Al Foil-Supported Carbon Nanosheets as Self-Supporting Electrodes for High Areal Capacitance Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Characterization of Samples
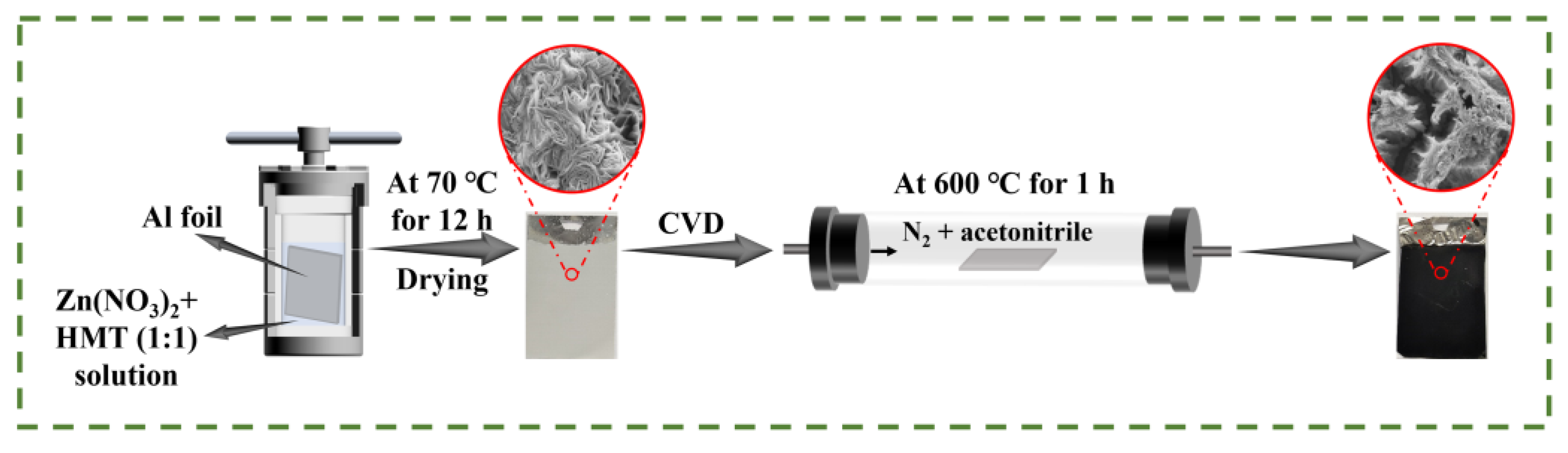
2.1.1. Diagram of the Preparation of Electrode Materials
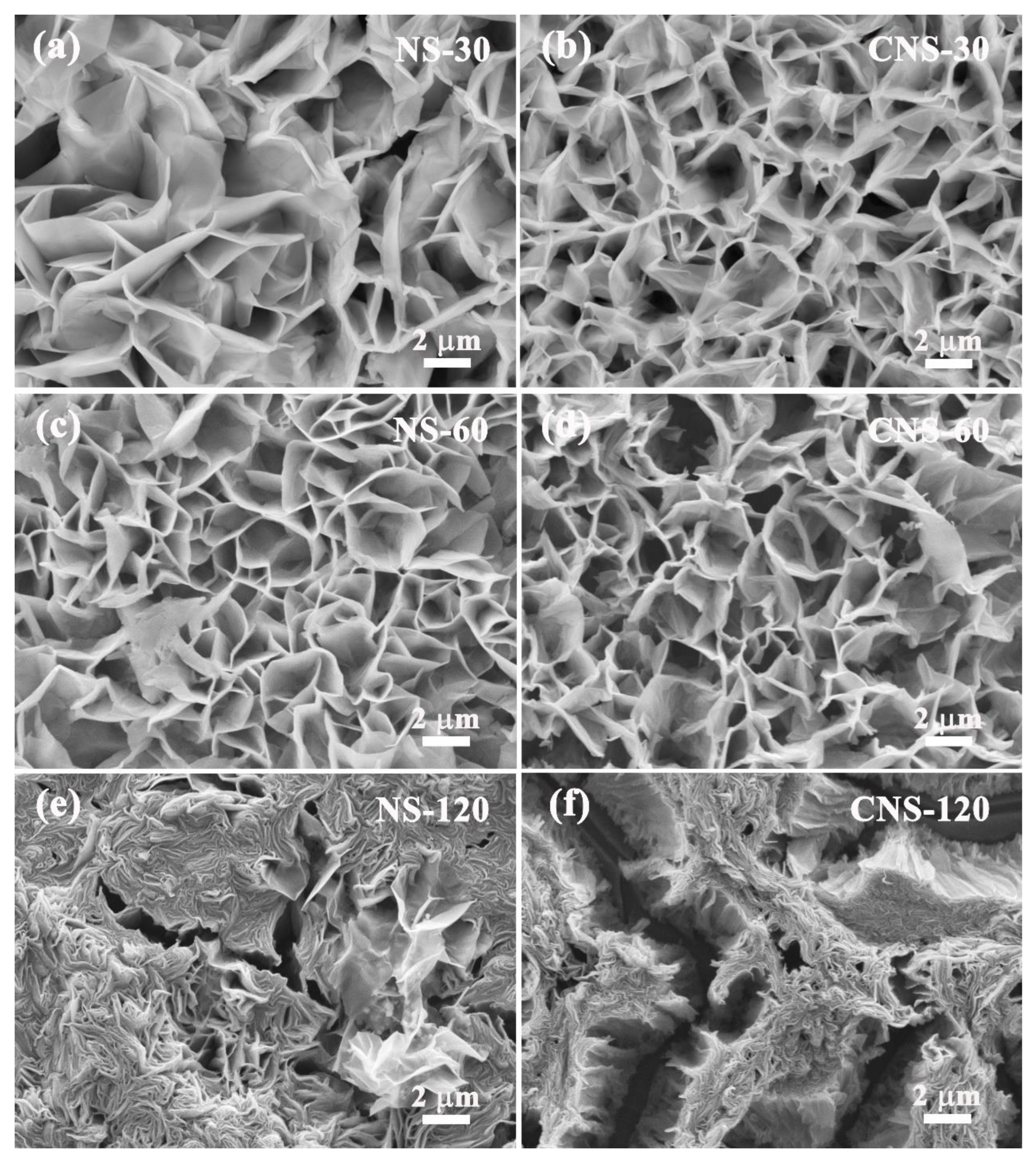
2.1.2. SEM
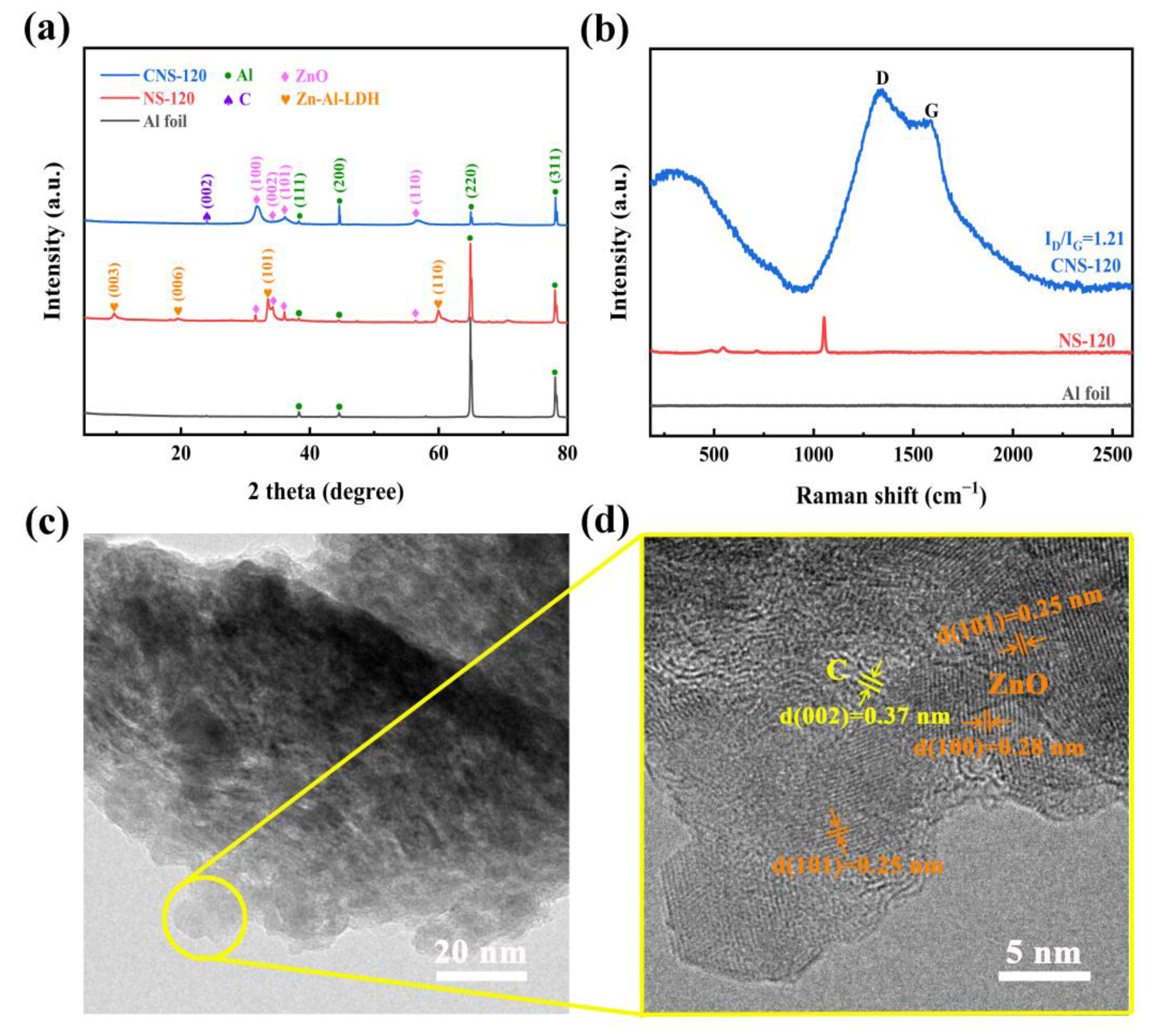
2.1.3. XRD, Raman, and TEM
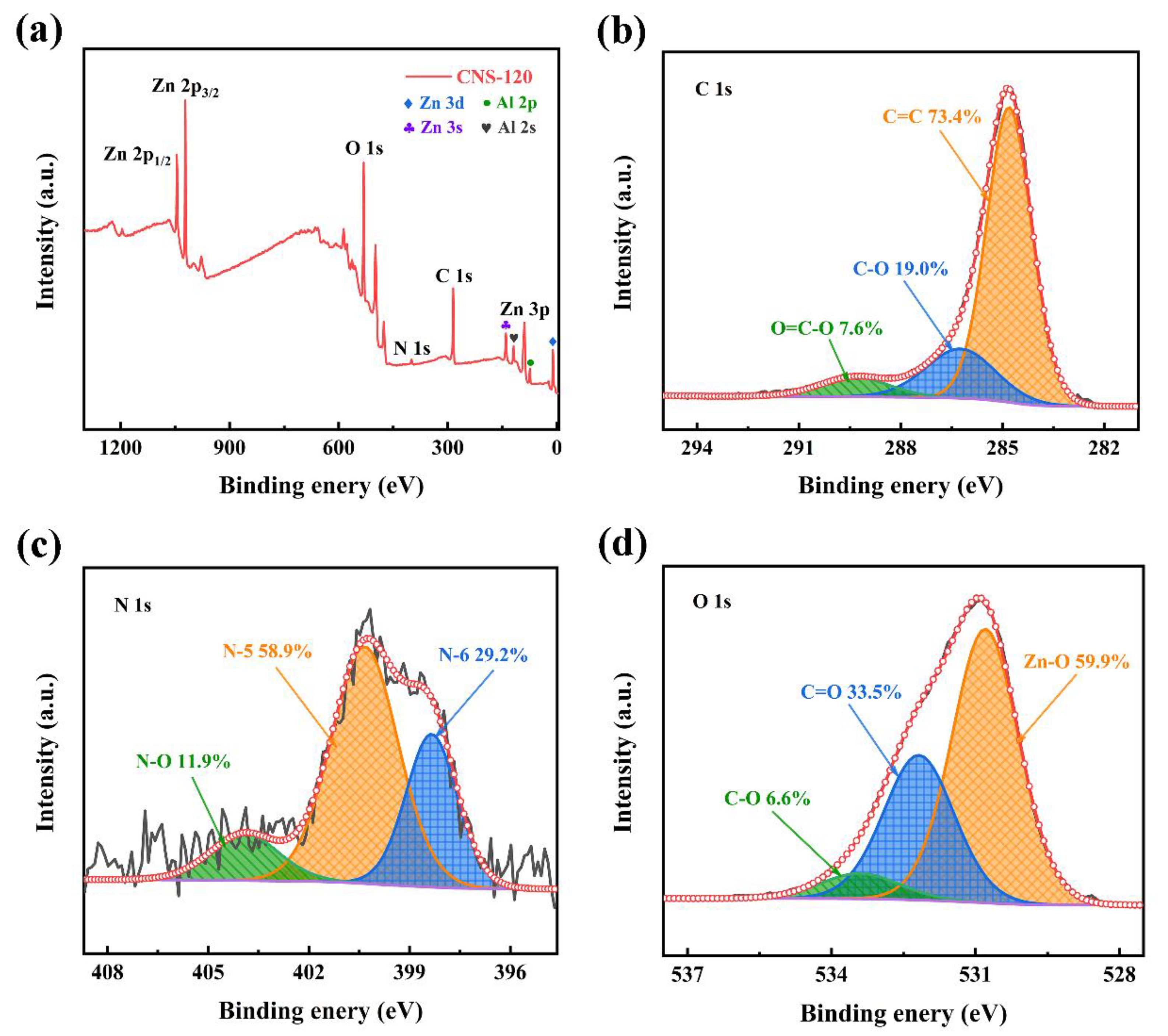
2.1.4. XPS
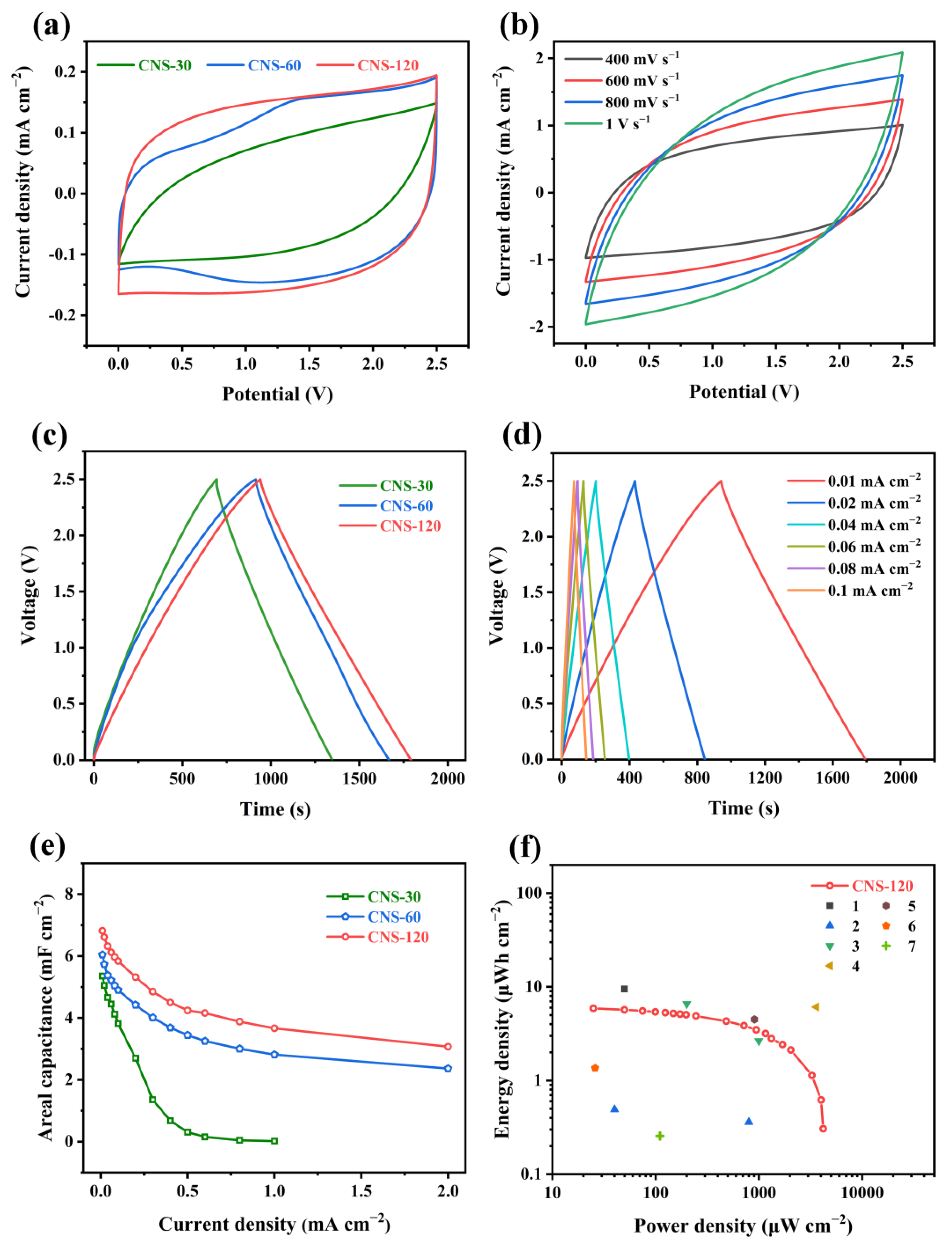
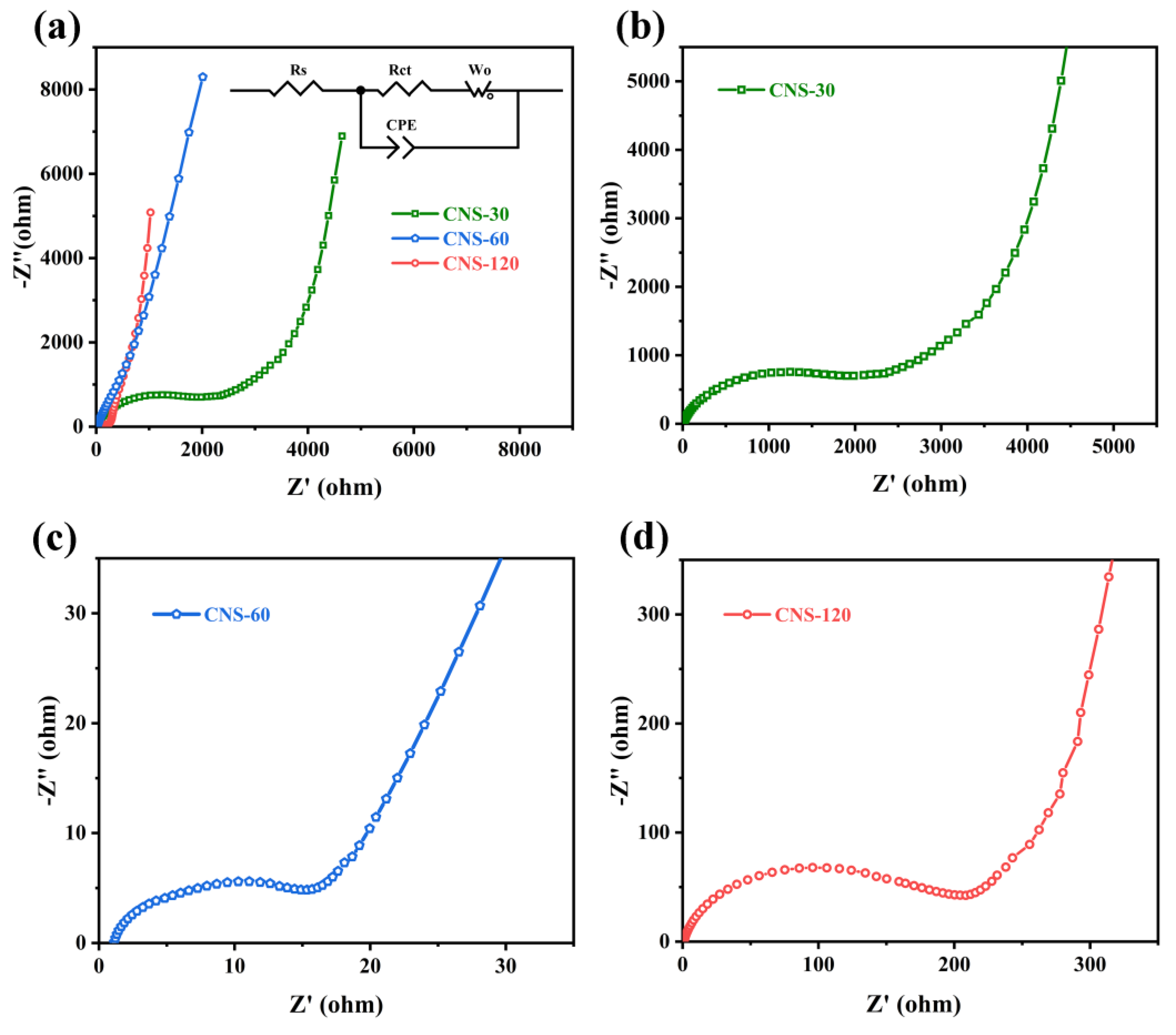
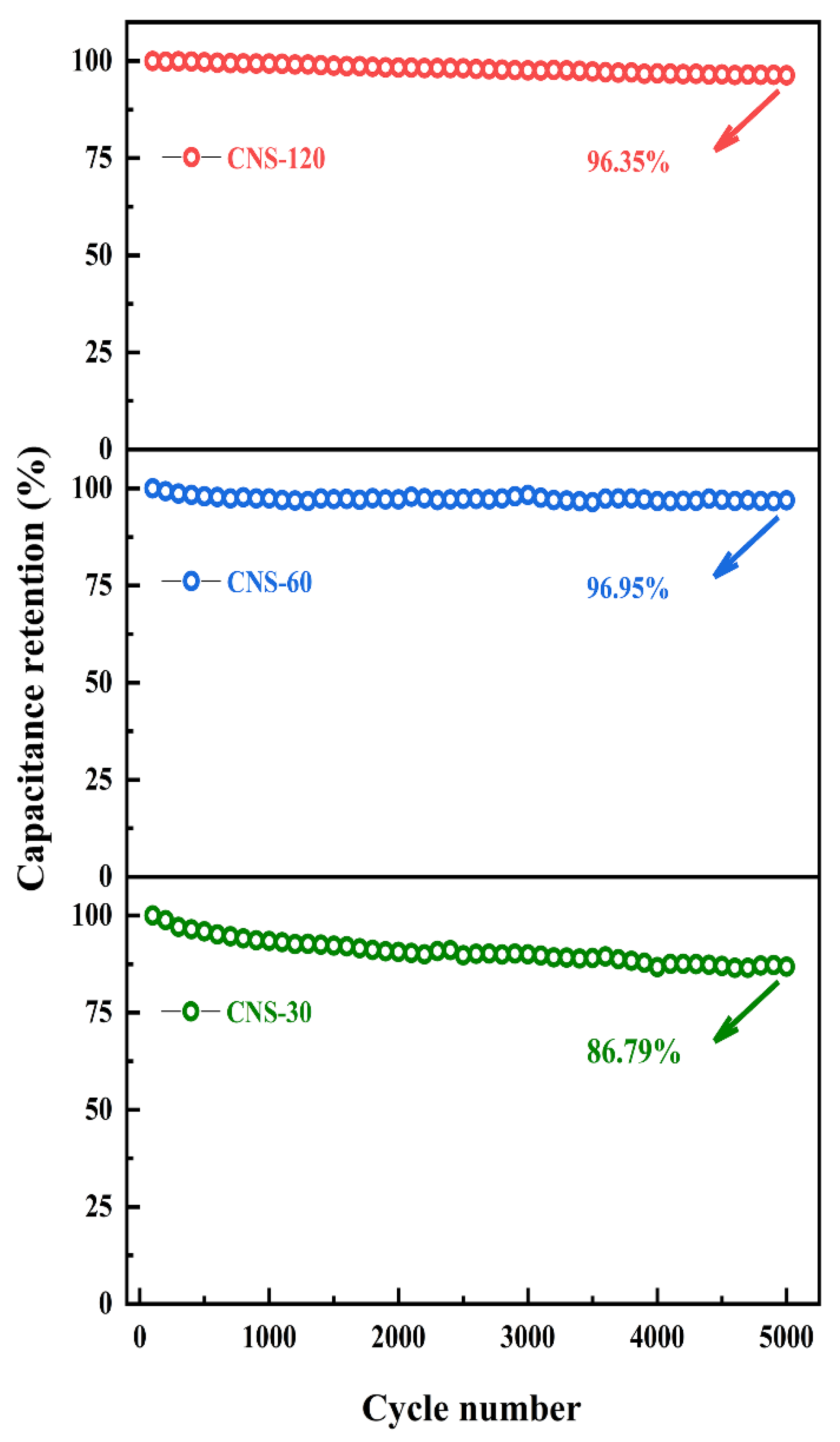
2.2. Electrochemical Performance of Coin-Type Supercapacitors
3. Materials and Methods
3.1. Materials
3.2. Preparation of Zinc Compound Nanosheets
3.3. Preparation of Al/ZnO/C Nanosheets
3.4. Characterization Methods
3.5. Electrochemical Performance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhong, M.; Zhang, M.; Li, X. Carbon nanomaterials and their composites for supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef]
- Mohamed, N.B.; El-Kady, M.F.; Kaner, R.B. Macroporous graphene frameworks for sensing and supercapacitor applications. Adv. Funct. Mater. 2022, 32, 2203101. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Dubal, D.P.; Nagar, B.; Ranc, V.; Tomanec, O.; Petr, M.; Datta, K.K.R.; Zboril, R.; Gomez-Romero, P.; Fischer, R.A. Ultrathin hierarchical porous carbon nanosheets for high-performance supercapacitors and redox electrolyte energy storage. Adv. Mater. 2018, 30, e1705789. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing flexible, smart and self-sustainable supercapacitors for portable/wearable electronics: From conductive polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Peng, H.; Yao, B.; Wei, X.; Liu, T.; Kou, T.; Xiao, P.; Zhang, Y.; Li, Y. Pore and heteroatom engineered carbon foams for supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-based materials for flexible all-solid-state supercapacitors. Adv. Mater. 2018, 30, e1705489. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, T.; Mou, J.; Zhang, W.; Jiang, Z.; Liu, J.; Huang, J.; Liu, M. Free-standing N-self-doped carbon nanofiber aerogels for high-performance all-solid-state supercapacitors. Nano Energy 2019, 63, 103836. [Google Scholar] [CrossRef]
- Xu, R.; Du, L.; Adekoya, D.; Zhang, G.; Zhang, S.; Sun, S.; Lei, Y. Well-defined nanostructures for electrochemical energy conversion and storage. Adv. Energy Mater. 2020, 11, 2001537. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of heterostructure materials in energy storage: A review. Adv. Mater. 2021, 33, e2100855. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Y.; Song, J.; Yang, W.; Wang, M.; Zhu, C.; Zhao, W.; Zheng, J.; Lin, Y. Self-supporting activated carbon/carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor. Carbon 2018, 129, 236–244. [Google Scholar] [CrossRef]
- Qing, Y.; Liao, Y.; Liu, J.; Tian, C.; Xu, H.; Wu, Y. Research progress of wood-derived energy storage materials. J. For. Eng. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, X.; Xu, T.; Bian, H.; Dai, H. Research progress on the preparation of lignin-derived carbon dots and graphene quantum dots. J. For. Eng. 2021, 6, 29–37. [Google Scholar] [CrossRef]
- He, S.; Zhang, R.; Zhang, C.; Liu, M.; Gao, X.; Ju, J.; Li, L.; Chen, W. Al/C/MnO2 sandwich nanowalls with highly porous surface for electrochemical energy storage. J. Power Sources 2015, 299, 408–416. [Google Scholar] [CrossRef]
- Han, X.; Huang, Z.-H.; Meng, F.; Jia, B.; Ma, T. Redox-etching induced porous carbon cloth with pseudocapacitive oxygenic groups for flexible symmetric supercapacitor. J. Energy Chem. 2022, 64, 136–143. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; Yang, W.; Han, J.; Jiang, S.; He, S. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Yin, B.-S.; Liu, C.; Wang, Z.-B.; Gu, D.-M. A lightweight, compressible and portable sponge-based supercapacitor for future power supply. Chem. Eng. J. 2018, 349, 509–521. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Lu, X.; Ma, Z.; Xie, C.; Zheng, Z. Chemical formation of soft metal electrodes for flexible and wearable electronics. Chem. Soc. Rev. 2018, 47, 4611–4641. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.-Y.; Yue, Y.-Y.; Zhang, N.; Feng, J.; Sun, H.-B. Flexible and transparent supercapacitor based on ultrathin Au/graphene composite electrodes. Appl. Surf. Sci. 2019, 467, 104–111. [Google Scholar] [CrossRef]
- Mehrabi-Matin, B.; Shahrokhian, S.; Iraji-Zad, A. Silver fiber fabric as the current collector for preparation of graphene- based supercapacitors. Electrochim. Acta 2017, 227, 246–254. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, C.; Xiong, Z.; Chen, H.Y.; Li, T.; Ding, G.; Yang, B.; Liao, Q.; Zhou, Y.; Han, S.T. Template-directed growth of hierarchical MOF hybrid arrays for tactile sensor. Adv. Funct. Mater. 2020, 30, 2001296. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Peng, Z.; Guan, S.; Fu, X. Ni foam-supported tin oxide nanowall array: An integrated supercapacitor anode. Molecules 2021, 26, 4517. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Yu, R.; Zhang, X.; Chen, X.; He, F.; Zhao, X.; Chen, N.; Ye, M.; Liu, X.Y. Making stretchable hybrid supercapacitors by knitting non-stretchable metal fibers. Adv. Funct. Mater. 2020, 30, 2003153. [Google Scholar] [CrossRef]
- Huang, S.; Du, X.; Li, X.; Ma, M.; Xiong, L. Ultrahigh-areal capacitance flexible supercapacitors based on laser assisted construction of hierarchical aligned carbon nanotubes. Adv. Funct. Mater. 2021, 31, 2104531. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Ye, Z.; Jin, Y.; Cui, C.; Xie, Q.; Wang, J.; Zhang, G.; Dong, Z.; Miao, Y.; et al. High energy and high power density supercapacitor with 3D Al foam-based thick graphene electrode: Fabrication and simulation. Energy Storage Mater. 2020, 33, 18–25. [Google Scholar] [CrossRef]
- Qi, J.L.; Lin, J.H.; Wang, X.; Guo, J.L.; Xue, L.F.; Feng, J.C.; Fei, W.-D. Low resistance VFG-microporous hybrid Al-based electrodes for supercapacitors. Nano Energy 2016, 26, 657–667. [Google Scholar] [CrossRef]
- Ahmed, F.; Almutairi, G.; Alotaibi, B.; Kumar, S.; Arshi, N.; Hussain, S.G.; Umar, A.; Ahmad, N.; Aljaafari, A. Binder-free electrode based on ZnO nanorods directly grown on aluminum substrate for high performance supercapacitors. Nanomaterials 2020, 10, 1979. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wang, H.M.; Li, G.R.; Su, W.X.; Zhang, Z.B.; Zhou, Z.C.; Dong, C. Microstructure and improved plasticity of (FeCoNi1.5CrCu)p/Al composites subject to adjusted deep cryogenic treatment (DCT). J. Alloys Compd. 2022, 895, 162690. [Google Scholar] [CrossRef]
- Zhang, Z.; Hua, Z.; Lang, J.; Song, Y.; Zhang, Q.; Han, Q.; Fan, H.; Gao, M.; Li, X.; Yang, J. Eco-friendly nanostructured Zn–Al layered double hydroxide photocatalysts with enhanced photocatalytic activity. CrystEngComm 2019, 21, 4607–4619. [Google Scholar] [CrossRef]
- Hu, K.; Wang, F.; Shen, Z.; Liu, H.; Zeng, W.; Wang, Y. Ar plasma treatment on ZnO–SnO2 heterojunction nanofibers and its enhancement mechanism of hydrogen gas sensing. Ceram. Int. 2020, 46, 21439–21447. [Google Scholar] [CrossRef]
- Sahoo, A.; Chowdhury, A.H.; Singha, P.; Banerjee, A.; Manirul Islam, S.; Bala, T. Morphology of ZnO triggered versatile catalytic reactions towards CO2 fixation and acylation of amines at optimized reaction conditions. Mol. Catal. 2020, 493, 111070. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Yan, K.; Duan, M. Growth mechanism of different morphologies of ZnO crystals prepared by hydrothermal method. J. Mater. Sci. Technol. 2011, 27, 153–158. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.M.; He, X.; Sun, F. Facile and large-scale production of ZnO/Zn−Al layered double hydroxide hierarchical heterostructures. J. Phys. Chem. B 2006, 110, 21865–21872. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, S.; Chen, X.; Tang, T.; Mijowska, E. Three dimensional graphene/carbonized metal-organic frameworks based high-performance supercapacitor. Carbon 2020, 157, 55–63. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Wang, R.; Zhang, Z.; Feng, Z.; Xie, X. Zn–Al layered double oxides as high-performance anode materials for zinc-based secondary battery. J. Mater. Chem. A 2015, 3, 7429–7436. [Google Scholar] [CrossRef]
- Wu, S.; Liang, H.; Zhang, Z.; Zhang, Q.; Han, Q.; Wang, J.; Gao, M.; Fan, H.; Yang, J.; Lang, J. The photocatalytic degradation and mechanism of rhodamine B by Zn–Al layered double hydroxide. Opt. Mater. 2022, 131, 112636. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Yang, Z.; Huang, M.; Zhang, Y.; Yang, C. The effect of TiO2@CoAl-LDH nanosphere on early hydration of cement and its photocatalytic depollution performance under UV–visible light. Constr. Build. Mater. 2022, 319, 126227. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, Z.; Li, J.; Yan, H.; Li, F. Preparation of lignin carbon/zinc oxide electrode material and its application in supercapacitors. Molecules 2021, 26, 3554. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, Y.; Ye, W.; Zhang, J.; Wang, Y.; Lin, Y.; Hou, L.; Wang, M.S.; Yuan, C. Unveiling intrinsic potassium storage behaviors of hierarchical nano Bi@N-doped carbon nanocages framework via In situ characterizations. Angew. Chem. Int. Ed. 2021, 60, 7180–7187. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Sun, G.; Su, F.; Guo, X.; Kong, Q.; Li, X.; Huang, X.; Wan, L.; Song, W.; Li, K.; et al. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Shah, J.; Kumar Kotnala, R. Rapid green synthesis of ZnO nanoparticles using a hydroelectric cell without an electrolyte. J. Phys. Chem. Solids 2017, 108, 15–20. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Luo, R.; Liu, C.; Qi, J.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Double -shelled hollow ZnO/carbon nanocubes as an efficient solid-phase microextraction coating for the extraction of broad-spectrum pollutants. Nanoscale 2019, 11, 2805–2811. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “adsorption–insertion” model: A new insight into the sodium storage mechanism of hard carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, F.; Xiao, X.; Chen, J.; Sun, J.; Gandla, D.; Ein-Eli, Y.; Tan, D.Q. Enhanced electrochemical performance of supercapacitors via atomic layer deposition of ZnO on the activated carbon electrode material. Molecules 2021, 26, 4188. [Google Scholar] [CrossRef]
- Yang, W.; Wang, P.; Tu, Z.; Hou, L.; Yan, L.; Jiang, B.; Zhang, C.; Huang, G.; Yang, F.; Li, Y. Heteroatoms-doped hierarchical porous carbon with multi-scale structure derived from petroleum asphalt for high-performance supercapacitors. Carbon 2022, 187, 338–348. [Google Scholar] [CrossRef]
- Hou, L.; Yang, W.; Li, Y.; Wang, P.; Jiang, B.; Xu, C.; Zhang, C.; Huang, G.; Yang, F.; Li, Y. Dual-template endowing N, O co-doped hierarchically porous carbon from potassium citrate with high capacitance and rate capability for supercapacitors. Chem. Eng. J. 2021, 417, 129289. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.; Gao, M.; Huang, L.; Li, L.; Liu, S.; Xie, C.; Zhang, Y.; Yu, S. N-doped porous carbon from different nitrogen sources for high-performance supercapacitors and CO2 adsorption. J. Alloys Compd. 2019, 786, 826–838. [Google Scholar] [CrossRef]
- Wong, S.I.; Lin, H.; Ma, T.; Sunarso, J.; Wong, B.T.; Jia, B. Binary ionic liquid electrolyte design for ultrahigh-energy density graphene-based supercapacitors. Mater. Rep. Energy 2022, 2, 100093. [Google Scholar] [CrossRef]
- Kamboj, N.; Purkait, T.; Das, M.; Sarkar, S.; Hazra, K.S.; Dey, R.S. Ultralong cycle life and outstanding capacitive performance of a 10.8 V metal free micro-supercapacitor with highly conducting and robust laser-irradiated graphene for an integrated storage device. Energy Environ. Sci. 2019, 12, 2507–2517. [Google Scholar] [CrossRef]
- Wu, W.; Ma, H.; Zhang, Z.; Zhang, Z.; Gu, Y.; Gao, W.; Zhou, W.; Zhang, R. In-situ synthesis of a unique 0D/2D porous carbon integrated architecture for high-performance flexible micro-supercapacitors. J. Power Sources 2022, 541, 231687. [Google Scholar] [CrossRef]
- Xie, X.; Guo, R.; Yang, B.; Li, H.; Yang, F.; Shen, B. Stencil-printed electrodes without current collectors and inactive additives on textiles for in-plane microsupercapacitors. J. Mater. Chem. A 2021, 9, 25042–25050. [Google Scholar] [CrossRef]
- Park, H.; Song, C.; Jin, S.W.; Lee, H.; Keum, K.; Lee, Y.H.; Lee, G.; Jeong, Y.R.; Ha, J.S. High performance flexible micro-supercapacitor for powering a vertically integrated skin-attachable strain sensor on a bio-inspired adhesive. Nano Energy 2021, 83, 105837. [Google Scholar] [CrossRef]
- Chang, Q.; Cao, C.; Qiao, H.; Hu, Y.; Xiao, G.; Shi, W.; Huang, L. Ink transfer for printed flexible microsupercapacitors. Carbon 2021, 178, 285–293. [Google Scholar] [CrossRef]
- Zaccagnini, P.; Di Giovanni, D.; Gomez, M.G.; Passerini, S.; Varzi, A.; Lamberti, A. Flexible and high temperature supercapacitor based on laser-induced graphene electrodes and ionic liquid electrolyte, a de-rated voltage analysis. Electrochim. Acta 2020, 357, 136838. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, G.; Shi, X.; Qiao, Y.; Liu, J.; Liu, H.; Ganesh, A.; Li, L. Direct graphene-carbon nanotube composite ink writing all-solid-state flexible microsupercapacitors with high areal energy density. Adv. Funct. Mater. 2020, 30, 1907284. [Google Scholar] [CrossRef]
- Ray, A.; Roth, J.; Saruhan, B. Laser-induced interdigital structured graphene electrodes based flexible micro-supercapacitor for efficient peak energy storage. Molecules 2022, 27, 329. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Pagaduan, J.N.; Yu, Y.G.; Einck, V.J.; Nuguri, S.; Katsumata, R.; Watkins, J.J. Large-pore ordered mesoporous turbostratic carbon films prepared using rapid thermal annealing for high-performance micro-pseudocapacitors. ACS Appl. Mater. Interfaces 2021, 13, 61027–61038. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Zhang, P.; Sun, L.; Ren, X.; Mi, H. Hierarchical hollow carbon spheres: Novel synthesis strategy, pore structure engineering and application for micro-supercapacitor. Carbon 2020, 157, 70–79. [Google Scholar] [CrossRef]
- Bräuniger, Y.; Lochmann, S.; Grothe, J.; Hantusch, M.; Kaskel, S. Piezoelectric inkjet printing of nanoporous carbons for micro-supercapacitor devices. ACS Appl. Energy Mater. 2021, 4, 1560–1567. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, H.J.; Shim, H.C.; Yoon, Y.; Ahn, J.; Lim, H.; Kim, G.; Choi, K.B.; Lee, J. Hierarchically porous, laser-pyrolyzed carbon electrode from black photoresist for on-chip microsupercapacitors. Nanomaterials 2021, 11, 2828. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, C.; Cao, Y.; Han, Q.; Parker, C.B.; Glass, J.T. Robust and high-performance electrodes via crumpled Au-CNT forests for stretchable supercapacitors. Matter 2020, 2, 1307–1323. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Xue, B.; Li, W.; Ding, Z.; Yang, X.; Qiu, J.; Wang, Z. Rice husk-based hierarchical porous carbon for high performance supercapacitors: The structure-performance relationship. Carbon 2020, 161, 432–444. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, J.; Zou, Z.; Li, J.; Wu, C.; Jiang, Y.; Chen, Y.; Zeng, Q.; Wu, X.; Sun, W.; et al. Labyrinth maze-like long travel-reduction of sulfur and polysulfides in micropores of a spherical honeycomb carbon to greatly confine shuttle effects in lithium-sulfur batteries. Mater. Rep. Energy 2022, 2, 100159. [Google Scholar] [CrossRef]
- Tahir, M.; He, L.; Yang, W.; Hong, X.; Haider, W.A.; Tang, H.; Zhu, Z.; Owusu, K.A.; Mai, L. Boosting the electrochemical performance and reliability of conducting polymer microelectrode via intermediate graphene for on-chip asymmetric micro-supercapacitor. J. Energy Chem. 2020, 49, 224–232. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Chi, M.; Guo, C.; Wang, S.; Min, D. Preparation and performance of different carbonized wood electrodes. J. For. Eng. 2022, 7, 127–135. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Han, J.; Zhang, C.; Yang, W.; Jiang, S.; He, S. Al Foil-Supported Carbon Nanosheets as Self-Supporting Electrodes for High Areal Capacitance Supercapacitors. Molecules 2023, 28, 1831. https://doi.org/10.3390/molecules28041831
Zheng J, Yan B, Feng L, Zhang Q, Han J, Zhang C, Yang W, Jiang S, He S. Al Foil-Supported Carbon Nanosheets as Self-Supporting Electrodes for High Areal Capacitance Supercapacitors. Molecules. 2023; 28(4):1831. https://doi.org/10.3390/molecules28041831
Chicago/Turabian StyleZheng, Jiaojiao, Bing Yan, Li Feng, Qian Zhang, Jingquan Han, Chunmei Zhang, Weisen Yang, Shaohua Jiang, and Shuijian He. 2023. "Al Foil-Supported Carbon Nanosheets as Self-Supporting Electrodes for High Areal Capacitance Supercapacitors" Molecules 28, no. 4: 1831. https://doi.org/10.3390/molecules28041831
APA StyleZheng, J., Yan, B., Feng, L., Zhang, Q., Han, J., Zhang, C., Yang, W., Jiang, S., & He, S. (2023). Al Foil-Supported Carbon Nanosheets as Self-Supporting Electrodes for High Areal Capacitance Supercapacitors. Molecules, 28(4), 1831. https://doi.org/10.3390/molecules28041831








