Glycyrrhizae radix et Rhizoma-Derived Carbon Dots and Their Effect on Menopause Syndrome in Ovariectomized Mice
Abstract
:1. Introduction
2. Results
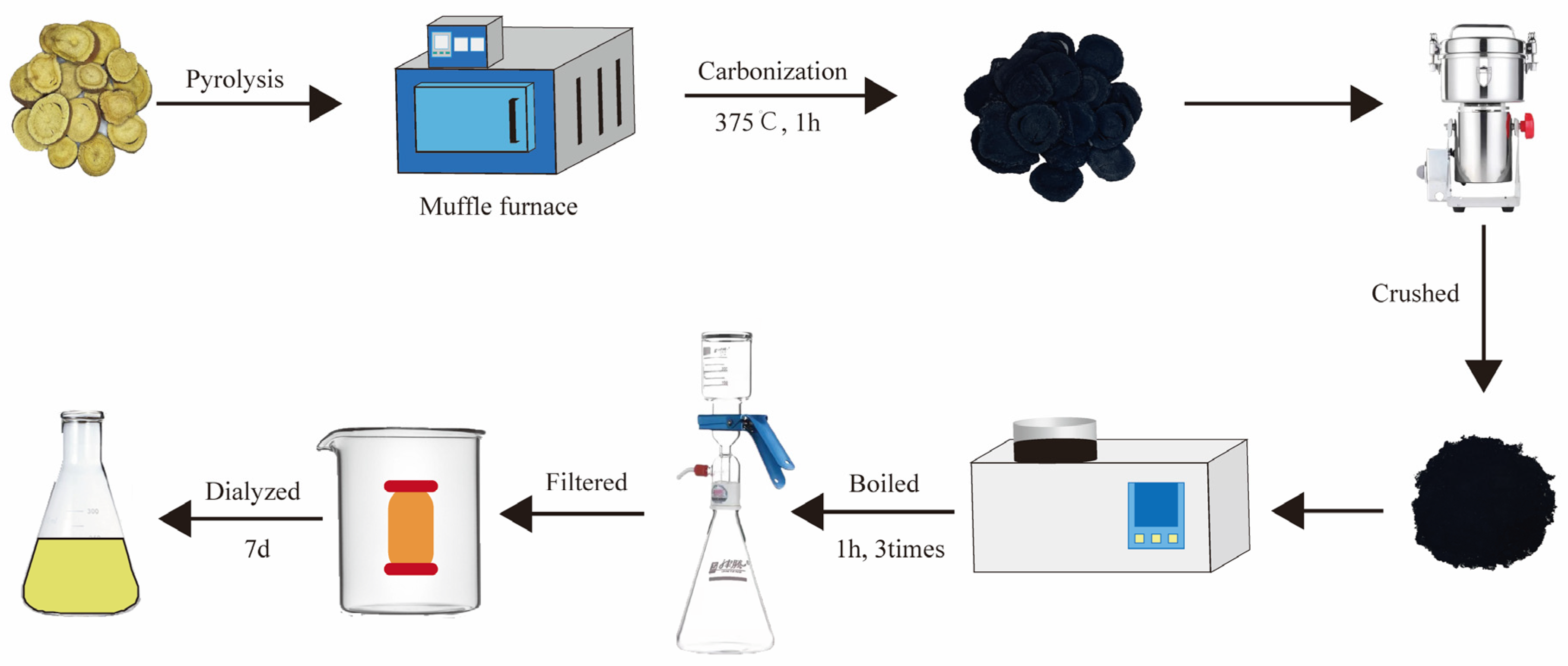
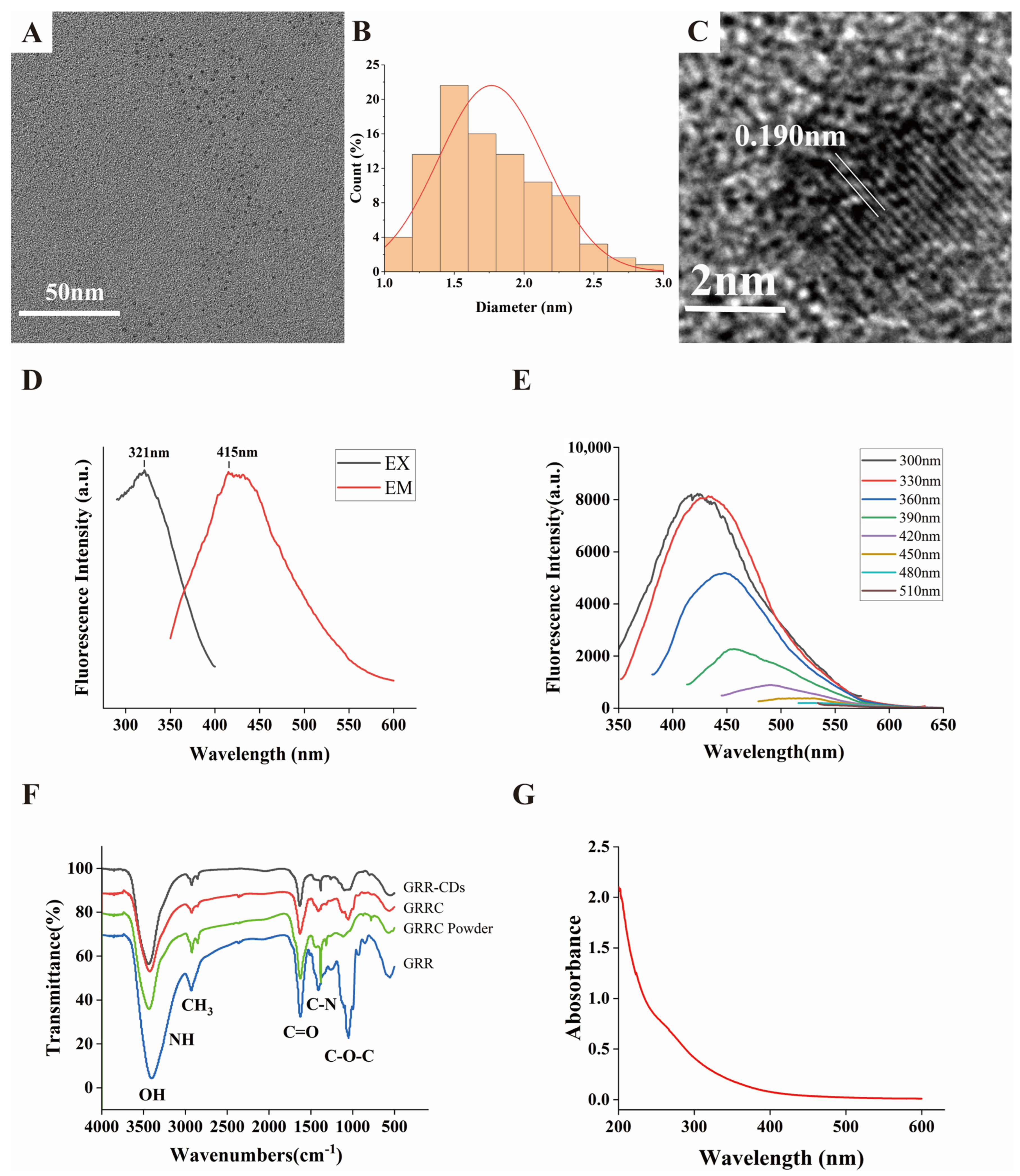
2.1. Characterization of GRR-CDs
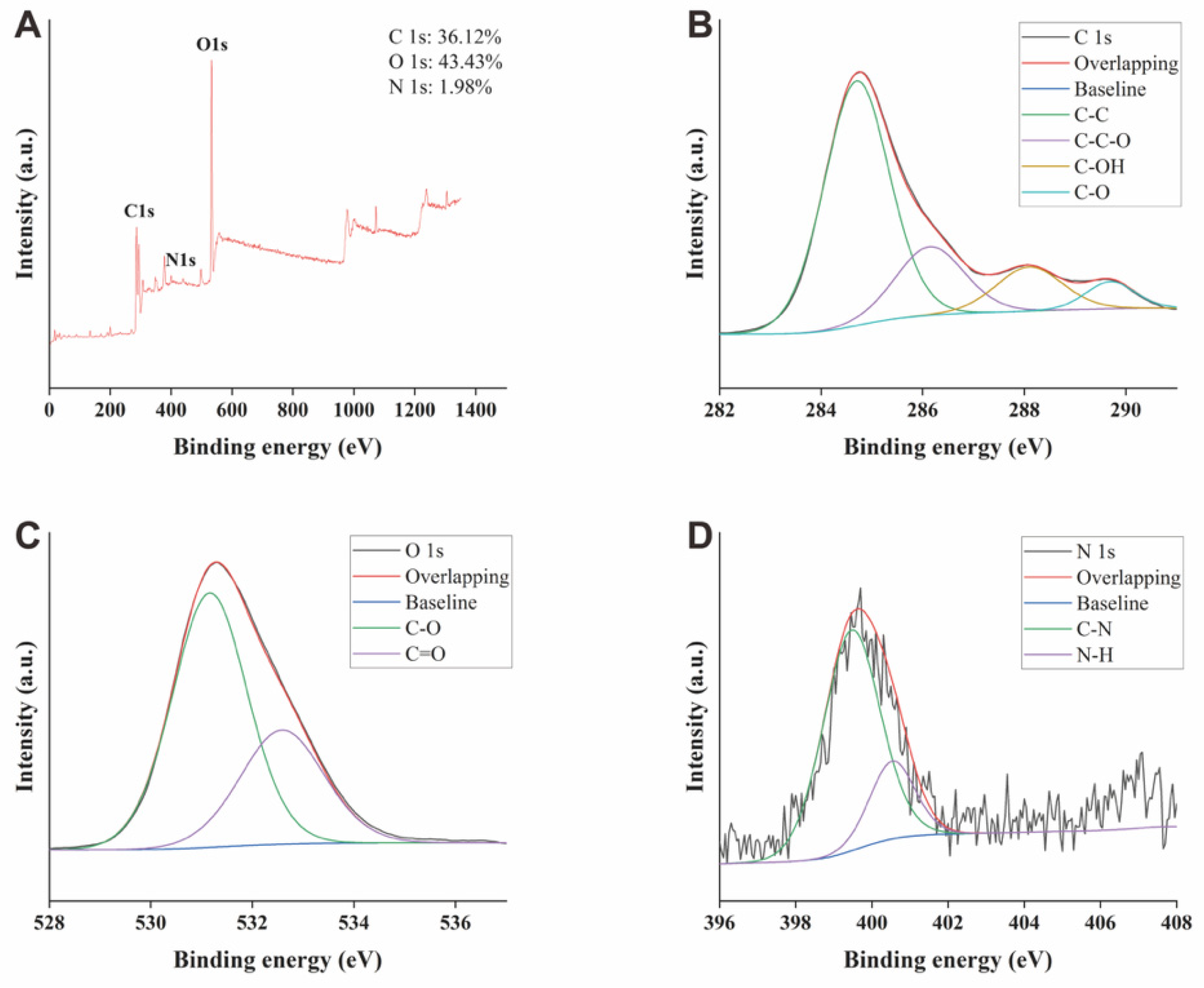
2.2. X-ray Photoelectron Spectroscopy of GRR-CDs
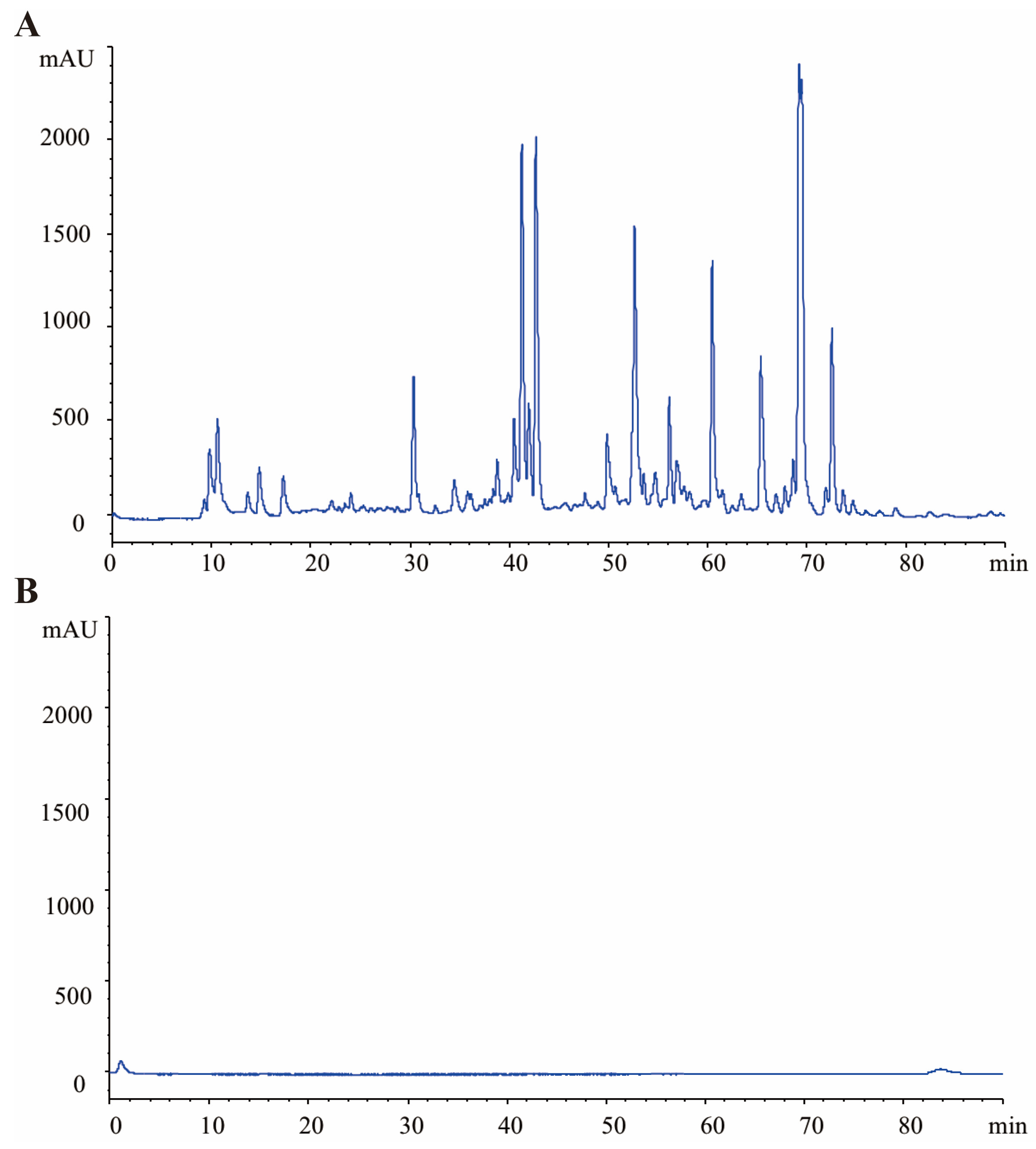
2.3. HPLC Profile of GRR and GRR-CDs
2.4. Effect of GRR-CDs on Sex Hormones in Healthy Female Mice
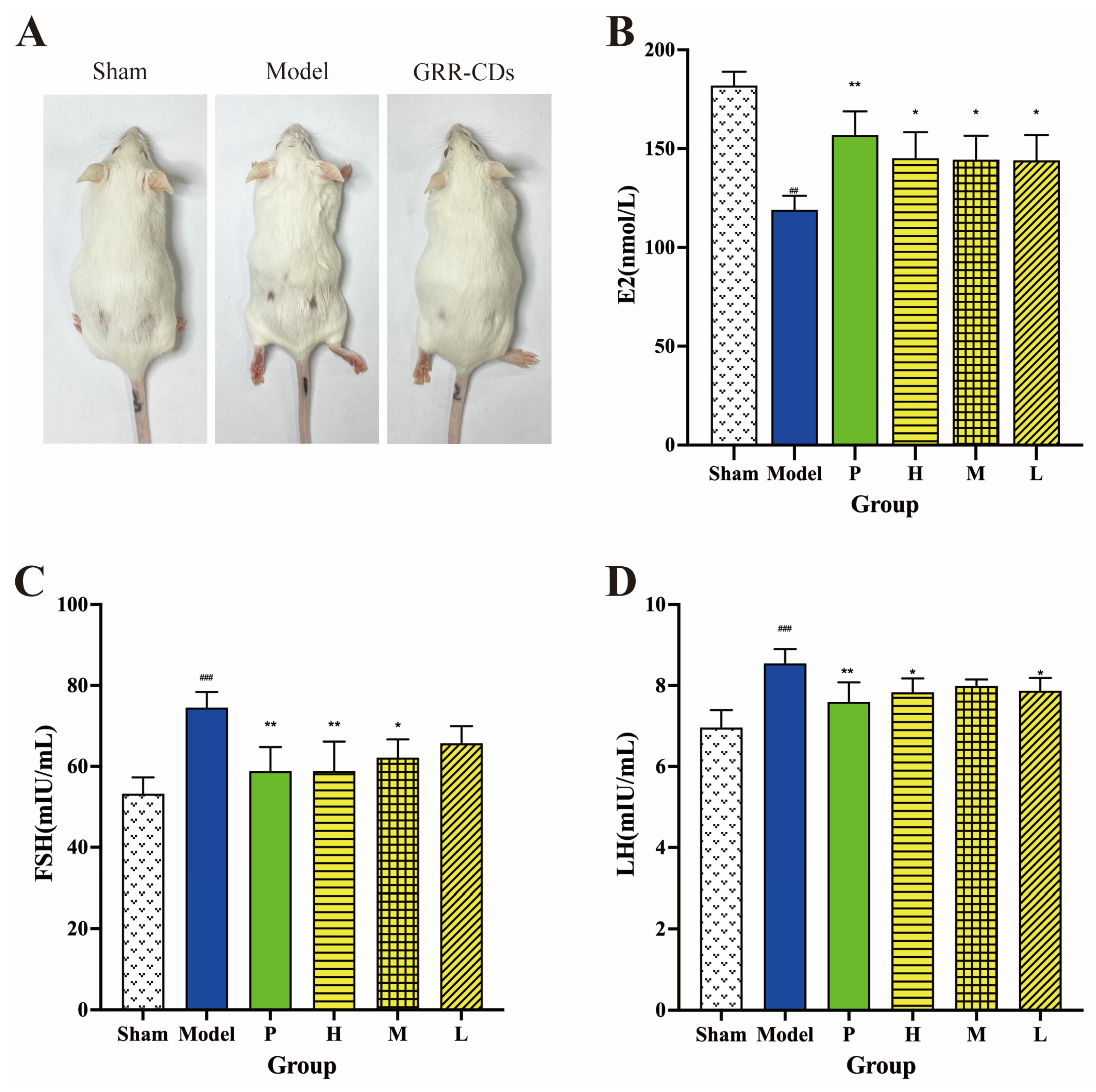
2.5. Effect of GRR-CDs on Sex Hormone in MPS Mice
2.6. Effect of GRR-CDs on Uterus Index
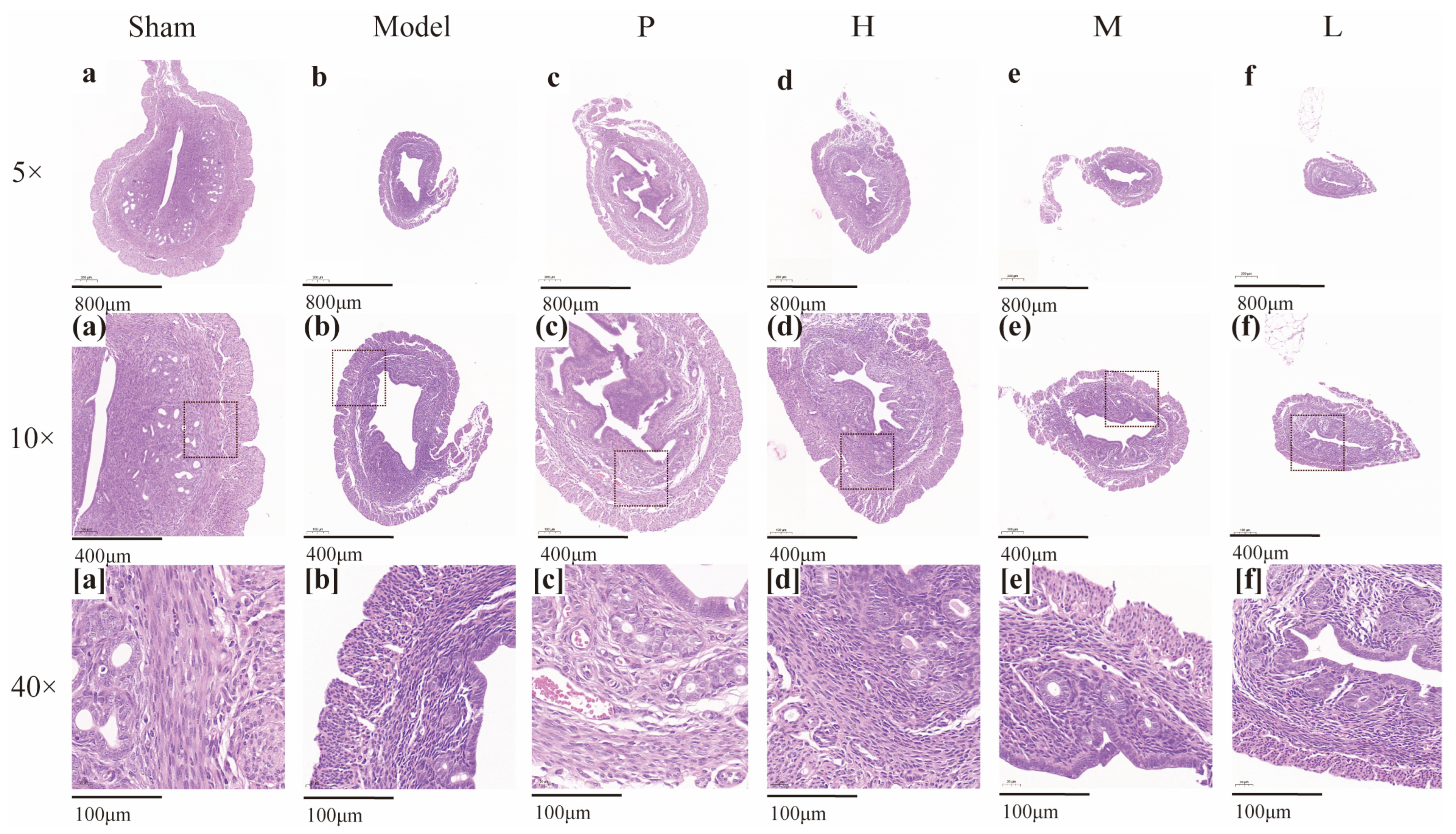
2.7. Effect of GRR-CDs on the Histopathological Examination of Uterine Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of GRR-CDs
4.4. Characterization of GRR-CDs
4.5. Fluorescence Quantum Yield of GRR-CDs
4.6. GRR-CDs Fingerprint Analysis
4.7. Treatment on Healthy Female Mice
4.8. MPS Mouse Model and Drug Treatment
4.9. Effect of GRR-CDs on the Uterus Index in MPS Mice
4.10. Detection of Sex Hormones in Serum
4.11. Histopathological Examination of Uterus Tissue
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Minkin, M.J. Menopause: Hormones, lifestyle, and optimizing aging. Obstet. Gynecol. Clin. N. Am. 2019, 46, 501–514. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal symptoms and their management. Endocrinol. Metab. Clin. North Am. 2015, 44, 497–515. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.S.; Soares, C.N.; Vitonis, A.F.; Otto, M.W.; Harlow, B.L. Risk for new onset of depression during the menopausal transition: The Harvard study of moods and cycles. Arch. Gen. Psychiatry 2006, 63, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Dennerstein, L.; Dudley, E.C.; Hopper, J.L.; Guthrie, J.R.; Burger, H.G. A prospective population-based study of menopausal symptoms. Obstet. Gynecol. 2000, 96, 351–358. [Google Scholar] [PubMed]
- Saunier, E.F.; Vivar, O.I.; Rubenstein, A.; Zhao, X.; Olshansky, M.; Baggett, S.; Staub, R.E.; Tagliaferri, M.; Cohen, I.; Speed, T.P.; et al. Estrogenic plant extracts reverse weight gain and fat accumulation without causing mammary gland or uterine proliferation. PLoS ONE 2011, 6, e28333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deligdisch-Schor, L.; Mareş Miceli, A. Hormonal biophysiology of the uterus. Adv. Exp. Med. Biol. 2020, 1242, 1–12. [Google Scholar] [PubMed]
- Lee, E.; Jang, M.; Lim, T.G.; Kim, T.; Ha, H.; Lee, J.H.; Hong, H.D.; Cho, C.W. Selective activation of the estrogen receptor-β by the polysaccharide from Cynanchum wilfordii alleviates menopausal syndrome in ovariectomized mice. Int. J. Biol. Macromol. 2020, 165, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.G.; Dluhy, R.G. Rethinking postmenopausal hormone therapy. N. Engl. J. Med. 2003, 348, 579–580. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Patil, A.; Thakur, S.; Kesharwani, P. Carbon dots: Emerging theranostic nanoarchitectures. Drug Discov. Today 2018, 23, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.T.; Wang, L.N.; Xu, J.; Huang, K.J.; Wu, X. Synthesis and modification of carbon dots for advanced biosensing application. Analyst 2021, 146, 4418–4435. [Google Scholar] [CrossRef]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, e1805087. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon dots as promising tools for cancer diagnosis and therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Anand, A.; Manavalan, G.; Mandal, R.P.; Chang, H.T.; Chiou, Y.R.; Huang, C.C. Carbon dots for bacterial detection and antibacterial applications-a minireview. Curr. Pharm. Des. 2019, 25, 4848–4860. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Sun, Z.; Lu, F.; Xiong, W.; Luo, J.; Kong, H.; Wang, Q.; Qu, H.; Zhao, Y. Hemostatic and hepatoprotective bioactivity of junci medulla carbonisata-derived carbon dots. Nanomedicine 2019, 14, 431–446. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Cheng, J.; Liu, X.; Wang, Y.; Yan, X.; Zhang, Y.; Lu, F.; Wang, Q.; Qu, H. Novel carbon dots derived from Schizonepetae Herba Carbonisata and investigation of their haemostatic efficacy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1562–1571. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Lu, F.; Zhang, M.; Kong, H.; Cheng, J.; Luo, J.; Zhao, Y.; Qu, H. The neuroprotective effect of pretreatment with carbon dots from Crinis Carbonisatus (carbonized human hair) against cerebral ischemia reperfusion injury. J. Nanobiotechnol. 2021, 19, 257. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.J.; Likis, F.E. Estrogen: Physiology, pharmacology, and formulations for replacement therapy. J. Midwifery Womens Health 2002, 47, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Montagner, A.; Fontaine, C.; Lenfant, F.; Arnal, J.F.; Gourdy, P. Nuclear and membrane actions of estrogen receptor alpha: Contribution to the regulation of energy and glucose homeostasis. Adv. Exp. Med. Biol. 2017, 1043, 401–426. [Google Scholar] [PubMed]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A nutraceutical approach to menopausal complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef] [Green Version]
- Küchler, E.C.; de Lara, R.M.; Omori, M.A.; Marañón-Vásquez, G.; Baratto-Filho, F.; Nelson-Filho, P.; Stuani, M.B.S.; Blanck-Lubarsch, M.; Schroeder, A.; Proff, P.; et al. Effects of estrogen deficiency during puberty on maxillary and mandibular growth and associated gene expression—AN μCT study on rats. Head Face Med. 2021, 17, 14. [Google Scholar] [CrossRef]
- Lizcano, F.; Guzmán, G. Estrogen deficiency and the origin of obesity during menopause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef] [Green Version]
- Hampson, E. Estrogens, aging, and working memory. Curr. Psychiatry Rep. 2018, 20, 109. [Google Scholar] [CrossRef] [Green Version]
- Bacon, J.L. The menopausal transition. Obstet. Gynecol. Clin. North Am. 2017, 44, 285–296. [Google Scholar] [CrossRef]
- Bruce, D.; Rymer, J. Symptoms of the menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 25–32. [Google Scholar] [CrossRef]
- Makanji, Y.; Harrison, C.A.; Robertson, D.M. Feedback regulation by inhibins A and B of the pituitary secretion of follicle-stimulating hormone. Vitam. Horm. 2011, 85, 299–321. [Google Scholar]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The menopause transition: Signs, symptoms, and management options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.A.; Pal, L.; Manson, J.E. Hormone therapy in menopause: Concepts, controversies, and approach to treatment. Endocr. Rev. 2021, 42, 720–752. [Google Scholar] [CrossRef] [PubMed]
- Honisett, S.Y.; Tangalakis, K.; Wark, J.; Apostolopoulos, V.; Stojanovska, L. The effects of hormonal therapy and exercise on bone turnover in postmenopausal women: A randomised double-blind pilot study. Pril 2016, 37, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. Jama 2002, 288, 321–333. [Google Scholar]
- Ghosal, K.; Ghosh, A. Carbon dots: The next generation platform for biomedical applications. Mater Sci. Eng. C Mater Biol. Appl. 2019, 96, 887–903. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, J.; Sun, Z.; Kong, H.; Zhang, Y.; Wang, S.; Wang, X.; Zhao, Y.; Qu, H. Protective effects of carbon dots derived from phellodendri chinensis cortex carbonisata against deinagkistrodon acutus venom-induced acute kidney injury. Nanoscale Res. Lett. 2019, 14, 377. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, M.; Cheng, J.; Zhang, Y.; Luo, J.; Liu, Y.; Kong, H.; Qu, H.; Zhao, Y. Effect of lonicerae japonicae flos carbonisata-derived carbon dots on rat models of fever and hypothermia induced by lipopolysaccharide. Int. J. Nanomed. 2020, 15, 4139–4149. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, F.; Cheng, J.; Zhang, M.; Zhang, Y.; Xiong, W.; Zhao, Y.; Qu, H. Haemostatic bioactivity of novel Schizonepetae Spica Carbonisata-derived carbon dots via platelet counts elevation. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S308–S317. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Yan, X.; Zhang, M.; Zhang, Y.; Cheng, J.; Lu, F.; Qu, H.; Wang, Q.; Zhao, Y. Novel phellodendri cortex (huang bo)-derived carbon dots and their hemostatic effect. Nanomedicine 2018, 13, 391–405. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, M.; Cheng, J.; Wu, S.; Xiong, W.; Kong, H.; Zhao, Y.; Qu, H. Hemostatic effect of novel carbon dots derived from Cirsium setosum Carbonisata. RSC Adv. 2018, 8, 37707–37714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kong, H.; Liu, X.; Luo, J.; Xiong, W.; Zhu, Y.; Zhang, Y.; Zhang, Y.; Zhao, Y.; Qu, H. Novel carbon dots derived from cirsii japonici herba carbonisata and their haemostatic effect. J. Biomed. Nanotechnol. 2018, 14, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, M.; Kong, H.; Wang, S.; Cheng, J.; Qu, H.; Zhao, Y. Novel carbon dots derived from puerariae lobatae radix and their anti-gout effects. Molecules 2019, 24, 4152. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, Y.; Bai, X.; Cheng, G.; Cao, T.; Dong, L.; Zhao, J.; Zhang, Y.; Qu, H.; Kong, H.; et al. Glycyrrhizae radix et Rhizoma-Derived Carbon Dots and Their Effect on Menopause Syndrome in Ovariectomized Mice. Molecules 2023, 28, 1830. https://doi.org/10.3390/molecules28041830
Zhang Y, Chen Y, Bai X, Cheng G, Cao T, Dong L, Zhao J, Zhang Y, Qu H, Kong H, et al. Glycyrrhizae radix et Rhizoma-Derived Carbon Dots and Their Effect on Menopause Syndrome in Ovariectomized Mice. Molecules. 2023; 28(4):1830. https://doi.org/10.3390/molecules28041830
Chicago/Turabian StyleZhang, Ying, Yumin Chen, Xue Bai, Guoliang Cheng, Tianyou Cao, Liyang Dong, Jie Zhao, Yue Zhang, Huihua Qu, Hui Kong, and et al. 2023. "Glycyrrhizae radix et Rhizoma-Derived Carbon Dots and Their Effect on Menopause Syndrome in Ovariectomized Mice" Molecules 28, no. 4: 1830. https://doi.org/10.3390/molecules28041830




