceedings of the 10th National Symposium on Biology; Atatürk University: Erzurum, Turkey, 1990; pp. 1–10. [Google Scholar]
- Cakilicioğlu, U.; Türkoğlu, I. An ethnobotanical survey of medicinal plants in Sivrice (Elaziğ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef]
- Erdoğan, M.; Konya, R.; Özhan, Y.; Sipahi, H.; Çinbilgel, İ.; Masullo, M.; Piacente, S.; Kırmızıbekmez, H. Secondary metabolites from Scutellaria brevibracteata subsp. subvelutina and their in vitro anti-inflammatory activities. S. Afr. J. Bot. 2021, 139, 12–18. [Google Scholar]
- Franzyk, H.; Rasmussen, J.H.; Jensen, S.R. Ozonolysis of Protected Iridoid Glucosides. EurJOC 1998, 2, 365–370. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Simmonds, M.S.; Rosselli, S.; Bruno, M.; Ložienė, K. Essential oils of three species of Scutellaria and their influence on Spodoptera littoralis. Biochem. Syst. Ecol. 2013, 48, 206–210. [Google Scholar] [CrossRef]
- Dogan, Z.; Telli, G.; Tel, B.C.; Saracoglu, I. Scutellaria brevibracteata Stapf and active principles with anti-inflammatory effects through regulation of NF-κB/COX-2/iNOS pathways. Fitoterapia 2022, 158, 105159. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.; Yilmaz, G.; Ciçek, M.; Sener, B. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem. Toxicol. 2010, 48, 781–788. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Production of Specific Flavonoids and Verbascoside in Shoot Cultures of Scutellaria baicalensis. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 249–272. [Google Scholar]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Endogenous production of specific flavonoids and verbascoside in agar and agitated microshoot cultures of Scutellaria lateriflora L. and biotransformation potential. Plant Cell Tissue Organ Cult. 2020, 142, 471–482. [Google Scholar] [CrossRef]
- Islam, N.; Downey, F.; Ng, C.Y.K. Comparative analysis of bioactive phytochemicals from Scutellaria baicalensis, Scutellaria lateriflora, Scutellaria racemosa, Scutellaria tomentosa and Scutellaria wrightii by LC-DAD-MS. Metabolomics 2011, 7, 446–453. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Tankhaeva, L.M. Phenolic compounds of Scutellaria baicalensis Georgi. Russ. J. Bioorganic Chem. 2010, 36, 816–824. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (American Skullcap) grown in vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upton, R.; d’Ayu, R.H. Skullcap Scutellaria lateriflora L.: An American nervine. J. Herb. Med. 2012, 2, 76–96. [Google Scholar] [CrossRef]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Albrecht, T.; Argueso, C.T. Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth–defence trade-off. Ann. Bot. 2017, 119, 725–735. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Kwiecień, I.; Miceli, N.; D’Arrigo, M.; Marino, A.; Ekiert, H. Antioxidant potential and enhancement of bioactive metabolite production in in vitro cultures of Scutellaria lateriflora L. by biotechnological methods. Molecules 2022, 27, 1140. [Google Scholar] [CrossRef]
- Pei, T.; Yan, M.; Huang, Y.; Wei, Y.; Martin, C.; Zhao, Q. Specific flavonoids and their biosynthetic pathway in Scutellaria baicalensis. Front. Plant Sci. 2022, 13, 866282. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Kochanek, B.; Sela, N.; Lerno, L.; Ebeler, S.E.; Lichter, A. Cytokinin but not gibberellin application had major impact on the phenylpropanoid pathway in grape. Hortic. Res. 2021, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Hidayathulla, S. Phytochemical profile of fresh and senescent leaves due to storage for Ficus deltoidea. Plant Biosyst. 2017, 151, 74–83. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Nadjafi, F.; Menichini, F. Angiotensin-converting enzyme inhibitory activity and antioxidant properties of Nepeta crassifolia Boiss & Buhse and Nepeta binaludensis Jamzad. Phytother. Res. 2013, 27, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, D.; Dryś, A.; Matkowski, A. Antiradical and antioxidant activity of flavones from Scutellariae baicalensis radix. Nat. Prod. Res. 2015, 29, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships between chemical structure and antioxidant activity of isolated phytocompounds from Lemon verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, E.I.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Gao, J.-J.; Kiharu Igalashi, K.; Nukina, M. Radical Scavenging Activity of Phenylpropanoid Glycosides in Caryopterisincana. Biosci. Biotechnol. Biochem. 1999, 63, 983–988. [Google Scholar] [CrossRef]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective Potential of Verbascoside Isolated from Acanthus mollis L. Leaves through Its Enzymatic Inhibition and Free Radical Scavenging Ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef]
- Pierre Luhata, L.; Usuki, T. Free radical scavenging activities of verbascoside and isoverbascoside from the leaves of Odontonema strictum (Acanthaceae). Bioorg. Med. Chem. Lett. 2022, 59, 128528. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, C.; Tatlı, I.; Orhan, I.; Akdemir, Z. Cholinesterase Inhibitory and Antioxidant Properties of Verbascum mucronatum Lam. and its Secondary Metabolites. Z. Naturforsch. C 2010, 65, 667–674. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Kędzia, A.; Kusiak, A.; Molęda-Ciszewska, B.; Wojtaszek-Słomińska, A.; Racka-Pilszak, B.; Czernecka, B. Sensitivity to Baikadent yeastlike fungi from genus candida isolated from orthodontic appliances wearers. Post. Fitoter. 2012, 3, 146–150. (In Polish) [Google Scholar]
- Kędzia, A.; Kufel, A.; Ciecierski, M.; Kwapisz, E.; Wierzbowska, M. Susceptibility to Baikadent microaerophilic bacteria isolated from atherosclerotic plaques. Post. Fitoter. 2014, 3, 144–149. (In Polish) [Google Scholar]
- Li, W.; Sun, H.; Zhou, J.; Zhang, Y.; Liu, L.; Gao, Y. Antibacterial activities, antioxidant contents and antioxidant properties of three traditional Chinese medicinal extracts. Bangladesh J. Pharmacol. 2015, 10, 131–137. [Google Scholar] [CrossRef]
- Blaszczyk, T.; Krzyzanowska, J.; Lamer-Zarawska, E. Screening for antimycotic properties of 56 traditional Chinese drugs. Phytother. Res. 2000, 14, 210–214. [Google Scholar] [CrossRef]
- Trinh, H.; Yoo, Y.; Won, K.H.; Ngo, H.T.T.; Yang, J.E.; Cho, J.G.; Lee, S.W.; Kim, K.Y.; Yi, T.H. Evaluation of in vitro antimicrobial activity of Artemisia apiacea H. And Scutellaria baicalensis G. extracts. J. Med. Microbiol. 2018, 67, 489–495. [Google Scholar] [CrossRef]
- Franzblau, S.G.; Cross, C. Comparative in vitro antimicrobial activity of Chinese medicinal herbs. J. Ethnopharmacol. 1986, 15, 279–288. [Google Scholar] [CrossRef]
- Tsao, T.F.; Newman, M.G.; Kwok, Y.Y.; Horikoshi, A.K. Effect of Chinese and Western antimicrobial agents on selected oral bacteria. J. Dent. Res. 1982, 61, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, B.S.F.; Arab, A.; Emami, S.A.; Asili, J.; Hasanzadeh-Khayyat, M.; Sahebkar, A. Antimicrobial and antioxidant activities of methanol, dichloromethane and ethyl acetate extracts of Scutellaria lindbergii Rech.f. Chiang Mai J. Sci. 2013, 40, 49–59. [Google Scholar]
- Tsai, C.C.; Lin, C.S.; Hsu, C.R.; Chang, C.M.; Chang, I.W.; Lin, L.W.; Hung, C.H.; Wang, J.L. Using the Chinese herb Scutellaria barbata against extensively drug-resistant Acinetobacter baumannii infections: In vitro and in vivo studies. BMC Complement. Altern. Med. 2018, 18, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Pant, C.C.; Melkani, A.B.; Mohan, L.; Dev, V. Composition and antibacterial activity of essential oil from Scutellaria grossa Wall ex Benth. Nat. Prod. Res. 2012, 26, 190–192. [Google Scholar] [CrossRef]
- Melkani, A.B.; Nailwal, M.; Mohan, L.; Pant, C.C.; Dev, V. Steam volatile oil from Scutellaria repens Buch-Ham. ex D. Don; its composition and antibacterial activity. J. Essent. Oil Res. 2013, 25, 368–371. [Google Scholar] [CrossRef]
- Shah, M.; Murad, W.; Ur Rehman, N.; Mubin, S.; Al-Sabahi, J.N.; Ahmad, M.; Zahoor, M.; Ullah, O.; Waqas, M.; Ullah, S. GC-MS analysis and biomedical therapy of oil from n-hexane fraction of Scutellaria edelbergii Rech. f.: In vitro, in vivo, and in silico approach. Molecules 2021, 26, 7676. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Mubin, S.; Hassan, S.S.u.; Tagde, P.; Ullah, O.; Rahman, M.H.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach. Biomolecules 2022, 12, 936. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Lazari, D.M.; Kyriazopoulos, P.; Golegou, S.; Triantaphyllidis, S.; Sokovic, M.; Kypriotakis, Z. Composition andantimicrobial activity of the essential oils of Scutellaria sieberia Benth. And Scutellaria rupestris Boiss. et Heldr. ssp. Adenotricha (Boiss. et Heldr.) Greuter et Burdet from Greece. J. Essent. Oil Res. 2005, 17, 232–235. [Google Scholar]
- Nan, Y.; Yuan, L.; Zhou, L.; Niu, Y. Study on the optimization of the technology for the extraction and purification of total flavone in Scutellaria baicalensis and its antibacterial activity. Afr. J. Microbiol. Res. 2011, 5, 5689–5696. [Google Scholar]
- Rajendran, N.; Subramaniam, S.; Christena, L.R.; Muthuraman, M.S.; Subramanian, N.S.; Pemiah, B.; Sivasubramanian, A. Antimicrobial flavonoids isolated from Indian medicinal plant Scutellaria oblonga inhibit biofilms formed by common food pathogens. Nat. Prod. Res. 2016, 30, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Bozov, P.; Girova, T.; Prisadova, N.; Hristova, Y.; Gochev, V. Antimicrobial activity of neo-clerodane diterpenoids isolated from Lamiaceae species against pathogenic and food spoilage microorganisms. Nat. Prod. Commun. 2015, 10, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Banik, K.; Khatoon, E.; Harsha, C.; Rana, V.; Parama, D.; Thakur, K.K.; Bishayee, A.; Kunnumakkara, A.B. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother. Res. 2022, 36, 1854–1883. [Google Scholar] [CrossRef]
- Wang, L.; Feng, T.; Su, Z.; Pi, C.; Wei, Y.; Zhao, L. Latest research progress on anticancer effect of baicalin and its aglycone baicalein. Arch. Pharm. Res. 2022, 45, 535–557. [Google Scholar] [CrossRef]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef] [PubMed]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997, 112, 127–133. [Google Scholar] [CrossRef]
- Parajuli, P.; Joshee, N.; Rimando, A.M.; Mittal, S.; Yadav, A.K. In vitro antitumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Med. 2009, 75, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; But, P.P.H. (Eds.) Pharmacology and Applications of Chinese Materia Medica; World Scientific: Singapore, 1987; Volume II. [Google Scholar]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically active compounds in four species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Ohnishi, M.; Morishita, H.; Iwahashi, H.; Shitzuo, T.; Yoshiaki, S.; Kimura, M.; Kido, R. Inhibitory effects of chlorogenic acid on linoleic acid, peroxidation and haemolysis. Phytochemistry 1994, 36, 579–583. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. JPN J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobson, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]



| BAP/NAA (mg/L) | 3.0/1.0 | 2.0/2.0 | 1.0/1.0 | 1.0/0.5 | 1.0/0.0 | 0.5/2.0 |
|---|---|---|---|---|---|---|
| Fresh biomass 1 (g) | 4.465 ± 0.767 | 4.616 ± 0.381 | 4.587 ± 0.570 | 4.040 ± 0.736 | 4.923 ± 0.512 | 4.042 ± 0.318 |
| Dry biomass (g) | 0.288 ± 0.051 | 0.296 ± 0.016 | 0.287 ± 0.022 | 0.274 ± 0.044 | 0.305 ± 0.024 | 0.275 ± 0.019 |
| Flavonoids (mg/g DW) | MS Medium Variant BAP/NAA (mg/L) | |||||
|---|---|---|---|---|---|---|
| 3.0/1.0 | 2.0/2.0 | 1.0/1.0 | 1.0/0.5 | 1.0/0.0 | 0.5/2.0 | |
| Baicalein | 14.52 ± 3.77 | 12.63 ± 2.22 | 13.45 ± 2.21 | 10.97 ± 1.08 | 13.42 ± 1.39 | 12.55 ± 2.79 |
| Baicalin | 104.51 ± 14.34 abc | 94.57 ± 5.29 abcd | 67.89 ± 10.93 ade | 70.36 ± 14.23 abde | 66.15 ± 11.70 abcde | 81.63 ± 9.35 acde |
| Wogonin | 41.58 ± 8.46 | 32.42 ± 5.80 | 36.63 ± 4.28 | 38.27 ± 7.29 | 38.76 ± 5.38 | 40.46 ± 12.89 |
| Wogonoside | 346.40 ± 53.05 ab | 330.44 ± 44.25 ab | 194.98 ± 29.78 ac | 275.34 ± 61.31 abc | 261.06 ± 67.19 abc | 273.87 ± 48.25 abc |
| Scutellarin | 35.37 ± 3.76 abc | 31.02 ± 5.37 ab | 35.67 ± 6.67 abc | 34.48 ± 3.87 abc | 40.69 ± 2.10 abc | 30.99 ± 3.78 ac |
| Chrysin | 32.46 ± 2.27 | 32.19 ± 3.61 | 35.97 ± 4.36 | 38.91 ± 5.80 | 38.00 ± 6.24 | 35.50 ± 7.75 |
| Total content | 574.84 ± 85.66 | 533.26 ± 66.54 | 384.59 ± 58.24 | 468.33 ± 93.58 | 458.08 ± 94.00 | 475.01 ± 84.81 |
| Phenolic Acids (mg/g DW) | MS Medium Variant BAP/NAA (mg/L) | |||||
|---|---|---|---|---|---|---|
| 3.0/1.0 | 2.0/2.0 | 1.0/1.0 | 1.0/0.5 | 1.0/0.0 | 0.5/2.0 | |
| p-Hydroxybenzoic acid | 2.09 ± 0.72 a | 0.56 ± 0.06 b | 0.62 ± 0.07 b | 0.79 ± 0.16 b | 0.59 ± 0.05 a | 1.65 ± 0.26 b |
| Caffeic acid | 2.57 ± 0.39 ab | 2.02 ± 0.18 abc | 2.20 ± 0.66 abc | 2.14 ± 0.21 abc | 1.31 ± 0.52 ab | 2.43 ± 0.28 ac |
| Ferulic acid | 7.01 ± 3.73 abc | 10.14 ± 1.16 ab | 6.55 ± 1.57 abc | 8.13 ± 0.16 abc | 7.93 ± 0.78 ab | 5.10 ± 0.39 ac |
| m-Coumaric acid | * | 2.76 ± 0.53 | * | * | * | 2.90 ± 0.55 |
| Total content | 11.67 ± 4.84 abc | 15.47 ± 1.93 bd | 9.37 ± 2.30 abc | 11.06 ± 0.53 abd | 9.83 ± 1.35 abd | 12.09 ± 1.48 abc |
| Phenylpropanoid Glycosides (mg/g DW) | MS Medium Variant BAP/NAA (mg/L) | |||||
|---|---|---|---|---|---|---|
| 3.0/1.0 | 2.0/2.0 | 1.0/1.0 | 1.0/0.5 | 1.0/0.0 | 0.5/2.0 | |
| Verbascoside | 440.55 ± 69.60 a | 364.20 ± 36.97 ab | 364.31 ± 39.65 ab | 362.12 ± 39.96 ab | 377.35 ± 45.08 ab | 349.73 ± 34.12 b |
| Isoerbascoside | 16.84 ± 3.54 | 17.19 ± 3.21 | 19.84 ± 4.91 | 17.60 ± 4.51 | 17.90 ± 0.00 | 15.34 ± 5.17 |
| Total content | 457.39 ± 73.13 | 381.40 ± 40.18 | 384.14 ± 44.55 | 379.72 ± 44.47 | 395.25 ± 45.08 | 365.07 ± 39.28 |
| Bacterial Strains | Species of Microorganisms | Antibiotic Activity (MIC–mg/mL) |
|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | 20.0 |
| Staphylococcus epidermidis | 30.0 | |
| Enterococcus faecalis | 30.0 | |
| Enterococcus faecium | 30.0 | |
| Bacillus subtilis | 10.0 | |
| Gram-negative bacteria | Escherichia coli | 30.0 |
| Enterobacter aerogenes | 30.0 | |
| Enterobacter cloacae | 30.0 | |
| Klebsiella pneumoniae | 30.0 | |
| Pseudomonas aeruginosa | 20.0 |
| Fungal Strains | Species of Microorganisms | Antibiotic Activity (MIC—mg/mL) |
|---|---|---|
| Yeast-like fungi | Candida albicans | 30.0 |
| Candida krusei | 30.0 | |
| Candida quilliermondii | 30.0 | |
| Moulds | Aspergillus flavus | 40.0 |
| Penicillium chrysogenum | 30.0 | |
| Dermatophytes | Trichophyton tonsurans | 7.5 |
| MS Medium Variant | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| PGR (mg/L) | BAP | 3.0 | 2.0 | 1.0 | 1.0 | 1.0 | 0.5 |
| NAA | 1.0 | 2.0 | 1.0 | 0.5 | 0.0 | 2.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiecień, I.; Łukaszyk, A.; Miceli, N.; Taviano, M.F.; Davì, F.; Kędzia, E.; Ekiert, H. In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites. Molecules 2023, 28, 1785. https://doi.org/10.3390/molecules28041785
Kwiecień I, Łukaszyk A, Miceli N, Taviano MF, Davì F, Kędzia E, Ekiert H. In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites. Molecules. 2023; 28(4):1785. https://doi.org/10.3390/molecules28041785
Chicago/Turabian StyleKwiecień, Inga, Aleksandra Łukaszyk, Natalizia Miceli, Maria Fernanda Taviano, Federica Davì, Elżbieta Kędzia, and Halina Ekiert. 2023. "In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites" Molecules 28, no. 4: 1785. https://doi.org/10.3390/molecules28041785
APA StyleKwiecień, I., Łukaszyk, A., Miceli, N., Taviano, M. F., Davì, F., Kędzia, E., & Ekiert, H. (2023). In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites. Molecules, 28(4), 1785. https://doi.org/10.3390/molecules28041785








