A Magnetic Surfactant Having One Degree of Unsaturation in the Hydrophobic Tail as a Shale Swelling Inhibitor
Abstract
:1. Introduction
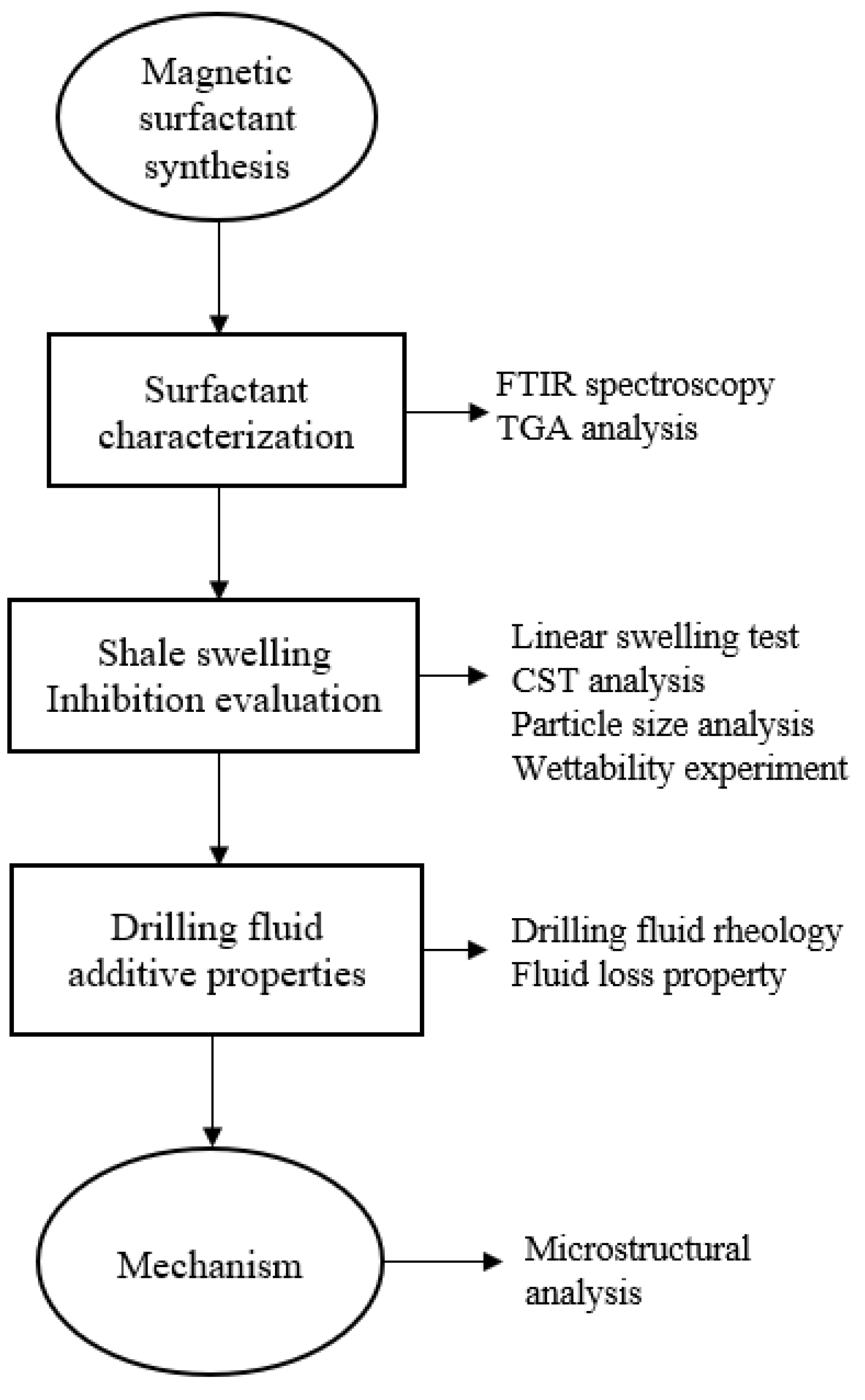
2. Methodology
2.1. Materials
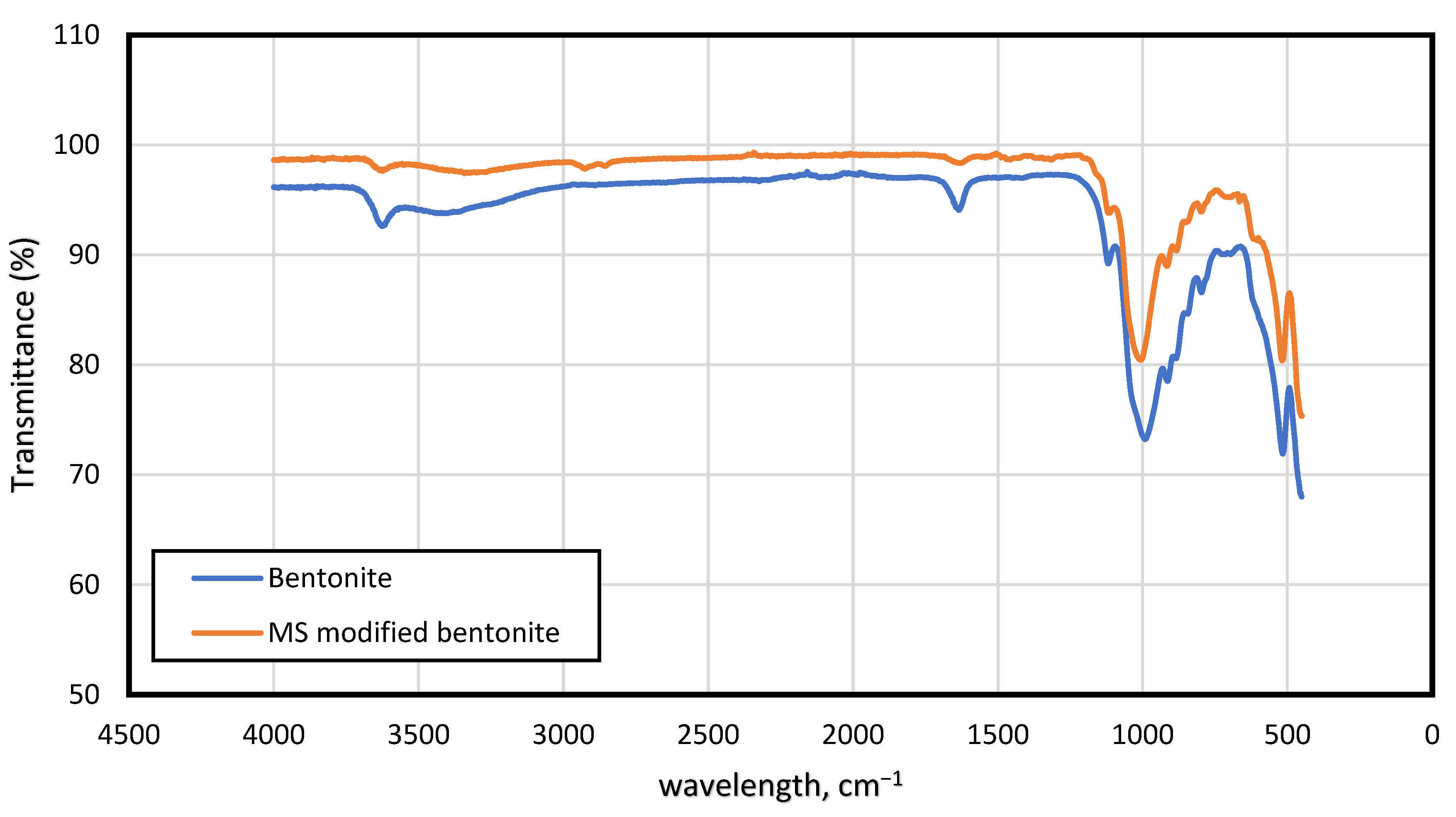
2.2. Surfactant Synthesis and Characterization
2.3. Solubility Test
2.4. Formulation of Water-Based Mud
2.5. Characterization of the Drilling Mud
2.6. Shale Inhibition and Swelling Experiment
2.7. Particle Size Distribution (PSD)
2.8. Contact Angle Measurement
2.9. Capillary Suction Time (CST) Test
2.10. Rheological Measurement
2.11. Filtration Test
2.12. Microstructural Analysis
3. Result and Discussion
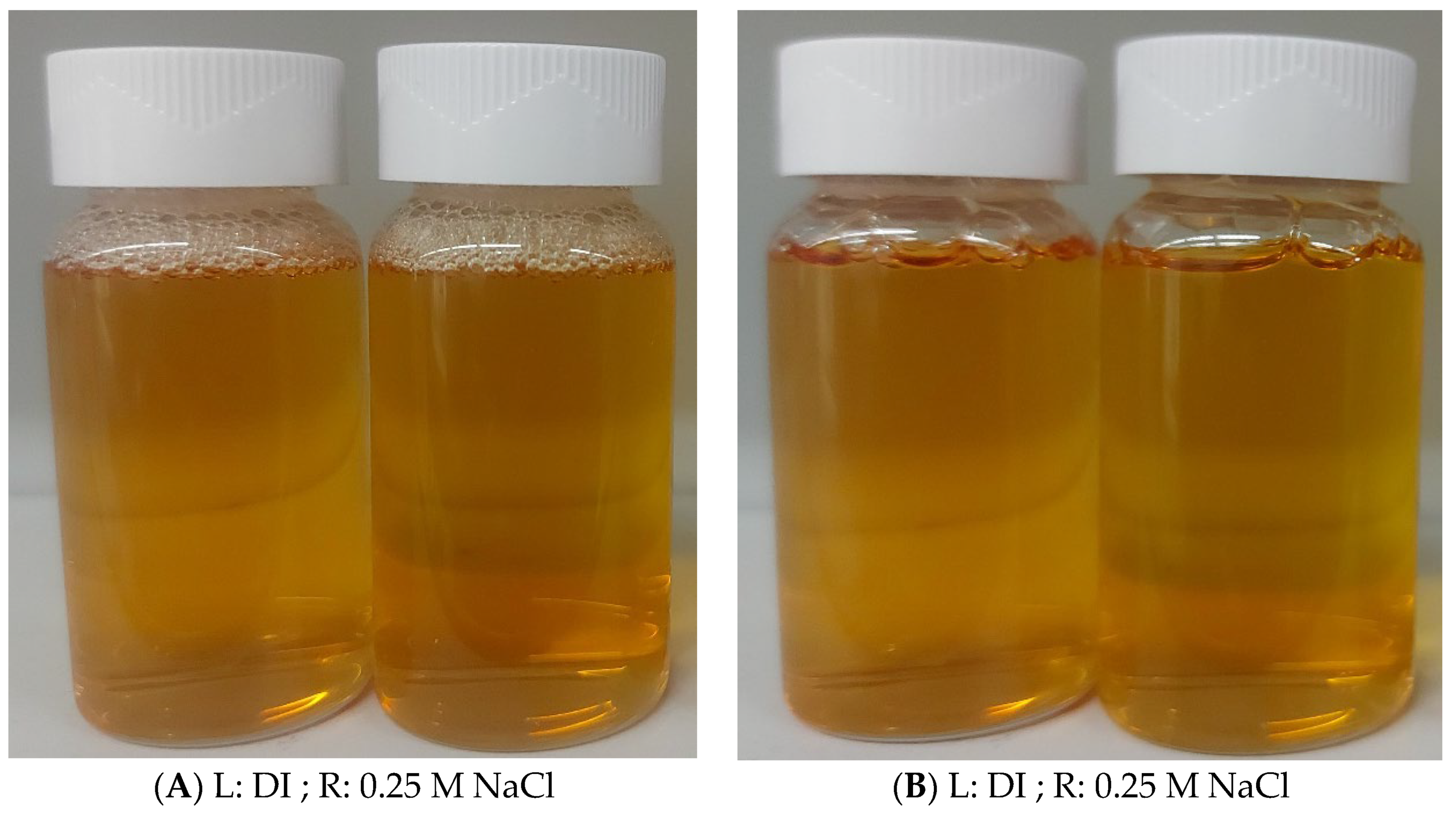
3.1. Solubility and Salt Tolerance of Magnetic Surfactant
3.2. Characterization of the Drilling Mud
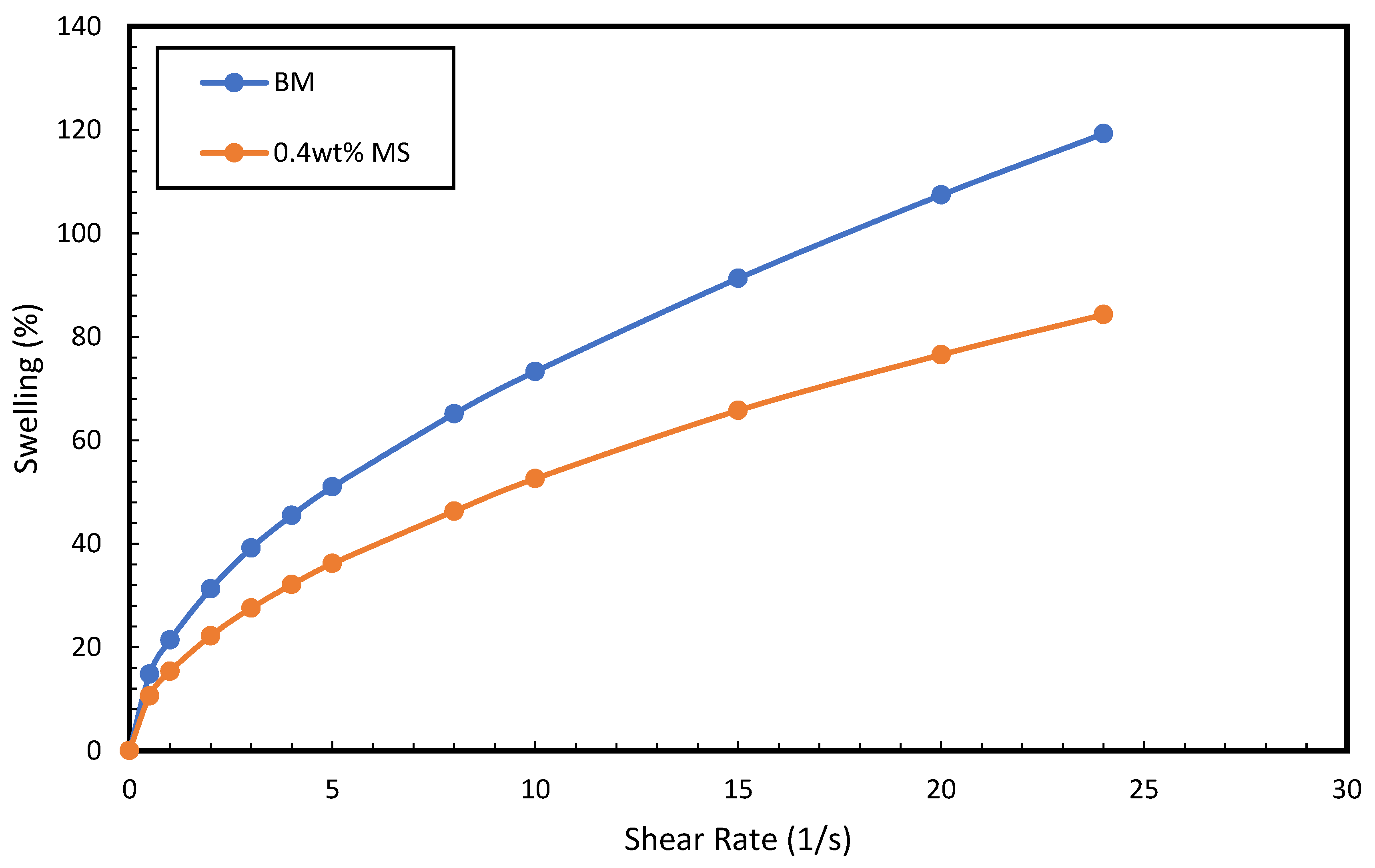
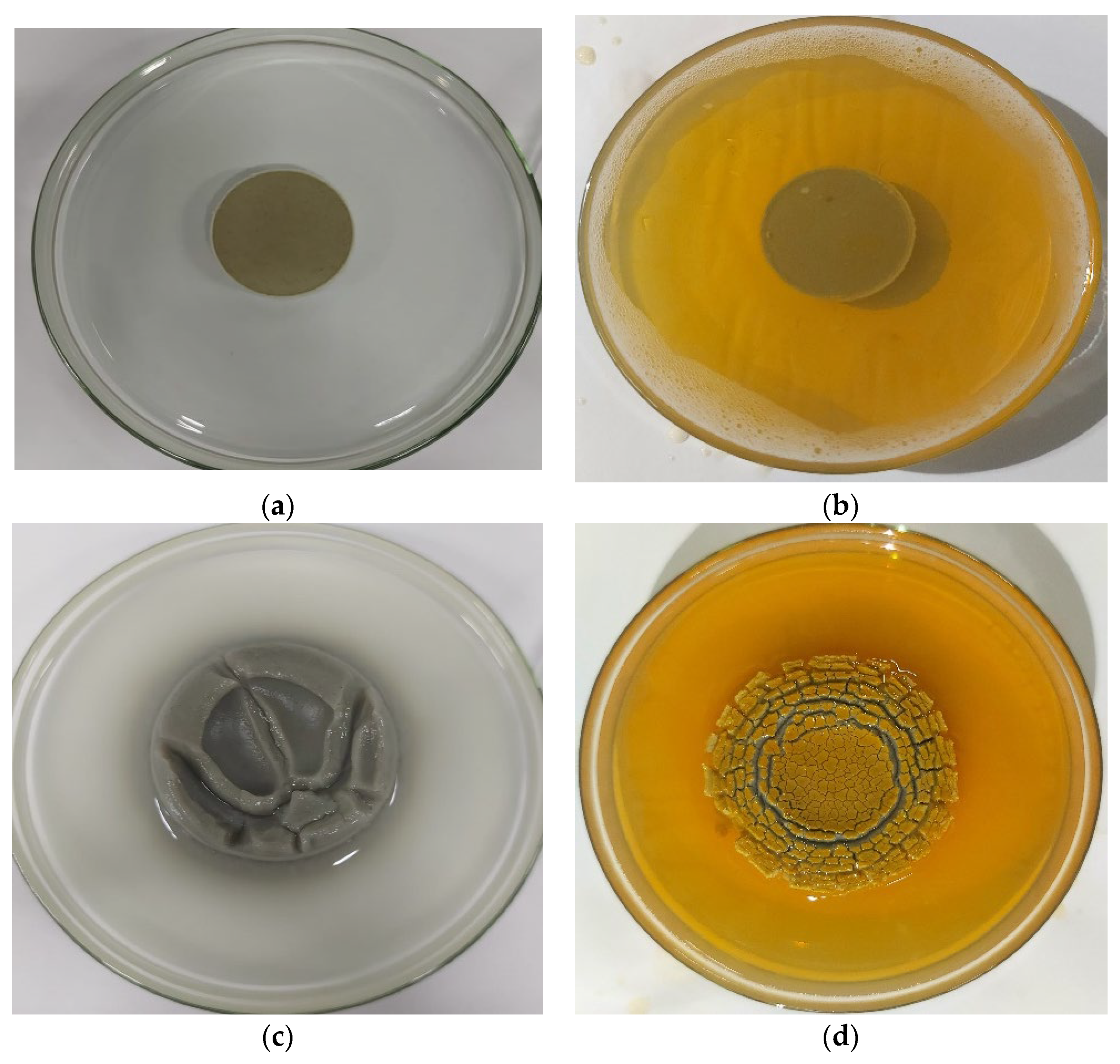
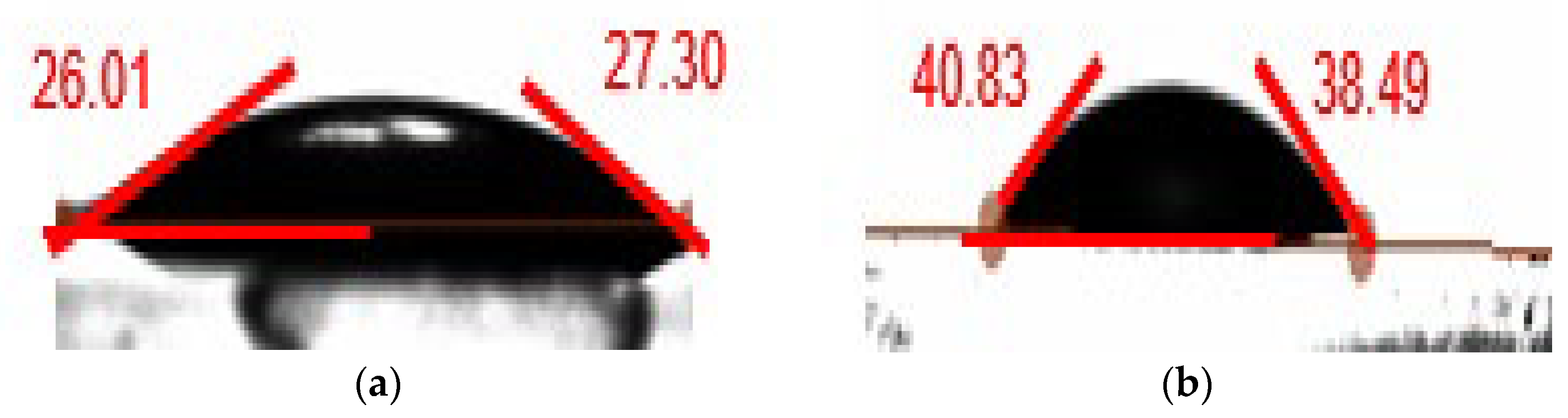
3.3. Shale Swelling Inhibition Property
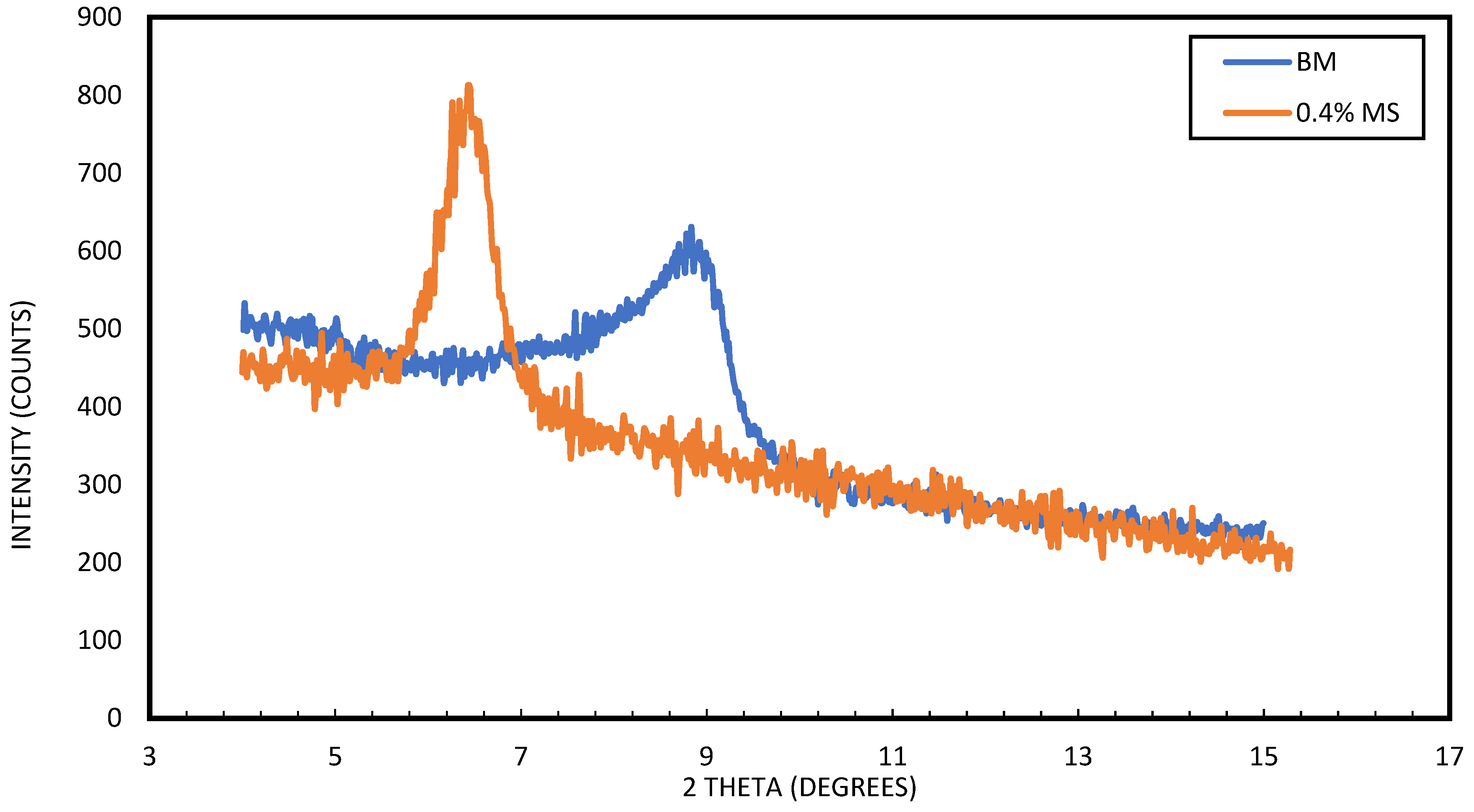
3.4. Particle Size Distribution Analysis
3.5. Wettability Test
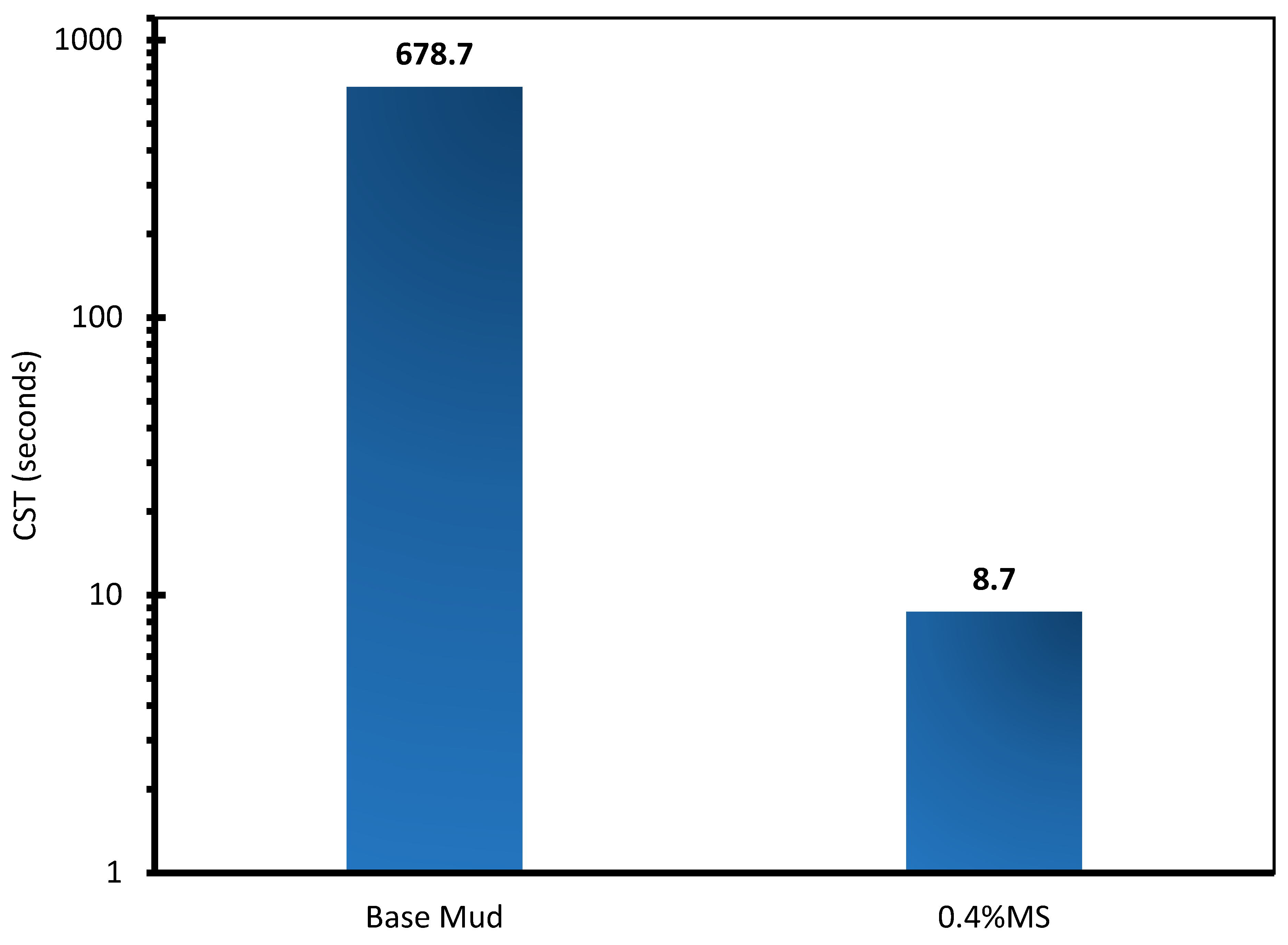
3.6. Capillary Suction Time (CST) Analysis
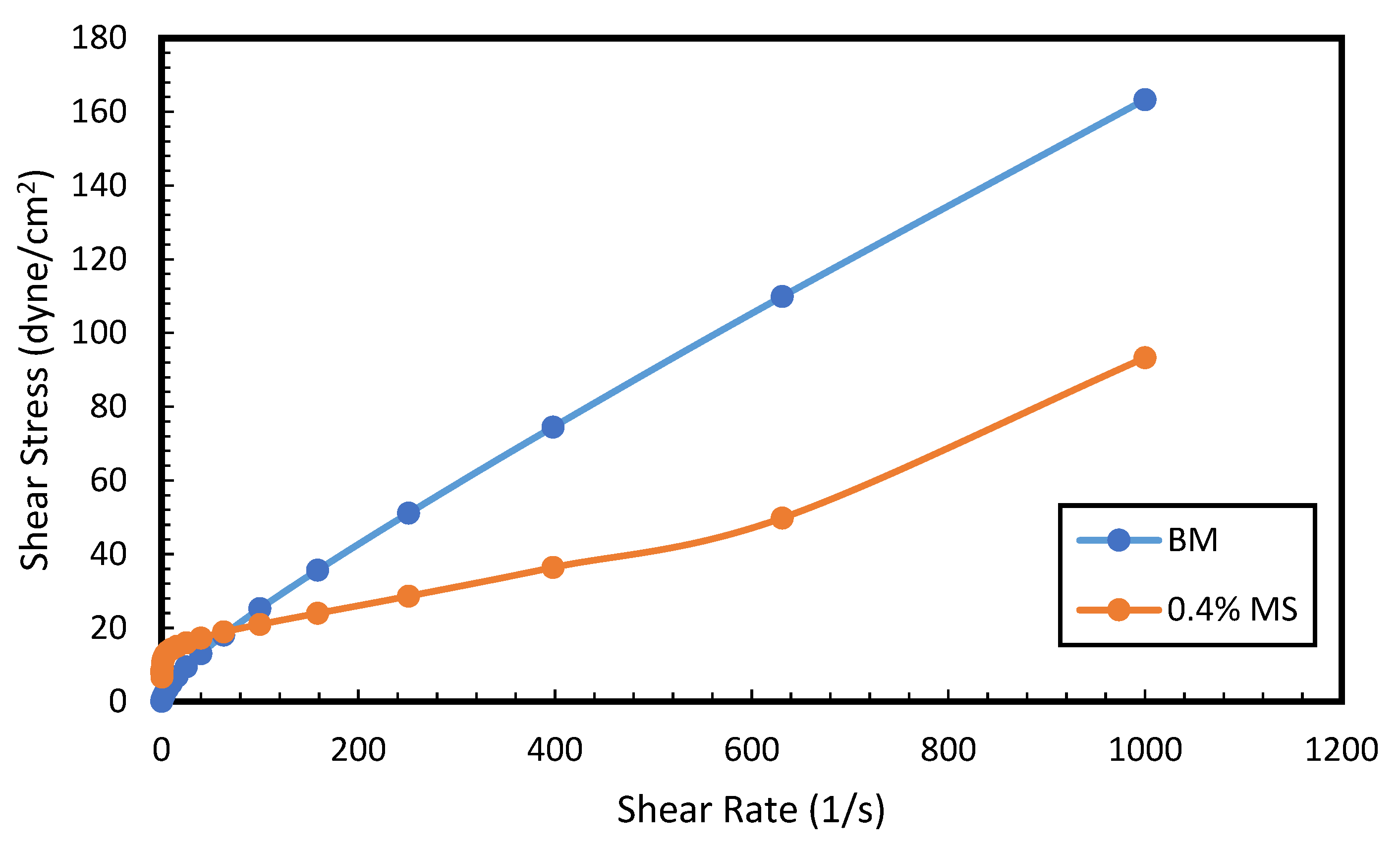
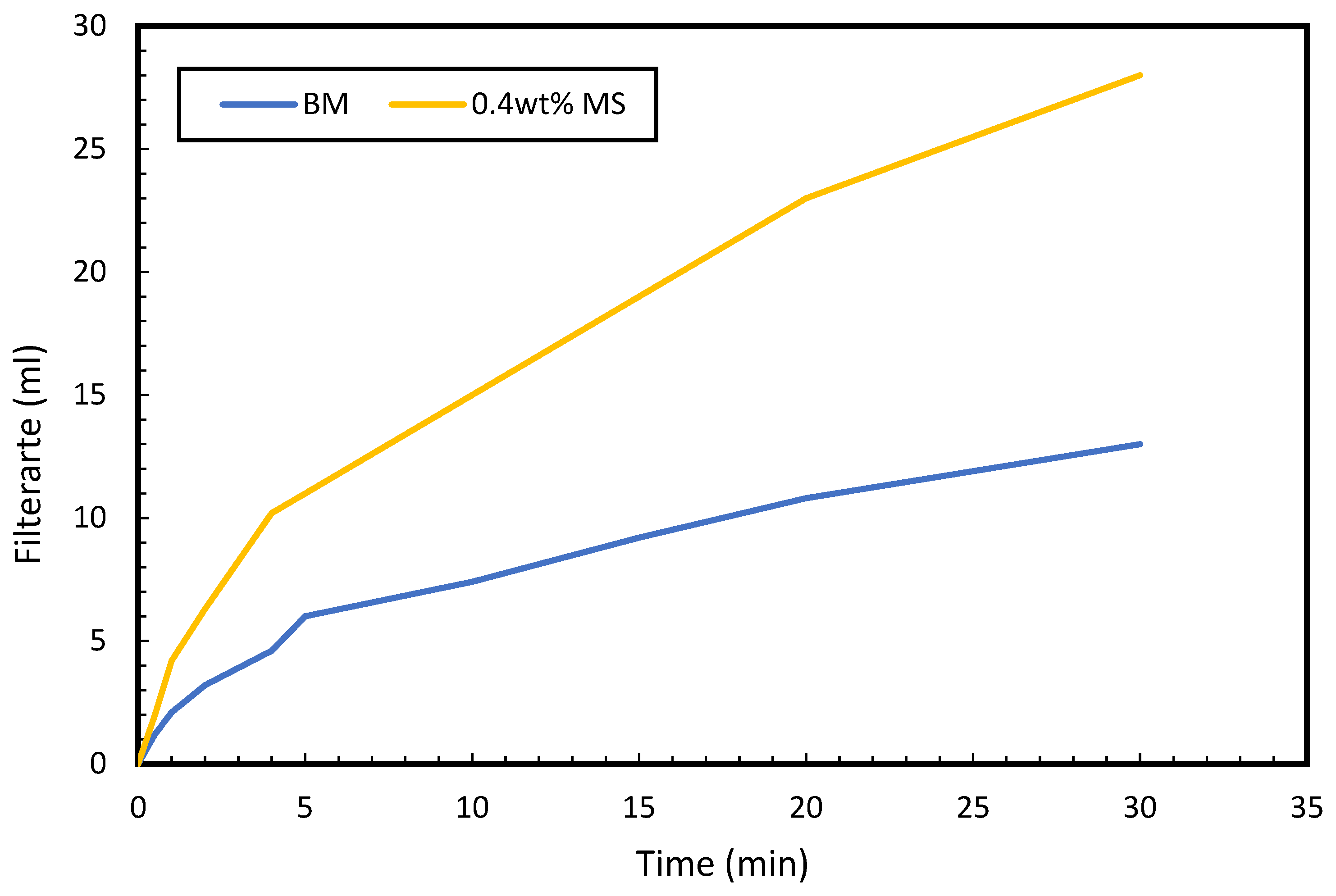
3.7. Rheology and Fluid Loss Properties
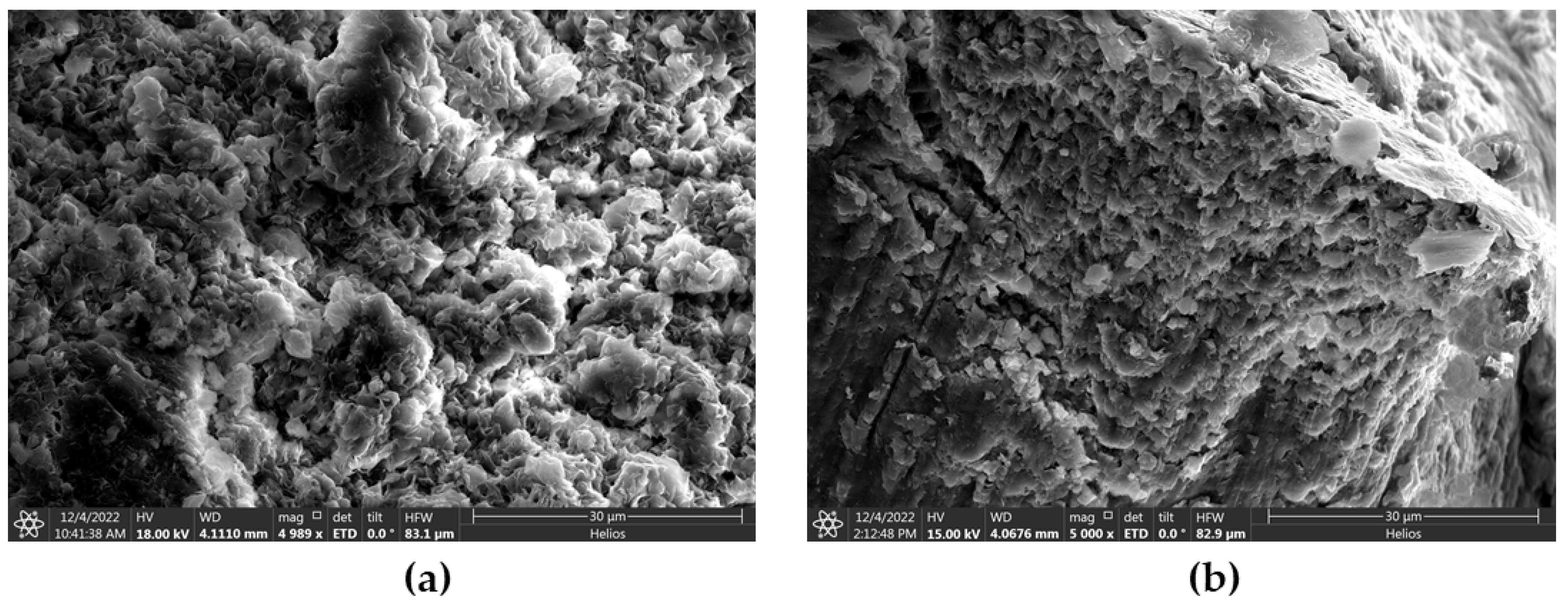
3.8. Microstructural Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bageri, B.S.; Adebayo, A.R.; Al Jaberi, J.; Patil, S.; Salin, R.B. Evaluating Drilling Fluid Infiltration in Porous Media—Comparing NMR, Gravimetric, and X-Ray CT Scan Methods. J. Pet. Sci. Eng. 2021, 198, 108242. [Google Scholar] [CrossRef]
- Blkoor, S.O.; Ismail, I.; Oseh, J.O.; Selleyitoreea, S.; Norddin, M.N.A.M.; Agi, A.; Gbadamosi, A.O. Influence of Polypropylene Beads and Sodium Carbonate Treated Nanosilica in Water-Based Muds for Cuttings Transport. J. Pet. Sci. Eng. 2021, 200, 108435. [Google Scholar] [CrossRef]
- Hossain, M.E.; Al-Majed, A.A. Fundamentals of Sustainable Drilling Engineering; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Liu, L.; Sun, J.; Wang, R.; Qu, Y.; Liu, F.; Yang, J.; Cheng, R.; Gao, S.; Huang, H. Synthesis of a New High Temperature and Salt Resistant Zwitterionic Filtrate Reducer and Its Application in Water-Based Drilling Fluid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129730. [Google Scholar] [CrossRef]
- Murtaza, M.; Tariq, Z.; Mahmoud, M.; Kamal, M.S.; Al Shehri, D. Application of Anhydrous Calcium Sulfate as a Weighting Agent in Oil-Based Drilling Fluids. ACS Omega 2021, 6, 21690–21701. [Google Scholar] [CrossRef]
- Oseh, J.O.; Mohd Norddin, M.N.A.; Ismail, I.; Ismail, A.R.; Gbadamosi, A.O.; Agi, A.; Ogiriki, S.O. Investigating Almond Seed Oil as Potential Biodiesel-Based Drilling Mud. J. Pet. Sci. Eng. 2019, 181, 106201. [Google Scholar] [CrossRef]
- Medved, I.; Gaurina-Međimurec, N.; Pašić, B.; Mijić, P. Green Approach in Water-Based Drilling Mud Design to Increase Wellbore Stability. Appl. Sci. 2022, 12, 5348. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Oseh, J.O.; Agi, A.; Yekeen, N.; Abdalla, Y.; Ogiriki, S.O.; Yusuff, A.S. Improving Hole Cleaning Efficiency Using Nanosilica in Water-Based Drilling Mud. In Proceedings of the Society of Petroleum Engineers—SPE Nigeria Annual International Conference and Exhibition NAIC 2018, Lagos, Nigeria, 6–8 August 2018. [Google Scholar]
- Gbadamosi, A.O.; Junin, R.; Abdalla, Y.; Agi, A.; Oseh, J.O. Experimental Investigation of the Effects of Silica Nanoparticle on Hole Cleaning Efficiency of Water-Based Drilling Mud. J. Pet. Sci. Eng. 2019, 172, 1226–1234. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A.; Rabiei, M.; Fakhari, N.; Balasubramaniam, P.; Rasouli, V.; Nagarajan, R. An Approach to Improve Wellbore Stability in Active Shale Formations Using Nanomaterials. Petroleum 2021, 7, 24–32. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J. Factors Affecting the Lower Limit of the Safe Mud Weight Window for Drilling Operation in Hydrate-Bearing Sediments in the Northern South China Sea. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 82. [Google Scholar] [CrossRef]
- Gamal, H.; Elkatatny, S.; Adebayo, A. Influence of Mud Filtrate on the Pore System of Different Sandstone Rocks. J. Pet. Sci. Eng. 2021, 202, 108595. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S. A Review on Clay Chemistry, Characterization and Shale Inhibitors for Water-Based Drilling Fluids. J. Pet. Sci. Eng. 2021, 206, 109043. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S.; Haq, B.; Patil, S. Insights into the Application of Surfactants and Nanomaterials as Shale Inhibitors for Water-Based Drilling Fluid: A Review. J. Nat. Gas Sci. Eng. 2021, 92, 103987. [Google Scholar] [CrossRef]
- Gholami, R.; Elochukwu, H.; Fakhari, N.; Sarmadivaleh, M. A Review on Borehole Instability in Active Shale Formations: Interactions, Mechanisms and Inhibitors. Earth Sci. Rev. 2018, 177, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Lv, K.; Liu, J.; Jin, J.; Sun, J.; Huang, X.; Liu, J.; Guo, X.; Hou, Q.; Zhao, J.; Liu, K.; et al. Synthesis of a Novel Cationic Hydrophobic Shale Inhibitor with Preferable Wellbore Stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128274. [Google Scholar] [CrossRef]
- Oseh, J.O.; Norddin, M.N.A.M.; Muhamad, H.N.; Ismail, I.; Gbadamosi, A.O.; Agi, A.; Ismail, A.R.; Blkoor, S.O. Influence of (3–Aminopropyl) Triethoxysilane on Entrapped Polypropylene at Nanosilica Composite for Shale Swelling and Hydration Inhibition. J. Pet. Sci. Eng. 2020, 194, 107560. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M. Polymeric and Low Molecular Weight Shale Inhibitors: A Review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Abbas, M.A.; Zamir, A.; Elraies, K.A.; Mahmood, S.M.; Rasool, M.H. A Critical Parametric Review of Polymers as Shale Inhibitors in Water-Based Drilling Fluids. J. Pet. Sci. Eng. 2021, 204, 108745. [Google Scholar] [CrossRef]
- Beg, M.; Singh, P.; Sharma, S.; Ojha, U. Shale Inhibition by Low-Molecular-Weight Cationic Polymer in Water-Based Mud. J. Pet. Explor. Prod. Technol. 2019, 9, 1995–2007. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Jiang, G.; Li, X.; He, Y.; Yang, L.; Cui, K.; Li, W. Application of Carboxylated Cellulose Nanocrystals as Eco-Friendly Shale Inhibitors in Water-Based Drilling Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127182. [Google Scholar] [CrossRef]
- Villada, Y.; Taverna, M.E.; Maffi, J.M.; Giletta, S.; Casis, N.; Estenoz, D. On the Use of Espina Corona Gum as a Polymeric Additive in Water-Based Drilling Fluid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129601. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmad, H.M.; Zhou, X.; Al-Shehri, D.; Mahmoud, M.; Shahzad Kamal, M. Okra Mucilage as Environment Friendly and Non-Toxic Shale Swelling Inhibitor in Water Based Drilling Fluids. Fuel 2022, 320, 123868. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Forson, K.; Zhang, J.; Zhang, C.; Chen, J.; Xu, N.; Wang, Y. Affecting Analysis of the Rheological Characteristic and Reservoir Damage of CO2 Fracturing Fluid in Low Permeability Shale Reservoir. Environ. Sci. Pollut. Res. 2022, 29, 37815–37826. [Google Scholar] [CrossRef] [PubMed]
- Bageri, B.S.; Adebayo, A.R.; Al Jaberi, J.; Patil, S. Effect of Perlite Particles on the Filtration Properties of High-Density Barite Weighted Water-Based Drilling Fluid. Powder Technol. 2020, 360, 1157–1166. [Google Scholar] [CrossRef]
- Bayat, A.E.; Moghanloo, P.J.; Piroozian, A.; Rafati, R. Experimental Investigation of Rheological and Filtration Properties of Water-Based Drilling Fluids in Presence of Various Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Ahmed Khan, R.; Murtaza, M.; Abdulraheem, A.; Kamal, M.S.; Mahmoud, M. Imidazolium-Based Ionic Liquids as Clay Swelling Inhibitors: Mechanism, Performance Evaluation, and Effect of Different Anions. ACS Omega 2020, 5, 26682–26696. [Google Scholar] [CrossRef] [PubMed]
- Oseh, J.O.; Norrdin, M.N.A.M.; Farooqi, F.; Ismail, R.A.; Ismail, I.; Gbadamosi, A.O.; Agi, A.J. Experimental Investigation of the Effect of Henna Leaf Extracts on Cuttings Transportation in Highly Deviated and Horizontal Wells. J. Pet. Explor. Prod. Technol. 2019, 9, 2387–2404. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Boru, X.; An, Y. Advanced Developments in Low-Toxic and Environmentally Friendly Shale Inhibitor: A Review. J. Pet. Sci. Eng. 2022, 208, 109578. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmed Khan, R.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M. Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants with Hydrophilic Spacers as Clay Stabilizers. Energy Fuels 2020, 34, 10619–10630. [Google Scholar] [CrossRef]
- Murtaza, M.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M.; Syed, N.A. Quaternary Ammonium Gemini Surfactants Having Different Spacer Length as Clay Swelling Inhibitors: Mechanism and Performance Evaluation. J. Mol. Liq. 2020, 308, 113054. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmad, H.M.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M.; Patil, S. Evaluation of Clay Hydration and Swelling Inhibition Using Quaternary Ammonium Dicationic Surfactant with Phenyl Linker. Molecules 2020, 25, 4333. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Murtaza, M.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M. Cationic Gemini Surfactants Containing Biphenyl Spacer as Shale Swelling Inhibitor. J. Mol. Liq. 2021, 325, 115164. [Google Scholar] [CrossRef]
- Lv, K.; Huang, X.; Li, H.; Sun, J.; Du, W.; Li, M. Modified Biosurfactant Cationic Alkyl Polyglycoside as an Effective Additive for Inhibition of Highly Reactive Shale. Energy Fuels 2020, 34, 1680–1687. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jalalifar, H.; Norouzi Apourvari, S.; Sakebi, M.R. Mechanistic Study of Improvement of Wellbore Stability in Shale Formations Using a Natural Inhibitor. J. Pet. Sci. Eng. 2019, 181, 106222. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Khezerloo-ye Aghdam, S.; Shahbazi, K.; Khezerloo-ye Aghdam, H.; Alboghobeish, F. Assessment of Swelling Inhibitive Effect of CTAB Adsorption on Montmorillonite in Aqueous Phase. Appl. Clay Sci. 2016, 127–128, 111–122. [Google Scholar] [CrossRef]
- Shehzad, F.; Hussain, S.M.S.; Adewunmi, A.A.; Mahboob, A.; Murtaza, M.; Kamal, M.S. Magnetic Surfactants: A Review of Recent Progress in Synthesis and Applications. Adv. Colloid Interface Sci. 2021, 293, 102441. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Alan Hatton, T.; Eastoe, J. Magnetic Surfactants. Curr. Opin. Colloid Interface Sci. 2015, 20, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Dong, S.; Hao, J. Recent Progress of Magnetic Surfactants: Self-Assembly, Properties and Functions. Curr. Opin. Colloid Interface Sci. 2018, 35, 81–90. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Kamal, M.S.; Mahboob, A. Synthesis and Evaluation of Magnetic Surfactants for High Temperature Oilfield Application. J. Mol. Liq. 2021, 340, 117216. [Google Scholar] [CrossRef]
- Chen, W.L.; Grabowski, R.C.; Goel, S. Clay Swelling: Role of Cations in Stabilizing/Destabilizing Mechanisms. ACS Omega 2022, 7, 3185–3191. [Google Scholar] [CrossRef]
- Sun, J.-S.; Wang, Z.-L.; Liu, J.-P.; Lv, K.-H.; Zhang, F.; Shao, Z.-H.; Dong, X.-D.; Dai, Z.-W.; Zhang, X.-F. Notoginsenoside as an Environmentally Friendly Shale Inhibitor in Water-Based Drilling Fluid. Pet. Sci. 2022, 19, 608–618. [Google Scholar] [CrossRef]
- Leerlooijer, K.; Kuijvenhoven, C.; Francis, P.A.; Rijswijk, S.R. Filtration Control, Mud Design and Well Productivity. In Proceedings of the SPE Formation Damage Control Symposium, Lafayette, LA, USA, 14–15 February 1996. [Google Scholar]
- Zhang, F.; Sun, J.; Chang, X.; Xu, Z.; Zhang, X.; Huang, X.; Liu, J.; Lv, K. A Novel Environment-Friendly Natural Extract for Inhibiting Shale Hydration. Energy Fuels 2019, 33, 7118–7126. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z.; Zhong, H.; Zhao, X.; Liu, Z.; Huang, W. Effects of Water-Based Drilling Fluid on Properties of Mud Cake and Wellbore Stability. J. Pet. Sci. Eng. 2022, 208, 109704. [Google Scholar] [CrossRef]



| Additives | Base Drilling Mud | Surfactant Mixed Drilling Mud |
|---|---|---|
| Water | 350 mL | 350 mL |
| Sodium hydroxide | 0.1 g | 0.1 g |
| Bentonite | 21 g | 21 g |
| Magnetic Surfactant | - | 0.4 wt% |
| Formulations | Particle Size Distribution (μm) | ||
|---|---|---|---|
| D10 | D50 | D90 | |
| BM | 1.06 | 2.57 | 7.09 |
| 0.4% MS | 1.22 | 4.39 | 16.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtaza, M.; Gbadamosi, A.; Ahmad, H.M.; Hussain, S.M.S.; Kamal, M.S.; Mahmoud, M.; Patil, S. A Magnetic Surfactant Having One Degree of Unsaturation in the Hydrophobic Tail as a Shale Swelling Inhibitor. Molecules 2023, 28, 1878. https://doi.org/10.3390/molecules28041878
Murtaza M, Gbadamosi A, Ahmad HM, Hussain SMS, Kamal MS, Mahmoud M, Patil S. A Magnetic Surfactant Having One Degree of Unsaturation in the Hydrophobic Tail as a Shale Swelling Inhibitor. Molecules. 2023; 28(4):1878. https://doi.org/10.3390/molecules28041878
Chicago/Turabian StyleMurtaza, Mobeen, Afeez Gbadamosi, Hafiz Mudaser Ahmad, Syed Muhammad Shakil Hussain, Muhammad Shahzad Kamal, Mohamed Mahmoud, and Shirish Patil. 2023. "A Magnetic Surfactant Having One Degree of Unsaturation in the Hydrophobic Tail as a Shale Swelling Inhibitor" Molecules 28, no. 4: 1878. https://doi.org/10.3390/molecules28041878
APA StyleMurtaza, M., Gbadamosi, A., Ahmad, H. M., Hussain, S. M. S., Kamal, M. S., Mahmoud, M., & Patil, S. (2023). A Magnetic Surfactant Having One Degree of Unsaturation in the Hydrophobic Tail as a Shale Swelling Inhibitor. Molecules, 28(4), 1878. https://doi.org/10.3390/molecules28041878










